Abstract
The present study evaluated the potential of recombinant binding region A of clumping factor A (rClfA-A) to be an effective component of a vaccine against mastitis induced by Staphylococcus aureus in the mouse. rClfA-A and inactivated S. aureus were each emulsified in Freund's adjuvant, mineral oil adjuvant, and Seppic adjuvant; phosphate-buffered saline was used as a control. Seven groups of 12 mice each were immunized intraperitoneally three times at 2-week intervals. The titers of IgG and subtypes thereof (IgG1 and IgG2a) in the rClfA-A-immunized group were more than 1,000-fold higher than those in the killed-bacteria-immunized group (P < 0.01). Of the three adjuvants used, mineral oil adjuvant induced the highest antibody levels for both antigens (P < 0.001). Furthermore, the anti-rClfA-A antibody capacities for bacterial adhesion and opsonizing phagocytosis were significantly greater in the rClfA-A-immunized group than in the killed-bacteria-immunized group (P < 0.05). Lactating mice immunized with either rClfA-A or inactivated vaccine were challenged with S. aureus via the intramammary route. The numbers of bacteria recovered from the murine mammary glands 24 h after inoculation were significantly lower in the rClfA-A group than in the killed-bacteria-immunized group (P < 0.001). Histologic examination of the mammary glands showed that rClfA-A immunization effectively preserved tissue integrity. Thus, rClfA-A emulsified in an oil adjuvant provides strong immune protection against S. aureus-induced mastitis in the mouse.
Staphylococcus aureus is the most common etiologic agent of contagious bovine mastitis, which results in a decline in milk production, culling of the dairy cow, and increased treatment costs (1, 29). In addition, food-borne S. aureus has become a major public health concern owing to the rapid evolution of resistant lineages (6). A vaccine against S. aureus-induced mastitis in dairy cows would represent a safe and ideal means of preventing and controlling mastitis (27).
S. aureus infection is a complex process that involves a series of events, resulting in malfunction or destruction of host tissues. Adherence of the microorganism to host tissues represents a critical first step. Nonadherent bacteria can be readily removed from the host by clearing mechanisms, such as peristalsis and excretion (3, 8). Thus, blocking bacterial adhesion to cells and colonization of the mucosal surface may be the most effective strategy for preventing S. aureus infection (19).
S. aureus clumping factor A (ClfA), which is usually covalently anchored to the peptidoglycan of the bacterial cell wall, is an important adhesin and a critical virulence factor. ClfA mediates the binding of S. aureus to fibrinogen on the host cell surface and promotes bacterial invasion into host tissues. An S. aureus clfA mutant displayed reduced virulence in mice (16). Stutzmann et al. (26) showed that introduction of the clfA gene into a less virulent organism, such as Streptococcus gordonii, significantly increased its infective capacity in a mouse endocarditis model. Tuchscherr et al. (30) showed that the passive transfer of antibodies to ClfA protected mice against mastitis. As ClfA does not show genetic polymorphism, it appears to be a suitable component of a novel vaccine against S. aureus infection and the resultant mastitis (2, 23). Josefsson et al. (10) demonstrated that the severity of arthritis was markedly reduced in mice immunized with ClfA. A DNA vaccine that encodes ClfA, as well as the fibronectin-binding motifs of FnBP, delivered twice and boosted once with recombinant fibronectin-binding motifs and ClfA proteins provided partial protection to the mammary gland against staphylococcal mastitis and produced better postchallenge conditions in vaccinated heifers (25). However, a safety concern is that the introduced DNA may be integrated into the host cell chromosomes by insertional mutagenesis (5). To enhance the immune responses induced by ClfA, the potent cytokine interleukin-18 (IL-18) has been used as an adjuvant (32).
The fragment of ClfA responsible for its activity lies within binding region A of ClfA (ClfA-A) (14). Hartford et al. (7) localized the fibrinogen-binding activity of ClfA to residues 221 to 559 of region A. Furthermore, the fibrinogen-binding sites P336 and Y338 of clumping factor A are crucial for S. aureus virulence (9). ClfA-A not only promotes bacterial binding to the cell surface but also camouflages S. aureus so as to inhibit phagocytosis (19). In addition, immunization with purified ClfA-A was found to protect against staphylococcus-mediated arthritis (10).
In the present study, ClfA-A was expressed and subunit vaccines were prepared as several combinations of recombinant ClfA-A (rClfA-A) and various adjuvants, and these were then evaluated in a BALB/c mouse model of mastitis. The results indicate that a vaccine formulation composed of rClfA-A and an appropriate adjuvant was effective in the prevention of S. aureus-induced mastitis.
MATERIALS AND METHODS
Bacteria and culture media.
S. aureus strain J9 was isolated and identified from a case of bovine mastitis in Dongxihu District, Wuhan City, China. The identity of the strain was confirmed by PCR and 16S rRNA sequencing. S. aureus strain J9 was grown in tryptic soy broth or agar (BD Difco, Sparks, MD). Escherichia coli strain DH5α grown in Luria-Bertani broth or agar (Difco) at 37°C in the presence of 50 μg/ml kanamycin when necessary was used as the host for cloning.
DNA extraction and PCR.
