Abstract
Streptococcus equi subsp. zooepidemicus has been linked to cases of acute fatal pneumonia in dogs in several countries. Outbreaks can occur in kenneled dog populations and result in significant levels of morbidity and mortality. This highly contagious disease is characterized by the sudden onset of clinical signs, including pyrexia, dyspnea, and hemorrhagic nasal discharge. The pathogenesis of S. equi subsp. zooepidemicus infection in dogs is poorly understood. This study systematically characterized the histopathological changes in the lungs of 39 dogs from a large rehoming shelter in London, United Kingdom; the dogs were infected with S. equi subsp. zooepidemicus. An objective scoring system demonstrated that S. equi subsp. zooepidemicus caused pneumonia in 26/39 (66.7%) dogs, and most of these dogs (17/26 [65.4%]) were classified as severe fibrino-suppurative, necrotizing, and hemorrhagic. Three recently described superantigen genes (szeF, szeN, and szeP) were detected by PCR in 17/47 (36.2%) of the S. equi subsp. zooepidemicus isolates; however, there was no association between the presence of these genes and the histopathological score. The lungs of S. equi subsp. zooepidemicus-infected dogs with severe respiratory signs and lung pathology did however have significantly higher mRNA levels of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and interleukin 8 (IL-8) than in uninfected controls, suggesting a role for an exuberant host immune response in the pathogenesis of this disease.
Infectious respiratory disease in dogs is usually caused by infection with one or more viruses, such as canine parainfluenza virus (CPIV) or canine herpesvirus (CHV), or bacteria, most notably Bordetella bronchiseptica, and results in the clinical syndrome termed canine infectious respiratory disease (CIRD) (7). CIRD has a multifactorial etiology and is most prevalent in shelters where stress, due to overcrowding, results in animals becoming more susceptible to infection (8). The disease is often highly contagious with high morbidity but is rarely fatal, and recovery from mild clinical signs usually occurs within a few weeks (8). The list of pathogens associated with CIRD is extensive and continues to grow; however, for many agents, the pathogenesis and specific associated lesions are poorly defined.
Streptococcus equi subsp. zooepidemicus has been shown to be associated with respiratory disease in dogs for a number of years (11); however, more recently, its significance has been highlighted by reports from several countries implicating the bacteria in a number of fatal outbreaks in shelter dogs (5, 6, 15, 24). Clinically, S. equi subsp. zooepidemicus causes severe acute respiratory distress, often with high morbidity and sometimes high mortality. At necropsy, affected dogs are usually diagnosed with severe, acute fibrino-suppurative, necrotizing, and/or hemorrhagic pneumonia.
Between 1999 and 2001, as part of a study into the causes of respiratory disease in a large rehoming kennel in London, United Kingdom, a kennel where respiratory disease was endemic, Chalker et al. (6) isolated S. equi subsp. zooepidemicus from the lungs of 48/215 dogs (22.3%) and showed that the presence of S. equi subsp. zooepidemicus was associated with increasing severity of clinical respiratory disease. Streptococcus canis was also present in some dogs but was not associated with respiratory disease (6).
The rapid onset of disease and fast deterioration in the clinical condition in many dogs infected with S. equi subsp. zooepidemicus are similar to human toxic shock syndrome caused by Streptococcus pyogenes (18). While the main site of inflammation in toxic shock syndrome is often the subcutaneous tissue, there are common clinical features of an acute illness characterized by pyrexia, hypovolemia, and coagulopathy (18).
Pyrogenic exotoxins produced by some streptococci, including S. pyogenes, act as superantigens by binding simultaneously to major histocompatibility complex (MHC) class II receptors on macrophages and T-cell receptors, bypassing conventional antigen presentation, and leading to the activation of a large proportion of T lymphocytes (9). The ensuing cytokine “avalanche” includes the production of proinflammatory cytokines, such as interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) (12, 20).
The presence of superantigen genes in strains of S. pyogenes has been linked to increased virulence and has also been suggested to contribute to the pathogenesis of “strangles,” a severe disease of horses caused by S. equi subsp. equi (4, 19, 21, 23). Superantigen genes have also been detected in some isolates of S. equi subsp. zooepidemicus (2, 13, 17). One canine isolate of S. equi subsp. zooepidemicus (isolate SzBHS5) was recently shown to encode three novel superantigen genes, szeF, szeN, and szeP, the products of which share 59%, 34%, and 49% amino acid sequence identity with the superantigens SpeH, SpeL, and SpeM of S. pyogenes, respectively (23a).
The aims of this study were (i) to characterize systematically the microscopic changes in canine lungs following infection with S. equi subsp. zooepidemicus, (ii) to test for possible associations between the severity of clinical signs, lung pathology, and the presence of S. equi subsp. zooepidemicus, and (iii) to investigate whether severe lung pathology is linked to the presence of superantigen genes in S. equi subsp. zooepidemicus isolates and/or the upregulation of the proinflammatory cytokines TNF-α, IL-8, and IL-6.