Genomic DNA extracted from S. aureus strain J9 using an extraction kit (Clontech, Palo Alto, CA) was used as the PCR template. Binding region A of ClfA was amplified using the forward primer ClfA-A-Fwd (5′-TATGGATCCGTAGCTGCAGATGCACC-3′), which includes a BamHI restriction site (underlined), and the reverse primer ClfA-A-Rev (5′-CGCGTCGACCTCTGGAATTGGTTCAATTTC-3′), which includes a SalI restriction site (underlined). The primers were specific for the sequence of the ClfA-A fragment, on the basis of the published sequence (GenBank accession no. BA000018). PCR was conducted under the following conditions: an initial 4-min denaturation at 95°C, followed by 30 cycles of 30 s at 95°C, 30 s at 50°C, and 1 min at 72°C and with a final extension step of 72°C for 10 min. The amplified products were purified using a QIAquick purification kit (Qiagen, Hilden, Germany).
Construction of recombinant plasmid.
The ClfA-A fragment was ligated into pMD-18T (Takara Bio, Ohtsu, Japan) to generate pMD-ClfA-A, excised following digestion with BamHI and SalI, and ligated into the similarly digested pET-28a(+) vector (Promega, Madison, WI) to produce pET-28a-ClfA-A. Restriction endonuclease digestion and automated DNA sequencing were used to verify coding sequence accuracy.
Expression and purification of recombinant proteins.
The recombinant plasmid was transformed into competent BL21(DE3) cells, and the transformants were subsequently cultivated to the mid-log phase at 37°C. Recombinant protein expression was induced by the addition of 0.8 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and the cells were harvested at 6 h postinduction. The cells were collected by centrifugation at 10,800 × g for 10 min at 4°C, washed in 0.1 M phosphate-buffered saline (PBS; pH 7.2), and disrupted by sonication. rClfA-A was purified by affinity chromatography using a nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen), according to the manufacturer's specifications. The protein concentration was determined by the bicinchoninic acid method.
Antigenicity analysis of recombinant proteins. (i) Western blot analysis.
Purified recombinant proteins separated by SDS-PAGE were transferred to nitrocellulose membranes (Bio-Rad, Mississauga, ON, Canada) and blocked for 1 h at room temperature in 5% skim milk (Oncogene, Milan, Italy) and PBS containing 0.05% (vol/vol) Tween 20. After several quick washes, the membranes were incubated for 1 h at room temperature with rabbit anti-S. aureus (1:1,500 dilution), mouse anti-rClfA-A (1:2,000 dilution), or anti-His (1:5,000 dilution) monoclonal antibodies (Southern Biotech, Birmingham, AL). After they were extensively washed, the bound antibodies were detected using horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Southern Biotech) or HRP-conjugated goat anti-rabbit IgG (Southern Biotech) antibodies.
(ii) Indirect immunofluorescence analysis.
Indirect immunofluorescence analysis was performed according to the method of Tunney et al. (31), to assess the self-made mouse anti-rClfA-A antibody recognition capacity of native ClfA. S. aureus strain J9 was cultivated to the mid-log phase at 37°C. Three washes with PBS were performed, and 20 μl of S. aureus strain J9 was applied to slides that were precoated with polylysine. The slides were then air dried, fixed in 100% methanol for 10 min at −20°C, and incubated with serum (diluted 1:20) obtained from mice immunized with the recombinant protein or the control for 1 h at 37°C. The slides were then washed again with PBS and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibodies (1:4,000; Sigma Chemical Co., St. Louis, MO) for 1 h at 37°C in the dark. After a final wash, the slides were mounted with glycerol-PBS and examined under a fluorescence microscope (Olympus, Tokyo, Japan).
Mouse immunization. (i) Animals.
Specific-pathogen-free BALB/c mice (28 males, 84 females; age, 7 to 9 weeks; weight, 20 to 22 g) were purchased from the Hubei Center for Disease Control, Wuhan, China. The mice were supplied with water and food ad libitum, according to Regulations for the Administration of Affairs Concerning Experimental Animals. The female mice were randomly allocated to seven treatment groups (n = 12 per group), as follows: (i) rClfA-A plus Freund's adjuvant (Sigma) (CF group), (ii) rClfA-A plus ISA (incomplete Seppic adjuvant) 206 adjuvant (Seppic, Paris, France) (CI group), (iii) rClfA-A plus oil adjuvant (Fuda, Hangzhou City, China) (CO group), (iv) inactivated S. aureus plus Freund's adjuvant (SF group), (v) inactivated S. aureus plus Seppic ISA 206 adjuvant (SI group), (vi) inactivated S. aureus plus oil adjuvant (SO group), and (vii) PBS (control group).
(ii) Preparation of bacterial and protein vaccines.
The inactivated S. aureus vaccine was prepared as follows. S. aureus strain J9 was cultured overnight at 37°C in tryptic soy broth medium, harvested by centrifugation, resuspended in sterilized PBS, and adjusted to 1 × 106 CFU/ml. The bacteria were then inactivated at 37°C for 24 h in 0.2% (wt/vol) formaldehyde. After confirmation of the absence of viable organisms, the bacteria were emulsified at a 1:1 ratio in Freund's complete adjuvant, oil adjuvant, or Seppic ISA 206 adjuvant for priming. The dosage of inactivated S. aureus vaccine (1 × 105 CFU) was determined in pilot trials and through determinations of protein content. The CF and SF groups were immunized with inactivated bacteria emulsified in Freund's incomplete adjuvant for boosting. Purified rClfA-A was dissolved in PBS and emulsified in the three adjuvants; the emulsion (100 μl) contained 40 μg of recombinant protein.
(iii) Vaccination schedule.
The mice were immunized at 2-week intervals (days 0, 14, and 28). Aliquots (100 μl) of the protein and inactivated S. aureus vaccine were administered intraperitoneally to the mice. Blood samples were collected on days 7, 21, and 35; and the sera were harvested and stored at −20°C. On day 24, female and male mice (3:1 ratio) were placed in cages, so that the female mice could become pregnant. The control group was injected with PBS following the same protocol.
Detection of specific antibodies by ELISA. (i) IgG detection.
Enzyme-linked immunosorbent assays (ELISAs) were used to determine the IgG titers against rClfA-A (17). Polystyrene Maxisorp 96-well plates (Nalgene; Nunc International Corp., Rochester, NY) were coated overnight at 4°C with 100 μl of recombinant protein at a concentration of 1 μg/ml in carbonate/bicarbonate buffer (pH 9.6). Following saturation of the plates with a skim milk solution (5%, wt/vol) overnight at 4°C, the serum samples were added at the specified dilution and incubated for 45 min at 37°C. HRP-conjugated goat anti-mouse IgG antibodies (1:4,000) were then added, and the mixture was incubated for 30 min at 37°C. One hundred microliters of chromogenic substrate solution (42 mM tetramethylbenzidine and 0.01% hydrogen peroxide) was added for 10 min. The enzymatic reaction was stopped by the addition of 50 μl of hydrofluoric acid. Three washes with PBS-0.05% Tween 20 were performed between each step. The optical density (OD) at 630 nm was read on a plate reader (BioTek Instruments, Winooski, VT). The method used to determine the titers of antibodies against S. aureus was the same as that described above for rClfA-A, except that the coated antigen was replaced with killed S. aureus.
(ii) Detection of IgG1 and IgG2a subtypes.
ELISAs were performed as before, except that the secondary antibody was either mouse anti-IgG1-HRP (Southern Biotech) or mouse anti-IgG2a-HRP (Southern Biotech). The dilutions used were 1:100 for the inactivated S. aureus groups and 1:102,400 for the rClfA-A groups.
(iii) S. aureus antibody binding assay.
An ELISA was used to determine the overall IgG binding capability in the CO and SO groups following the third immunization against S. aureus (2). Briefly, 96-well flat-bottomed plates were coated with killed S. aureus and incubated overnight at 4°C. The rest of the procedure was as described above. The experiment was repeated three times.
In vitro phagocytosis by neutrophilic leukocytes.
Neutrophilic leukocytes from a healthy cow in early lactation were isolated using the method described by Diarra et al. (4). The cells were resuspended in Dulbecco's modified Eagle's medium (DMEM) to a concentration of 5 × 106 cells/ml. The phagocytosis assay was performed as described previously, with minor modifications (21). Overnight cultures of S. aureus were washed twice with sterile PBS and collected by centrifugation. The pellets were resuspended in DMEM without serum or antibiotics, and the suspension was then adjusted to 1 × 109 CFU/ml (determined by counting of viable cells). The bacteria were preincubated for 30 min at 37°C with sera from the CO, SO, and PBS control groups after the third immunization at a bacterium/serum ratio of 100:1 (vol/vol). A volume of 200 μl (2 × 108 CFU) of preincubated bacteria was added to the washed neutrophils at a 40:1 ratio (bacteria/neutrophils), and the mixture was incubated for 30 min at 37°C with slow shaking, to allow phagocytosis to proceed. Phagocytosis was stopped by the addition of 1 ml of cold sterile PBS. Extracellular bacteria were removed by four washes with PBS and centrifugation at 250 × g at 4°C for 5 min, and the pellets were resuspended in DMEM (2 ml). Each sample was divided into two equal portions. One portion was incubated at 37°C for 5 min (total incubation time, 35 min), while the other portion was incubated at 37°C for 30 min (total incubation time, 60 min). After phagocytosis of the bacteria was completed, the cells were lysed by adding sterile distilled water. The numbers of bacteria that were phagocytosed by neutrophils were determined by dilution plating. The CFU/ml values were calculated as the means of triplicate samples. In addition, a HeLa cell control group was used to verify the efficiency of removing extracellular S. aureus by washing.
Challenge of lactating mice and bacterial recovery from the mammary glands.
Four days after delivery, the female mice in the CO and SO groups were lightly anesthetized, and S. aureus mastitis was induced as described previously (3, 12). The teat canals were on the left side from anterior to posterior were designated L1 to L4, and the corresponding canals on the right side were designated R1 to R4. The L4 and R4 teat canals of each animal were inoculated with 50 μl of an S. aureus J9 suspension, corresponding to 5 × 106 CFU. After 24 h, the mice were killed, and their mammary glands were removed aseptically. The glands were placed in sterile PBS (1:10, wt/vol) and homogenized using a sterile Polytron homogenizer. Appropriate dilutions were then seeded in Baird-Parker agar with egg yolk supplement to determine the number of CFU per gland.
Assessment of IL-4 and IL-6 in mammary glands.
At 24 h after inoculation, mammary gland homogenates from the experimental mice were centrifuged at 13,000 × g for 30 min, and the supernatants were collected. IL-4 and IL-6 were quantified using ELISA kits (BioSource, Camarillo, CA), according to the manufacturer's instructions.
Histological examination of mammary glands.