We demonstrate the following. (i) S. equi subsp. zooepidemicus in dogs most often causes severe fibrino-suppurative, necrotizing, and hemorrhagic pneumonia. (ii) Marked elevation of the mRNA of proinflammatory cytokines TNF-α, IL-8, and IL-6 is observed in dogs with S. equi subsp. zooepidemicus-induced pneumonia. (iii) As many as three superantigen genes are prevalent in canine isolates of the bacterium.
MATERIALS AND METHODS
Overview.
In this study, 39 dogs with Streptococcus equi subsp. zooepidemicus isolated from a postmortem lung wash and 16 control dogs with a sterile necropsy lung wash were studied. The S. equi subsp. zooepidemicus isolates were further analyzed by PCR for superantigen genes. Postmortem lung tissue samples of all dogs were examined microscopically and investigated by quantitative reverse transcription-PCR (RT-PCR) for mRNA levels of the proinflammatory cytokines TNF-α, IL-6, and IL-8.
Study population.
Previously, during a large study of respiratory disease in dogs at a well-established rehoming kennel in London, United Kingdom, a kennel where respiratory disease was endemic, necropsies were carried out on dogs of different ages, breeds, and sexes, euthanized for reasons ranging from behavioral problems to severe respiratory disease (8). During the postmortem examination, lung washes were performed, and the bronchoalveolar fluid obtained was used for bacterial culture by the method of Chalker et al. (6). In addition, standardized tissue samples of apical and diaphragmatic lung lobes were collected and stored frozen and as formalin-fixed paraffin-embedded tissue.
For this study, 39 dogs with positive S. equi subsp. zooepidemicus culture from the necropsy lung wash were selected. A further 16 dogs from the same study with no clinical signs of respiratory disease and no S. equi subsp. zooepidemicus cultured from their lung washes were included as controls. All dogs were screened for other potential respiratory pathogens, known to occur at the premises, including viruses (canine parainfluenza virus [CPIV], canine herpesvirus [CHV], canine respiratory coronavirus [CRCoV], canine adenovirus [CAV], and canine distemper virus [CDV]) and bacteria (Mycoplasma spp. and Bordetella bronchiseptica) by PCR on lung tissue according to previously published methods (7, 8, 10, 16).
Study groups and histopathological scoring.
The dogs within this study (n = 55) were assigned to four groups (groups 0 to 3) based on the clinical respiratory signs, which had been recorded by veterinarians immediately antemortem, and the presence or absence of S. equi subsp. zooepidemicus in the postmortem lung wash (Table 1). For each dog within the study groups, sections of apical and diaphragmatic lung lobes were scored blindly by a veterinary pathologist for the presence and relative abundance of the following parameters: hemorrhage, neutrophils, fibrin exudation, histiocytes, and tissue necrosis (Table 2). The presence of bacteria and vascular thrombosis was also noted. Individual histological scores for each lung lobe were added together to give a total histopathological score for each dog. The parameters of the grading scheme (Table 3) enabled the objective diagnosis of the presence or absence of pneumonia and type of pneumonia. If the apical and diaphragmatic lung lobes differed in regard to certain scoring parameters, the lobe with the highest individual score was used for pneumonia classification.
TABLE 1.
Scoring scheme for clinical respiratory signs
| Study group (no. of dogs) | Score for clinical signs | S. equi subsp. zooepidemicus isolated | Clinical sign(s) |
|---|---|---|---|
| 0 (16) | 0 | No | None |
| 1 (7) | 1 | Yes | None |
| 2 (17) | 2 | Yes | Cough and nasal discharge |
| 3 (15) | 3 | Yes | Cough, nasal discharge, and pyrexia |
TABLE 2.
Histopathological scoring of lung parameters according to their presence and severity
| Parameter | Definition of histopathological score of: |
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Red blood cells (% parenchyma) | None | <10% parenchyma with >3 cells or 1 to 3 cells in <50% parenchyma | 10 to 50% parenchyma with >3 cells or 1 to 3 cells in >50% parenchyma | >50% parenchyma with >3 cells |
| Neutrophils (% parenchyma) | None | <10% parenchyma with >3 cells or 1 to 3 cells in <50% parenchyma | 10 to 50% parenchyma with >3 cells or 1 to 3 cells in >50% parenchyma | >50% parenchyma with >3 cells |
| Fibrin exudation (% parenchyma) | None | <10% | 10 to 50% | >50% |
| No. of histiocytes (per alveolus) | <3 cells | 3 to 10 cells | >10 cells | |
| Tissue necrosis | None | 10% | 10 to 50% | >50% |
TABLE 3.
Pneumonia classification based on histopathological scoresa
| Pneumonia category | Criteria for pneumonia category (histopathological scores) |
|---|---|
| No pneumonia | Neutrophil score of ≤1 and fibrin score of ≤1 and hemorrhage score of ≤1 |
| Pneumonia | |
| Fibrinous | Fibrin score of ≥2 or fibrin score of ≥1 and necrosis or fibrin score of ≥1 and hemorrhage score of 1 and necrosis |
| Hemorrhagic | Hemorrhage score of ≥2 or hemorrhage score of ≥1 and necrosis |
| Fibrino-suppurative | Fibrin score of ≥2 and neutrophil score of ≥1 |
| Fibrino-suppurative and necrotizing | Fibrin score of ≥2 and neutrophil score of ≥1 and necrosis |
| Fibrino-suppurative, necrotizing, and hemorrhagic | Fibrin score of ≥2 and neutrophil score of ≥1 and hemorrhage score of ≥2 |
Statistical analysis of histopathological scoring.
The total histopathological scores for apical and diaphragmatic lung lobes were compared by the Wilcoxon test. The total histopathological scores for S. equi subsp. zooepidemicus-infected and uninfected dogs were compared using a Mann-Whitney independent sample test, and this was repeated for other respiratory pathogens (CPIV, CHV, CRCoV, Mycoplasma spp., and B. bronchiseptica). In addition, histopathological scores for study groups were compared by the Kruskal-Wallis test, and posthoc pairwise comparisons were made by using the Mann-Whitney test with Bonferroni's correction for multiple comparisons. The histopathological score was also compared to the length of time in the kennel (days from admission to death) using the Spearman rank correlation coefficient. All statistical tests were performed using SPSS Statistics 17.0 (SPSS Inc.), and for all analyses, a P value of <0.05 was considered significant.
PCR for superantigen genes in S. equi subsp. zooepidemicus.
S. equi subsp. zooepidemicus isolates, obtained from necropsy lung washes, were available from 38/39 dogs scored microscopically. A further 9 isolates were available from additional dogs within the same population which did not have sections available for histopathological evaluation. Each isolate was recultured from cryopreserved stock (−70°C) on blood agar. After 48 h of culture, individual colonies were selected, placed in Todd-Hewitt broth (Oxoid, Basingstoke, United Kingdom), and further incubated overnight in a shaking incubator (200 rpm) at 37°C. Overnight cultures were centrifuged (800 × g for 10 min), the supernatant was removed, and the bacteria in the pellet were lysed using the DNeasy blood and tissue kit according to the manufacturer's protocol (Qiagen, Crawley, United Kingdom). To confirm adequate DNA quality and quantity, a superoxidase dismutase A (sodA) gene PCR was performed on the extracted DNA (3).
A single canine isolate of S. equi subsp. zooepidemicus (SzBHS5) from one of the necropsied dogs was previously sequenced at the Centre for Genomic Research, University of Liverpool, United Kingdom (23a). PCR primers were designed to amplify the szeF, szeN, and szeP genes (Table 4). Two sets of primers were designed for each gene: one pair to amplify the full-length gene (primers 1 and 2) and another pair to amplify a shorter internal sequence (primers 3 and 4). PCR amplification was performed in a 50-μl reaction mixture containing 1× Taq amplification buffer (Promega, Southampton, United Kingdom), 0.8 mM deoxynucleoside triphosphate (dNTP) mixture (Bioline, London, United Kingdom), 2.0 mM MgCl2 (Promega), 0.5 μM internal primers, and 1.25 units GoTaq DNA polymerase (Promega). Cycling conditions were as follows: (i) denaturation for 5 min at 95°C; (ii) 30 cycles of PCR amplification, with 1 cycle consisting of denaturation for 1 min at 95°C, annealing for 30 s at 50°C, and extension for 45 s at 72°C; (iii) a final extension for 5 min at 72°C. Detection of the amplified products was carried out by electrophoresis in an agarose gel stained with SafeView (NBS Biologicals, Huntingdon, United Kingdom). Each experiment included two negative controls (a blank sample consisting of reaction mixture without DNA and another with S. canis DNA) and a positive control (isolate SzBHS5).
TABLE 4.
Primer sequences for amplification of szeF, szeN, and szeP superantigen genes
| Gene and primera | Sequence (5′ to 3′) |
|---|---|
| szeF | |
| F1 | ATC-CGA-ATG-TAT-ACT-GAC-GAA-AAG |
| F2 | TTG-CTA-GCA-GAT-GAC-AGG-AAG-ATA |
| F3 | TTT-TAT-CTT-GTG-GCT-TTC-GTT-A |
| F4 | CTA-TTT-CTT-GGG-CTG-TTA-CTA-T |
| szeN | |
| N1 | AAA-TGC-TCA-AAA-GTG-CGA-CAG-G |
| N2 | ATA-GAA-GGT-AGA-GCC-CCA-AGA-TAA-GAT-A |
| N3 | AAT-GTA-AGT-TTA-TCC-GAA-GAA |
| N4 | AAT-ATC-CAG-TTG-AGA-AAT-CC |
| szeP | |
| P1 | TGT-CGC-ACT-TTT-GAG-CAT-TTT-G |
| P2 | CTG-AGC-GAT-TTT-AAC-ATA-GTA-GTC |
| P3 | AAG-CGA-GTT-AAT-GGG-ATA-CGA-T |
| P4 | TCA-CCC-TTT-ACA-AAT-TTA-CCT-T |
Two sets of primers were designed for each gene: one pair to amplify the full-length gene (primers 1 and 2 [e.g., primers F1 and F2 for the szeF gene]) and another pair to amplify a shorter internal sequence (primers 3 and 4 [e.g., primers F3 and F4 for the szeF gene]).
PCR product cloning and sequencing.