At 24 h after inoculation, the mammary glands were fixed in 4% formaldehyde in PBS for 24 h and then embedded in paraffin wax. Sections (5 μm thick) were cut and stained with hematoxylin-eosin. Light microscopy (Eclipse 80i; Nikon, Tokyo, Japan) was performed, and histology micrographs were obtained.
Statistical analysis.
The arithmetic mean and standard error of the mean were calculated for each treatment group. The data were analyzed using the analysis of variance (ANOVA) contained in the SAS software package (SAS Institute, Cary, NC). Differences between treatment means were evaluated by Dunnett's post hoc test. P values of <0.05 were considered significant.
Nucleotide sequence accession number.
The sequence of the ClfA-A gene fragment was deposited in the GenBank database under accession no. HM042450.
RESULTS
Cloning, expression, and purification of recombinant ClfA-A.
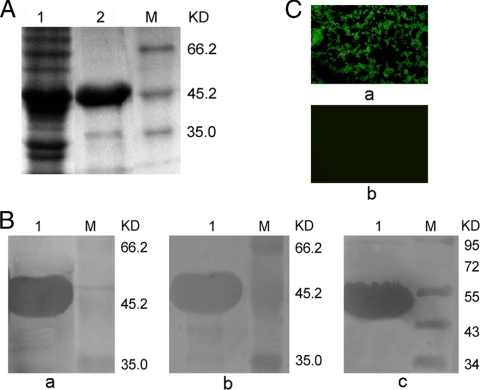
Five colonies of E. coli DH5α that contained pET-28a(+)-ClfA-A were selected, and the recombinant plasmids were extracted and sequenced. The results of the sequence analysis confirmed that the ClfA-A gene fragment was inserted into the vector in the correct orientation, and this sequence was deposited in the GenBank database (accession no. HM042450). Recombinant bacteria that contained pET-28a-ClfA-A were induced with IPTG after 6 h in culture and subsequently lysed by ultrasonic disruption. The recombinant protein was purified by His tag Ni affinity chromatography. The rClfA-A proteins before and after purification were analyzed by 10% SDS-PAGE, and the protein had the expected molecular mass of 48 kDa and was expressed in soluble form at a yield of 2.23 mg/ml (Fig. 1A).
FIG. 1.
(A) Analysis of rClfA-A expression by SDS-PAGE. Lane 1, E. coli containing pET-28a-ClfA-A after induction; lane 2, rClfA-A purified by His-affinity chromatography; lane M, proteins with reference molecular masses (KD, kilodaltons). (B) Western blotting for rClfA-A. Lane 1, purified rClfA-A; lane M, prestained protein marker. a, the primary antibody was mouse anti-rClfA-A serum; b, the primary antibody was rabbit anti-S. aureus serum; c, the primary antibody was an anti-His monoclonal antibody. (C) Assessment of the mouse anti-rClfA-A antibody recognition capacity of native ClfA by indirect immunofluorescence assay. S. aureus was applied to slides and incubated with immunized serum. The slides were incubated with FITC-conjugated goat anti-mouse IgG antibodies and examined with a fluorescence microscope. a, S. aureus was incubated with serum from the rClfA-A immunization group; b, S. aureus was incubated with serum from the PBS control group.
Determination of recombinant protein antigenicity.
The immunogenicity of rClfA-A was further determined by Western blot analysis (Fig. 1B). Mouse antisera to rClfA-A detected rClfA-A as a single band of 48 kDa. Furthermore, the rabbit antiserum to S. aureus reacted with rClfA-A, indicating that the protein retained its natural configuration. In addition, the commercial mouse anti-His monoclonal antibody showed immunoreactivity with rClfA-A, which supported the previous finding that rClfA-A is immunogenic. The mouse antiserum to rClfA-A reacted with S. aureus in an indirect immunofluorescence experiment (Fig. 1C). Preincubation of S. aureus with the sera from the rClfA-A-vaccinated groups produced a strong green fluorescence, whereas the mock-vaccinated groups showed no fluorescence. The fact that the sera from the immunized mice bound to the bacteria indicates that the binding region A of ClfA has potent antigenicity.
Humoral immune responses induced by rClfA-A and inactivated vaccines. (i) IgG production.
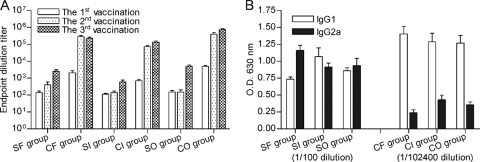
The IgG titers in the mice were measured after three rounds of vaccination (Fig. 2A). The titers of antibodies against rClfA-A and S. aureus peaked at the third immunization. Comparison of the three adjuvants used with the rClfA-A antigen revealed that after the third immunization, the antibody titers in the CO group (760,685 ± 154,814) were higher than those in either the CF group (234,057 ± 77,407) or the CI group (136,533 ± 52,879) (P < 0.001). In the inactivated-vaccine group, the oil adjuvant stimulated the highest IgG levels after immunization.
FIG. 2.
IgG antibody titers (A) and serum IgG1 and IgG2a levels (B) determined by ELISA. The mice were immunized three times at 2-week intervals, and sera were obtained from the mice 1 week after each vaccination. SF, SI, and SO represent the mice immunized with inactivated S. aureus emulsified in Freund's adjuvant, Seppic adjuvant, and oil adjuvant, respectively. CF, CI, and CO represent the mice immunized with rClfA-A emulsified in Freund's adjuvant, Seppic adjuvant, and oil adjuvant, respectively. Results are shown as means ± standard deviations.