PCR amplification of the full-length superantigen genes (szeF, szeN, and szeP) was performed using external primers (Table 4) in a 50-μl reaction mixture using the LongRange PCR system (Qiagen), according to the manufacturer's protocol. Cycling conditions were as follows: (i) denaturation for 3 min at 93°C; (ii) 35 cycles of PCR amplification, with 1 cycle consisting of denaturation for 15 s at 93°C, annealing for 30 s at 50°C, and extension for 1 min at 68°C; (iii) a final extension for 5 min at 68°C. PCR products were purified using the QIAquick gel extraction kit (Qiagen) and then cloned into the pGEM-T Easy plasmid vector (Promega), both following the manufacturer's protocol. The plasmid was used to transform Escherichia coli XL1-Blue competent cells (Stratagene, Stockport, United Kingdom), and successful transformants were selected on LB agar supplemented with ampicillin (100 μg ml−1) (Sigma) and also containing isopropyl-β-d-thiogalactopyranoside (IPTG) (0.5 mM) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (80 μg ml−1) (Promega). Purified plasmid was obtained from selective (ampicillin [100 μg ml−1]) LB broth cultures of transformed cells using the QIAprep spin miniprep kit (Qiagen) according to the manufacturer's protocol. DNA sequencing was performed by the Sequencing Service, School of Life Sciences, University of Dundee. Sequences were aligned using MegAlign (DNASTAR, Madison, WI), and a sequence similarity search was performed using FASTA.
Quantitative RT-PCR for the detection of proinflammatory cytokine mRNA.
The mRNA levels of the proinflammatory cytokines TNF-α, IL-6, and IL-8 were compared in samples of lung tissue from 10 dogs of study group 3 (i.e., severe clinical respiratory signs and lung pathology and S. equi subsp. zooepidemicus isolated) and 10 dogs of study group 0 (i.e., no clinical respiratory signs or lung pathology and a sterile postmortem lung wash).
Samples of lung tissue (3 mm3) (stored at −70°C) were homogenized using a ball mill (MM 300; Retsch, Leeds, United Kingdom) for 4 min at 20 Hz, and RNA was extracted using the RNeasy minikit (Qiagen) following the manufacturer's protocol. Genomic DNA was removed using RQ1 RNA-free DNase (Promega) according to the manufacturer's protocol, and cDNA synthesis was performed as follows. One microgram of RNA was added to 1 μl of pd(N)6 random hexamers (0.5 mg ml−1) (GE Healthcare, Little Chalfont, United Kingdom) and incubated at 70°C for 10 min. cDNA was synthesized using the ImProm-II reverse transcription system (Promega) and quantified using Quant-iT PicoGreen double-stranded RNA reagent (Invitrogen, Paisley, United Kingdom) according to the manufacturer's protocol. Quantification of IL-6, TNF-α, and IL-8 mRNA was performed by previously published methods using the GoTaq quantitative PCR master mix (Promega) according to the manufacturer's protocol (25). cDNA samples and standards were processed in duplicate, and a negative control and two internal controls (previously quantified cDNA samples) were included. Quantification was performed using standard curves produced by the Opticon Monitor software v. 3.1 (Bio-Rad, Hemel Hempstead, United Kingdom). The absolute mRNA copy number was normalized to cDNA concentration per gram of tissue. Mean cytokine mRNA copies for control and S. equi subsp. zooepidemicus-infected dogs were compared by a t test (SPSS Statistics).
RESULTS
Lung tissue samples from all dogs included in this study were screened by PCR for known respiratory pathogens, and the results are summarized in Table 5. All dogs were negative for canine adenovirus (CAV) and canine distemper virus (CDV) by PCR.
TABLE 5.
Average histopathological lung scores for all dogs infected with S. equi subsp. zooepidemicus and for dogs coinfected with S. equi subsp. zooepidemicus and common respiratory viruses and bacteria
| Dog infection categorya | Avg histopathological score for: |
P value | |
|---|---|---|---|
| Infected dogs (no. of dogs) | Uninfected dogs (no. of dogs) | ||
| All dogs infected with SZ | 14.5 (39) | 4.9 (16) | 0.0003 |
| Dogs infected with SZ and another virus (CHV and/or CPIV and/or CRCoV) | 11.7 (14) | 13.6 (16) | 0.5947 |
| Canine parainfluenza virus (CPIV) | 10.2 (5) | 14.9 (29) | 0.3357 |
| Canine respiratory coronavirus (CRCoV) | 12.5 (6) | 13.2 (23) | 0.8816 |
| Canine herpesvirus (CHV) | 16.5 (6) | 14.1 (33) | 0.5859 |
| Dogs infected with SZ and bacteria | |||
| Mycoplasma spp. | 14.6 (17) | 14.4 (21) | 0.9610 |
| Mycoplasma cynos | 16.0 (7) | 14.2 (31) | 0.6598 |
| B. bronchiseptica | 9.0 (10) | 16.4 (29) | 0.0354 |
SZ, S. equi subsp. zooepidemicus.
Within groups with positive S. equi subsp. zooepidemicus isolation (groups 1 to 3), 66.7% (26/39) of dogs were diagnosed with pneumonia, whereas only 6.3% (1/16) of the control dogs (group 0) showed microscopic evidence of pneumonia (one case of hemorrhagic pneumonia). The types of pneumonia diagnosed among S. equi subsp. zooepidemicus-infected dogs were fibrino-suppurative, necrotizing, and hemorrhagic (65.4%; n = 17), fibrinous (15.4%; n = 4), hemorrhagic (15.4%; n = 4), and fibrino-suppurative (3.8%; n = 1). Interestingly, within group 3 (the group with the most severe clinical respiratory signs), 80.0% (12/15) of dogs had the most severe form of pneumonia, i.e., fibrino-suppurative, necrotizing, and hemorrhagic pneumonia (Fig. 1), whereas only 23.5% (4/17) and 14.3% (1/7) of dogs in groups 2 and 1, respectively, were assigned this diagnosis.
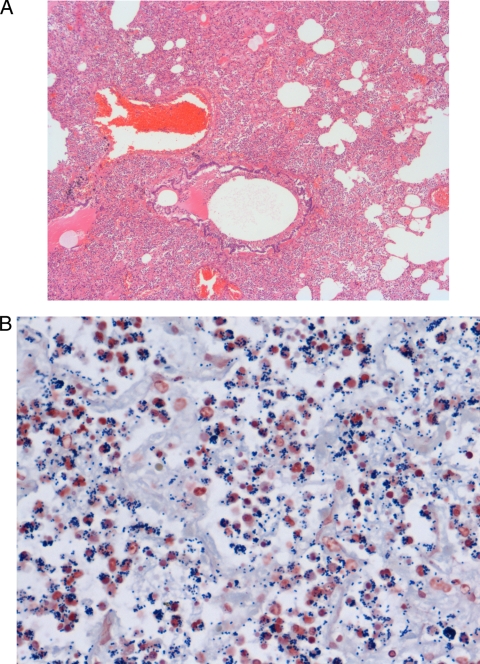
FIG. 1.
Canine lung sections. (A) Typical fibrino-suppurative, necrotizing, and hemorrhagic pneumonia due to S. equi subsp. zooepidemicus, characterized by diffuse filling and obliteration of alveolar spaces by numerous neutrophils, fibrin, and proteinaceous fluid. Bronchioles contain hemorrhaged blood, proteinaceous fluid, and sloughed necrotic cells. This section was stained with hematoxylin and eosin. Magnification, ×20. (B) Neutrophils frequently contain numerous intracellular Gram-positive cocci. This section was Gram stained. Magnification, ×40.
The total histopathological scores for apical and diaphragmatic lobes were not found to be significantly different (P = 0.7), thus scores were combined to give an overall histopathological lung score for each dog that was used for all subsequent analyses.
Statistically significant differences in total histopathological scores were recorded for S. equi subsp. zooepidemicus-infected dogs versus uninfected dogs (P < 0.001). Similar analyses were performed for coinfections with other common respiratory pathogens, although no significant differences in the histopathological score were identified (Table 5).
The mean histopathological scores for each study group were compared, and statistically significant differences were identified between groups 0 and 3 (P < 0.001) and groups 2 and 3 (P < 0.001). There was no statistically significant correlation between the length of time in the kennel and the histopathological score (P = 0.3).
PCR amplification and sequencing of superantigen genes.
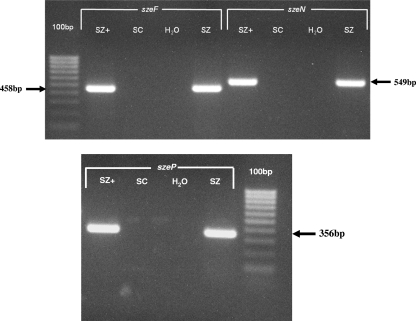
All isolates were positive for sodA gene and PCR amplification using internal primer pairs for the szeF, szeN, and szeP genes was performed on genomic DNA from 47 canine isolates of S. equi subsp. zooepidemicus (Fig. 2). Specifically, 36.2% (17/47) of isolates were positive for all three genes; the remaining 63.8% (30/47) of isolates were negative for all three genes. There was no significant association between the presence or absence of genes and the total histopathological score (P = 0.4) or the respiratory score (P = 0.3). For one isolate that was PCR positive for all three genes (isolate SzBHS7), sequences were amplified using external primer pairs, cloned into a plasmid vector, and sequenced. The sequences were compared to that of isolate SzBHS5; DNA and amino acid sequence identity were 100% for SzeF, SzeN, and SzeP (23a).
FIG. 2.
PCR amplification of S. equi subsp. zooepidemicus DNA using internal primers to the superantigen genes szeF, szeN, and szeP. Lanes: SZ+, S. equi subsp. zooepidemicus isolate SzBHS5; SC, S. canis control; H2O, water control with no DNA added; SZ, S. equi subsp. zooepidemicus isolate from one dog (isolate SzBHS7).
Canine cytokine and chemokine mRNA quantification.