Comparison of the two antigens with the same adjuvant after the third immunization revealed that rClfA-A induced a stronger antibody response. The mean antibody titer of the CO group (760,685 ± 154,814) was higher than that of the SO group (5,200 ± 2,221) (P < 0.001), the mean antibody titer of the CF group (234,057 ± 77,407) was higher than that of the SF group (2,514 ± 1,982) (P < 0.01), and the mean antibody titer of the CI group (136,533 ± 52,879) was higher than that of the SI group (580 ± 405) (P < 0.05). In addition, rClfA-A stimulated a more rapid antibody rise than the inactivated S. aureus. The mean antibody titers of all the rClfA-A groups were significantly higher after the second vaccination than after the first vaccination, whereas the antibody titers were significantly enhanced in all the inactivated vaccine groups only after the third immunization.
(ii) IgG1 and IgG2a levels.
The serum levels of IgG1 and IgG2a directed against rClfA-A following the administration of rClfA-A emulsified in different adjuvants were measured (Fig. 2B). The serum dilution was 1:100 for the inactivated S. aureus groups and 1:102,400 for the rClfA-A groups. The subtype of IgG induced by rClfA-A emulsified in all three adjuvants was predominantly IgG1. Of the three adjuvants, Freund's adjuvant stimulated the highest IgG1 titers, as evidenced by the OD values (1.40 ± 0.25; P < 0.001). The IgG1 titers of the CI and CO groups were 1.28 ± 0.28 and 1.27 ± 0.25, respectively. In contrast, no significant differences in IgG subtypes were observed for the inactivated S. aureus groups.
(iii) S. aureus antibody binding assay.
The binding capacities of the antibodies to S. aureus that were induced were compared between the rClfA-A and inactivated S. aureus groups. The immune serum binding capacity decreased as the dilution increased. The binding capacity of the rClfA-A-specific antibody was significantly higher than that of the S. aureus-specific antibody at dilutions of 1:100 (2.33 ± 0.02 and 0.62 ± 0.19, respectively; P < 0.01), 1:200 (2.25 ± 0.08 and 0.43 ± 0.07, respectively; P < 0.05), and 1:400 (2.10 ± 0.11 and 0.33 ± 0.08, respectively; P < 0.05).
Opsonic activities of antibodies.
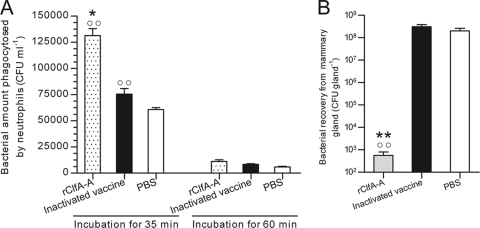
After preincubation of S. aureus with pools of antisera from the CO, SO, and PBS control groups, ingestion of the bacteria by bovine neutrophils was detected (Fig. 3A). The number of bacteria ingested by bovine neutrophils in the presence of the antisera from the CO group was 1.31 × 105 ± 1.16 × 104 CFU/ml, significantly higher than the numbers for both the SO group (7.53 × 104 ± 9.24 × 103 CFU/ml) and the PBS group (6.07 × 104 ± 3.06 × 103 CFU/ml) (P < 0.001). Over a period of 1 h, the numbers of bacteria ingested by bovine neutrophils in the presence of antisera from the CO, SO, and PBS control groups were 1.12 × 104 ± 2.62 × 103 CFU/ml, 8.30 × 103 ± 1.25 × 103 CFU/ml, and 5.97 × 103 ± 9.50 × 102 CFU/ml, respectively. Few bacteria were obtained from the lysed HeLa cells. These results indicate that the extracellular S. aureus cells were washed off efficiently, that the bacteria opsonized with antisera immunized with rClfA-A were easier to eliminate, and that the antibodies induced in the rClfA-A-immunized group had the strongest opsonic activities.
FIG. 3.
(A) In vitro phagocytosis of S. aureus after opsonization. Bacteria were preincubated with sera from rClfA-A-immunized mice, inactivated vaccine-immunized mice, or the control group. Then, the bacteria were added to neutrophils and incubated for 35 or 60 min. The numbers of bacteria phagocytosed by neutrophils were determined by dilution plating. (B) The numbers of bacteria recovered from the mammary glands of rClfA-A-immunized mice, inactivated S. aureus-immunized mice, or the control group were determined after an S. aureus challenge. Results are shown as mean ± standard deviations. *, P < 0.05 versus the inactivated vaccine group; **, P < 0.01 versus the inactivated vaccine group; ○○, P < 0.01 versus the PBS group.
Bacterial recovery from mammary glands after intramammary challenge with S. aureus.
The mammary glands of the mice were inoculated with S. aureus strain J9, and bacterial counts were performed 24 h later (Fig. 3B). There were fewer bacteria in the rClfA-A group (5.40 × 102 ± 2.45 × 102 CFU/gland) than in the inactivated vaccine group (2.96 × 108 ± 1.10 × 108 CFU/gland, P<0.01) or control group (4.13 × 109 ± 6.95 × 108 CFU/gland, P<0.001).
Histopathologic examination.