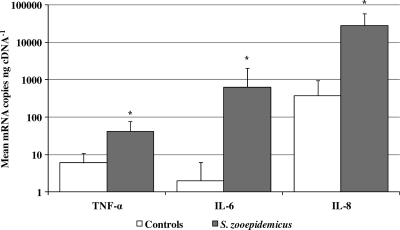
Quantification of mRNA copies of canine TNF-α, IL-6, and IL-8 in samples of lung tissue from 10 control dogs and 10 dogs with S. equi subsp. zooepidemicus-associated pneumonia showed that S. equi subsp. zooepidemicus-infected dogs had significantly higher levels of TNF-α, IL-6, and IL-8 mRNA than uninfected control dogs did (P = 0.002, P < 0.001, and P < 0.001, respectively). Specifically, the mean number of mRNA copies (per nanogram of cDNA) were 5.8 and 42.3 (TNF-α), 2.4 and 634.9 (IL-6), and 380.5 and 27929.2 (IL-8) for control and S. equi subsp. zooepidemicus-infected animals, respectively, corresponding to 7.3-, 264.5-, and 73.4-fold increases for TNF-α, IL-6, and IL-8, respectively (Fig. 3).
FIG. 3.
Mean number of canine cytokine and chemokine mRNA copies in samples of lung tissue from control and S. equi subsp. zooepidemicus-infected dogs. Values that are statistically significantly different (P < 0.005) in the control lung and infected lung are indicated by an asterisk. The values are means plus 1 standard deviation (error bars).
DISCUSSION
This study systematically characterized pneumonia due to S. equi subsp. zooepidemicus in dogs using a specifically designed and objective histopathological scoring scheme. The majority of S. equi subsp. zooepidemicus-infected dogs with pneumonia had severe fibrino-suppurative, necrotizing, and hemorrhagic pneumonia, which is strongly associated with severe clinical signs. A small number of dogs (group 1) had no clinical signs of respiratory disease despite isolation of S. equi subsp. zooepidemicus from a postmortem lung wash. It is possible that these dogs were either carriers of S. equi subsp. zooepidemicus, perhaps they had previously been infected and recovered, or bacterial and/or host factors contributed to the dogs not developing clinical disease.
While a number of other viral and bacterial pathogens were present in the dog population studied, none of these pathogens were associated with pneumonia as assessed by using the histopathological score. Moreover, histopathological lung lesions typical for infection with canine herpesvirus (CHV), canine parainfluenza virus (CPIV), canine adenovirus (CAV), and canine distemper virus (CDV) were absent. Several of these other agents, in particular canine respiratory coronavirus (CRCoV), are associated with upper respiratory tract infections, and thus, their involvement in pneumonia would not be expected. Interestingly, dogs coinfected with B. bronchiseptica had a significantly lower histopathological score (P = 0.04) than dogs not coinfected with B. bronchiseptica. This should be interpreted with caution because of the borderline significance of the association; however, this is supportive of B. bronchiseptica having a tropism for the canine upper respiratory tract. In addition, the presence of two different bacteria competing within the same organ system could reduce the overall lung load of S. equi subsp. zooepidemicus. An interesting potential implication of this result is that the widespread use of kennel cough vaccines, containing B. bronchiseptica, may have created a “niche” for S. equi subsp. zooepidemicus infection in dogs. Of the mycoplasmas, only Mycoplasma cynos has been associated with lower respiratory tract infections (28). However, in this study, there was no association between the presence of this bacterium and the histopathological score.
Three superantigen genes, szeF, szeN, and szeP, the products of which have 59%, 34%, and 49% amino acid sequence identity to SpeH, SpeL, and SpeM of S. pyogenes, respectively, were previously identified in the genome of isolate SzBHS5 (23a). In order to try to explain differences in both the clinical signs and lung pathology of dogs known to be infected with S. equi subsp. zooepidemicus, isolates obtained from postmortem lung washes were screened for the presence of these genes by PCR. Thirty-six percent of isolates in this study were positive, and the identity of the amplified products of one strain was confirmed by sequencing. The remaining 64% of isolates were negative for all three genes, and there was no association between the presence or absence of these genes and clinical respiratory or histopathological scores. This suggests that either these proteins do not have superantigen activity in vivo or that other, as yet unidentified, virulence factors may be associated with severe S. equi subsp. zooepidemicus-associated disease in dogs.
The mRNA levels of TNF-α, IL-8, and IL-6 were significantly increased in the lungs of dogs with severe pneumonia induced by S. equi subsp. zooepidemicus than in uninfected dogs without lung pathology, suggesting that raised levels of these proinflammatory cytokines contribute to the pathogenesis of the infection in canine lungs. Studies in humans have shown a direct correlation between serum levels of proinflammatory cytokines, including TNF-α and IL-6 and the severity of streptococcal toxic shock syndrome (STSS) (22). It has recently been shown that plasma levels of IL-6 in dogs are predictive for the severity of sepsis and systemic inflammatory response syndrome (SIRS) (26). The role of these cytokines in severe pneumonia is also documented for other bacteria and in other species, particularly in pigs with Mycoplasma hyopneumoniae (1, 27). Host inflammatory factors are involved in lung lesion development in porcine Actinobacillus pleuropneumoniae infection, where TNF-α together with IL-1 stimulates the synthesis and rapid release of IL-8 by pulmonary macrophages following acute insult (14). IL-8 is strongly chemotactic for neutrophils and given the presence of numerous neutrophils observed microscopically, would be expected to be significantly elevated. The presence of large numbers of neutrophils adds to the pulmonary injury via the release of inflammatory mediators, e.g., leukotrienes and proteases, a process termed acute respiratory distress syndrome (ARDS) (29).