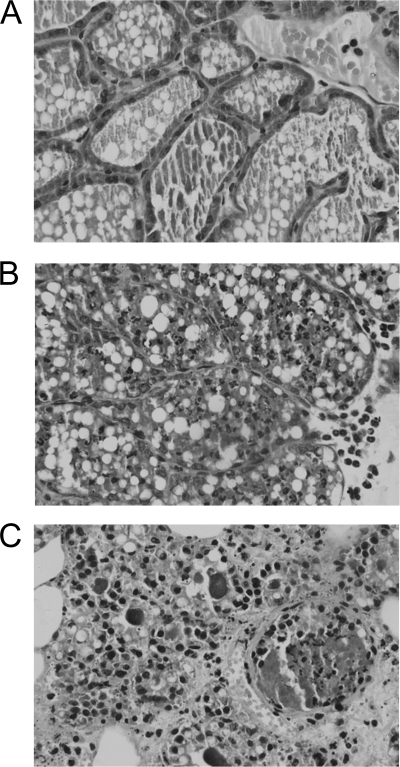
The acinar structure of the mammary glands of mice immunized with the rClfA-A vaccine was similar to that of a normal murine mammary gland. There were only a few epithelial cells shedding in partial vision (Fig. 4A). In the mice immunized with inactivated S. aureus, the mammary acinar structure was destroyed. Many glandular epithelial cells appeared to be necrotic and were shed into the alveoli. Small capillaries were congested, and mild interstitial edema and polymorphonuclear leukocyte (PMN) infiltration into the alveoli were observed (Fig. 4B). In contrast, in the control group, the mammary structures were completely destroyed and interstitial edema was significant and widespread. Numerous degenerate epithelial cells were observed, along with scattered PMNs and mononuclear cells (Fig. 4C).
FIG. 4.
Microscopic images of mammary gland in the rClfA-A-immunized group (A), inactivated S. aureus-immunized group (B), and PBS control group (C). Female BALB/c mice were immunized with rClfA-A or inactivated vaccine just before pregnancy. Four days after delivery, the lactating mice were challenged with S. aureus in the R4 and L4 mammary abdominal glands. The histology of the mammary glands was assessed 24 h after inoculation. The mammary acinar structure was intact, with a few epithelial cells shedding in partial vision in the rClfA-A group. The mammary acinar structure was destroyed, and a lot of glandular epithelial cells were necrotic and shed into the alveoli in the inactivated vaccine group. The mammary structures were completely destroyed and a large number of degenerated epithelial cells and neutrophils infiltrated in the PBS control group. Magnifications, ×400.
Levels of IL-4 and IL-6 in mammary glands.
To elucidate the mechanism underlying the immune protection against mastitis, the levels of IL-4 and IL-6 in the mammary glands were determined. Intramammary challenge with S. aureus resulted in increased concentrations of IL-4 and IL-6 in the murine mammary glands of both the rClfA-A-vaccinated and inactive S. aureus-vaccinated groups (Fig. 5). The concentration of IL-4 was significantly higher in the rClfA-A group than in the inactive S. aureus group (P < 0.01), whereas the concentration of IL-6 was lower in the rClfA-A group than in the inactive S. aureus group (P < 0.05).
FIG. 5.
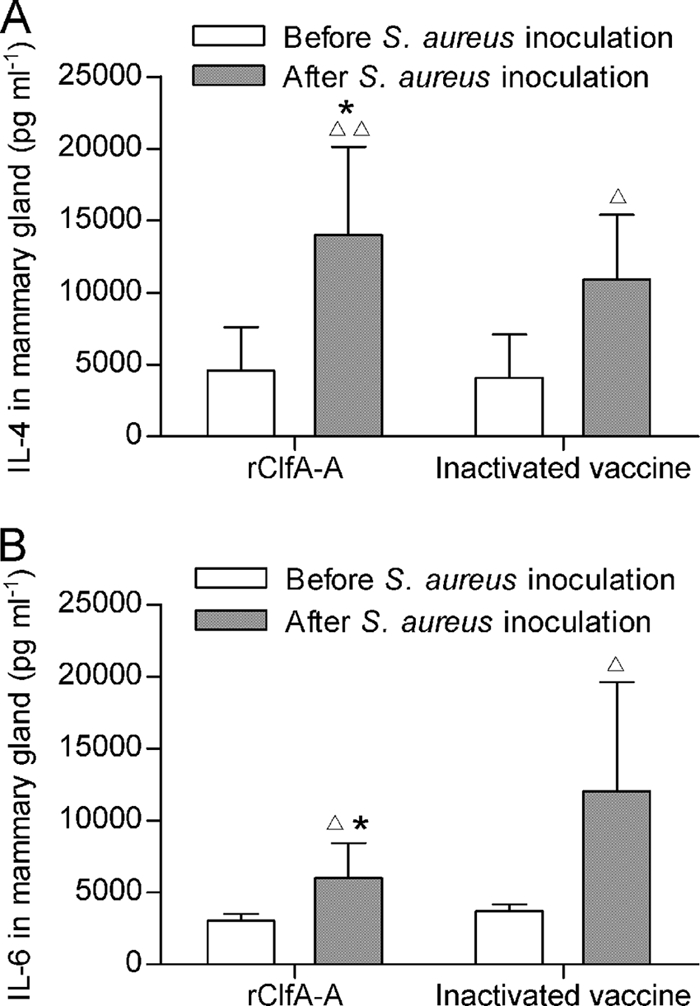
Levels of IL-4 (A) and IL-6 (B) from mammary glands before and after S. aureus challenge. Female BALB/c mice were immunized with rClfA-A or inactivated vaccine just before pregnancy. Lactating mice in the rClfA-A-immunized, inactivated vaccine-immunized, and PBS control groups were challenged with S. aureus in the R4 and L4 mammary abdominal glands. IL-4 and IL-6 production in the supernatant of the mammary glands was analyzed by ELISA. Results are shown as means ± standard deviations. ▵, P < 0.05 versus before S. aureus challenge; ▵▵, P < 0.01 versus before S. aureus challenge; *, P < 0.05 versus the inactivated vaccine-immunized group.
DISCUSSION
The mouse is an appropriate experimental animal for studying the pathogenesis of mammary infection. Although reports have indicated that bacterial challenge can be performed either via the mammary papilla or by intraperitoneal injection (2, 33), the present study demonstrates that in the mouse model of mastitis, mammary gland injection of bacteria more closely resembles natural infection than does intraperitoneal injection (18). Furthermore, the fourth pair of mammary glands in mice is similar to that in cows. Therefore, in our challenge experiments, S. aureus was inoculated into the mammary gland directly via the mammary papilla. Comprehensive analyses, which included histopathologic observations and the counting of bacteria, confirmed that mastitis was successfully established in the S. aureus-inoculated mice. The infection was found to be associated with complete disruption of the mammary acinar structure, necrosis of the epithelium, extensive neutrophil infiltration, and serious interstitial edema (Fig. 4C). These results establish a foundation for further evaluations of the candidate vaccines.
Adhesins play important roles in S. aureus infection and invasion, and vaccines that target adhesins have shown promise in terms of preventing S. aureus-induced mastitis (27). ClfA, one of the more important adhesins, is an ideal vaccine candidate owing to its conservative nature. The present study demonstrates that the ClfA-A subunit expresses many of the critical functions of the ClfA protein. The ClfA-A of the S. aureus J9 strain, which was isolated from the milk of a local cow with mastitis, was cloned and expressed. Therefore, ClfA-A was used in formulations with three different adjuvants (Freund's adjuvant, Seppic adjuvant, and oil adjuvant). Tollersrud et al. (28) have suggested that vaccination with intact staphylococcal cells prevents staphylococcal mastitis in cows. However, attempts to produce killed whole-cell vaccines for S. aureus infections have proven unsuccessful (15). In contrast, vaccines that contain purified bacterial surface components have been shown to provide protection against experimental infections in animals (10). In the present study, the rClfA-A subunit vaccine provided significantly better protection against S. aureus-induced mastitis than did either the inactivated S. aureus vaccine or mock immunization.
The mechanism underlying this enhanced protection was explored. Crucially, rClfA-A stimulated stronger humoral immune responses, which were characterized by higher IgG titers (significantly higher IgG1 and IgG2a titers), dominance by the IgG1 subtype, and the enhanced ability of the induced antibodies to bind and opsonize the bacteria, as well as to inhibit bacterial growth.
In the present study, the numbers of bacteria recovered from mammary glands were significantly lower in the rClfA-A-vaccinated group than in either the inactivated S. aureus-immunized group or the control group. In the rClfA-A-immunized group, the structure of the mammary glands remained intact and only a slight focal lesion was observed. Compared to the PBS control group, immunization with inactivated S. aureus gave some protection in terms of preserving tissue integrity after challenge with S. aureus. However, immunization with rClfA-A was much more effective in this respect.
Analysis of the IgG responses revealed that rClfA-A immunization produced more than 1,000-fold higher levels of IgG than did immunization with inactivated S. aureus. Further experiments showed that the antisera from the rClfA-A-vaccinated animals were more potent in promoting ingestion of the bacteria by bovine neutrophilic leukocytes than the inactivated S. aureus or PBS control. These results confirm that antisera against ClfA promote phagocytosis and are in line with the recovery of fewer bacteria from the mammary glands. These results also suggest that anti-ClfA antibodies enhance the clearance of staphylococci in vivo.
The isotype profiles revealed that rClfA-A immunization predominantly induced the IgG1 subtype. This represents a Th2-dominant response. Regarding immunization with inactivated S. aureus, there was only a slight difference between the IgG1 and IgG2a titers. The induction of a higher proportion of IgG1 is a typical feature of protein immunization (13). In addition, the IgG1 subtype is the most efficient opsonization isotype for neutrophils (24). Therefore, the higher titers of IgG and IgG1 dominance may be linked to the superior protection conferred by immunization with rClfA-A compared to that conferred by immunization with inactivated S. aureus.
IL-4 and IL-6 favor the development of Th2 lymphocytes. In the present study, rClfA-A immunization significantly stimulated the production of these cytokines. This result, combined with the dominant IgG1 subtype, confirms that rClfA-A immunization induces Th2 responses that allow the host to fight invading bacteria (22). The higher levels of IL-6 observed in the inactivated S. aureus group probably reflect a severe inflammatory response (17, 20).
Adjuvants are important components of vaccines. In the present study, three adjuvants (Freund's adjuvant, Seppic adjuvant, and oil adjuvant) were chosen for use in the two formulations (rClfA-A and killed S. aureus). The oil adjuvant induced antibody production the most rapidly and efficiently in the protein antigen groups. This oil adjuvant has several functions, including sequestering antigens and their slow release, prolonging antigen stimulation, as well as activating complement pathways, antigen-presenting cells, and T cells (11). Therefore, the oil adjuvant groups were selected to evaluate further the immunologic effects in the challenge experiments.