S. equi subsp. zooepidemicus is frequently associated with acute and often fatal clinical disease in dogs. This study has shown that when pneumonia does occur, it is usually histopathologically severe and extensive. On the basis of detailed histopathological examinations, the szeF, szeN, and szeP genes are not essential factors in pathogenesis of the disease; however, the significant increases in proinflammatory cytokine mRNA within the lungs suggest that this bacteria can evoke potentially detrimental immune dysregulation in dogs. Histopathology alone may not be sensitive enough to establish the involvement of superantigens in the disease, and further investigations will be required to explore the many unanswered questions regarding the high pathogenicity of this organism in dogs.
Acknowledgments
The work of A.C.D. was funded by The RCVS Trust.
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Ahn, K. K., D. Kwon, K. Jung, Y. Ha, M. J. Seo, S. H. Kim, M. Y. Kim, K. D. Cho, B. H. Lee, and C. Chae. 2009. Identification of interleukin-1, tumor necrosis factor-alpha, and interleukin-6 expression in lungs from pigs naturally infected with Mycoplasma hyopneumoniae by in situ hybridization. J. Vet. Med. Sci. 71:441-445. [DOI] [PubMed] [Google Scholar]
- 2.Alber, J., A. El-Sayed, S. Estoepangestie, C. Lammler, and M. Zschock. 2005. Dissemination of the superantigen encoding genes seeL, seeM, szeL and szeM in Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus. Vet. Microbiol. 109:135-141. [DOI] [PubMed] [Google Scholar]
- 3.Alber, J., A. El-Sayed, C. Lammler, A. A. Hassan, R. Weiss, and M. Zschock. 2004. Multiplex polymerase chain reaction for identification and differentiation of Streptococcus equi subsp. zooepidemicus and Streptococcus equi subsp. equi. J. Vet. Med. B Infect. Dis. Vet. Public Health 51:455-458. [DOI] [PubMed] [Google Scholar]
- 4.Anzai, T., A. S. Sheoran, Y. Kuwamoto, T. Kondo, R. Wada, T. Inoue, and J. F. Timoney. 1999. Streptococcus equi but not Streptococcus zooepidemicus produces potent mitogenic responses from equine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 67:235-246. [DOI] [PubMed] [Google Scholar]
- 5.Byun, J. W., S. S. Yoon, G. H. Woo, B. Y. Jung, and Y. S. Joo. 2009. An outbreak of fatal hemorrhagic pneumonia caused by Streptococcus equi subsp. zooepidemicus in shelter dogs. J. Vet. Sci. 10:269-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalker, V. J., H. W. Brooks, and J. Brownlie. 2003. The association of Streptococcus equi subsp. zooepidemicus with canine infectious respiratory disease. Vet. Microbiol. 95:149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erles, K., E. J. Dubovi, H. W. Brooks, and J. Brownlie. 2004. Longitudinal study of viruses associated with canine infectious respiratory disease. J. Clin. Microbiol. 42:4524-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erles, K., C. Toomey, H. W. Brooks, and J. Brownlie. 2003. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 310:216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser, J. D., and T. Proft. 2008. The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 225:226-243. [DOI] [PubMed] [Google Scholar]
- 10.Frisk, A. L., M. Konig, A. Moritz, and W. Baumgartner. 1999. Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. J. Clin. Microbiol. 37:3634-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnett, N. L., R. S. Eydelloth, M. M. Swindle, S. L. Vonderfecht, J. D. Strandberg, and M. B. Luzarraga. 1982. Hemorrhagic streptococcal pneumonia in newly procured research dogs. J. Am. Vet. Med. Assoc. 181:1371-1374. [PubMed] [Google Scholar]
- 12.Hackett, S. P., and D. L. Stevens. 1992. Streptococcal toxic shock syndrome: synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J. Infect. Dis. 165:879-885. [DOI] [PubMed] [Google Scholar]
- 13.Holden, M. T., Z. Heather, R. Paillot, K. F. Steward, K. Webb, F. Ainslie, T. Jourdan, N. C. Bason, N. E. Holroyd, K. Mungall, M. A. Quail, M. Sanders, M. Simmonds, D. Willey, K. Brooks, D. M. Aanensen, B. G. Spratt, K. A. Jolley, M. C. Maiden, M. Kehoe, N. Chanter, S. D. Bentley, C. Robinson, D. J. Maskell, J. Parkhill, and A. S. Waller. 2009. Genomic evidence for the evolution of Streptococcus equi: host restriction, increased virulence, and genetic exchange with human pathogens. PLoS Pathog. 5:e1000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang, H., A. A. Potter, M. Campos, F. A. Leighton, P. J. Willson, D. M. Haines, and W. D. Yates. 1999. Pathogenesis of porcine Actinobacillus pleuropneumonia, part II: roles of proinflammatory cytokines. Can. J. Vet. Res. 63:69-78. [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, M. K., H. Jee, S. W. Shin, B. C. Lee, B. Pakhrin, H. S. Yoo, J. H. Yoon, and D. Y. Kim. 2007. Outbreak and control of haemorrhagic pneumonia due to Streptococcus equi subspecies zooepidemicus in dogs. Vet. Rec. 161:528-530. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, H., K. Yamamoto, M. Eguchi, M. Kubo, S. Nakagami, S. Wakisaka, M. Kaizuka, and H. Ishii. 1995. Rapid detection of mycoplasma contamination in cell cultures by enzymatic detection of polymerase chain reaction (PCR) products. J. Vet. Med. Sci. 57:769-771. [DOI] [PubMed] [Google Scholar]
- 17.Korman, T. M., A. Boers, T. M. Gooding, N. Curtis, and K. Visvanathan. 2004. Fatal case of toxic shock-like syndrome due to group C streptococcus associated with superantigen exotoxin. J. Clin. Microbiol. 42:2866-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lappin, E., and A. J. Ferguson. 2009. Gram-positive toxic shock syndromes. Lancet Infect. Dis. 9:281-290. [DOI] [PubMed] [Google Scholar]
- 19.Luca-Harari, B., K. Ekelund, M. van der Linden, M. Staum-Kaltoft, A. M. Hammerum, and A. Jasir. 2008. Clinical and epidemiological aspects of invasive Streptococcus pyogenes infections in Denmark during 2003 and 2004. J. Clin. Microbiol. 46:79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller-Alouf, H., J. E. Alouf, D. Gerlach, J. H. Ozegowski, C. Fitting, and J. M. Cavaillon. 1994. Comparative study of cytokine release by human peripheral blood mononuclear cells stimulated with Streptococcus pyogenes superantigenic erythrogenic toxins, heat-killed streptococci, and lipopolysaccharide. Infect. Immun. 62:4915-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musser, J. M., A. R. Hauser, M. H. Kim, P. M. Schlievert, K. Nelson, and R. K. Selander. 1991. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc. Natl. Acad. Sci. U. S. A. 88:2668-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norrby-Teglund, A., K. Pauksens, M. Norgren, and S. E. Holm. 1995. Correlation between serum TNF alpha and IL6 levels and severity of group A streptococcal infections. Scand. J. Infect. Dis. 27:125-130. [DOI] [PubMed] [Google Scholar]
- 23.Paillot, R., C. Robinson, K. Steward, N. Wright, T. Jourdan, N. Butcher, Z. Heather, and A. S. Waller. 2010. Contribution of each of four superantigens to Streptococcus equi-induced mitogenicity, gamma interferon synthesis, and immunity. Infect. Immun. 78:1728-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Paillot, R., A. C. Darby, C. Robinson, N. L. Wright, K. F. Steward, E. A. Anderson, K. Webb, M. T. G. Holden, A. Efstratiou, K. Broughton, K. A. Jolley, S. L. Priestnall, M. C. M. Campi, M. A. Hughes, A. Radford, K. Erles, and A. S. Waller. 2010. Identification of three novel superantigen-encoding genes in Streptococcus zooepidemicus, szeF, szeN, and sze. Infect. Immun. 78:4817-4827. [DOI] [PMC free article] [PubMed]
- 24.Pesavento, P. A., K. F. Hurley, M. J. Bannasch, S. Artiushin, and J. F. Timoney. 2008. A clonal outbreak of acute fatal hemorrhagic pneumonia in intensively housed (shelter) dogs caused by Streptococcus equi subsp. zooepidemicus. Vet. Pathol. 45:51-53. [DOI] [PubMed] [Google Scholar]
- 25.Priestnall, S. L., J. A. Mitchell, H. W. Brooks, J. Brownlie, and K. Erles. 2009. Quantification of mRNA encoding cytokines and chemokines and assessment of ciliary function in canine tracheal epithelium during infection with canine respiratory coronavirus (CRCoV). Vet. Immunol. Immunopathol. 127:38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rau, S., B. Kohn, C. Richter, N. Fenske, H. Kuchenhoff, K. Hartmann, S. Hartle, B. Kaspers, and J. Hirschberger. 2007. Plasma interleukin-6 response is predictive for severity and mortality in canine systemic inflammatory response syndrome and sepsis. Vet. Clin. Pathol. 36:253-260. [DOI] [PubMed] [Google Scholar]
- 27.Redondo, E., A. J. Masot, A. Fernandez, and A. Gazquez. 2009. Histopathological and immunohistochemical findings in the lungs of pigs infected experimentally with Mycoplasma hyopneumoniae. J. Comp. Pathol. 140:260-270. [DOI] [PubMed] [Google Scholar]
- 28.Rosendal, S. 1978. Canine mycoplasmas: pathogenicity of mycoplasmas associated with distemper pneumonia. J. Infect. Dis. 138:203-210. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler, A. P., and G. R. Bernard. 2007. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369:1553-1564. [DOI] [PubMed] [Google Scholar]