In summary, the results of our mouse immunization experiments, challenge experiments, and in vitro assay indicate that rClfA-A induces a significantly stronger and more effective immune response than killed S. aureus in an S. aureus-induced mouse mastitis model. Therefore, rClfA-A is a promising candidate for use in a novel subunit vaccine against S. aureus-induced mastitis in cow populations in which the predominant infecting staphylococci are known to express ClfA.
Acknowledgments
This work was supported by National Eleventh Five-Years Science and Technology Support Program of China—Key Special Project for Dairy Industry (grant nos. 2006BAD04A05 and 2006BAD04A12) and the Program for Changjiang Scholars and Innovative Research Team in University of China (PCSIRT) (grant no. IRT0726).
Footnotes
Published ahead of print on 8 September 2010.
REFERENCES
- 1.Brouillette, E., and F. Malouin. 2005. The pathogenesis and control of Staphylococcus aureus-induced mastitis: study models in the mouse. Microbes Infect. 7:560-568. [DOI] [PubMed] [Google Scholar]
- 2.Brouillette, E., P. Lacasse, L. Shkreta, J. Bélanger, G. Grondin, M. S. Diarra, S. Fournier, and B. G. Talbot. 2002. DNA immunization against the clumping factor A (ClfA) of Staphylococcus aureus. Vaccine 20:2348-2357. [DOI] [PubMed] [Google Scholar]
- 3.Castagliuolo, I., R. Piccinini, E. Beggiao, G. Palù, C. Mengoli, F. Ditadi, G. Vicenzoni, and A. Zecconi. 2006. Mucosal genetic immunization against four adhesins protects against Staphylococcus aureus-induced mastitis in mice. Vaccine 24:4393-4402. [DOI] [PubMed] [Google Scholar]
- 4.Diarra, M. S., D. Petitclerc, E. Deschênes, N. Lessard, G. Grondin, B. G. Talbot, and P. Lacasse. 2003. Lactoferrin against Staphylococcus aureus mastitis. Lactoferrin alone or in combination with penicillin G on bovine polymorphonuclear function and mammary epithelial cells colonisation by Staphylococcus aureus. Vet. Immunol. Immunopathol. 95:33-42. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira, G. N., G. A. Monteiro, D. M. Prazeres, and J. M. Cabral. 2000. Downstream processing of plasmid DNA for gene therapy and DNA vaccine applications. Trends Biotechnol. 18:380-388. [DOI] [PubMed] [Google Scholar]
- 6.Grundmann, H., M. Aires-de-Sousa, J. Boyce, and E. Tiemersma. 2006. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368:874-885. [DOI] [PubMed] [Google Scholar]
- 7.Hartford, O. M., E. R. Wann, M. Höök, and T. J. Foster. 2000. Identification of residues in the Staphylococcus aureus fibrinogen-binding MSCRAMM clumping factor A (ClfA) that are important for ligand binding. J. Biol. Chem. 276:2466-2473. [DOI] [PubMed] [Google Scholar]
- 8.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Höök. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211-223. [DOI] [PubMed] [Google Scholar]
- 9.Josefsson, E., J. Higgins, T. J. Foster, and A. Tarkowski. 2008. Fibrinogen binding sites P336 and Y338 of clumping factor A are crucial for Staphylococcus aureus virulence. PLoS One 3:e2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Josefsson, E., O. Hartford, L. O'Brien, J. M. Patti, and T. Foster. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 184:1572-1580. [DOI] [PubMed] [Google Scholar]
- 11.Kim, S. K., G. Ragupathi, C. Musselli, S. J. Choi, Y. S. Park, and P. O. Livingston. 1999. Comparison of the effect of different immunological adjuvants on the antibody and T-cell response to immunization with MUC1-KLH and GD3-KLH conjugate cancer vaccines. Vaccine 18:597-603. [DOI] [PubMed] [Google Scholar]
- 12.Mamo, W., M. Bodén, and J. I. Flock. 1994. Vaccination with Staphylococcus aureus fibrinogen binding proteins (FgBPs) reduces colonisation of S. aureus in a mouse mastitis model. FEMS Immunol. Med. Microbiol. 10:47-53. [DOI] [PubMed] [Google Scholar]
- 13.Maurer, M., and E. von Stebut. 2004. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 36:1882-1886. [DOI] [PubMed] [Google Scholar]
- 14.McDevitt, D., T. Nanavaty, K. House-Pompeo, E. Bell, N. Turner, L. McIntire, T. Foster, and M. Höök. 1997. Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. Eur. J. Biochem. 247:416-424. [DOI] [PubMed] [Google Scholar]
- 15.Middleton, J. R. 2008. Staphylococcus aureus antigens and challenges in vaccine development. Expert Rev. Vaccines 7:805-815. [DOI] [PubMed] [Google Scholar]
- 16.Moreillon, P., J. M. Entenza, P. Francioli, D. McDevitt, T. J. Foster, P. François, and P. Vaudaux. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63:4738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima, Y., E. Momotani, T. Murakami, Y. Ishikawa, M. Morimatsu, M. Saito, H. Suzuki, and K. Yasukawa. 1993. Induction of acute phase protein by recombinant human interleukin-6 (IL-6) in calves. Vet. Immunol. Immunopathol. 35:385-391. [DOI] [PubMed] [Google Scholar]
- 18.Notebaert, S., and E. Meyer. 2006. Mouse models to study the pathogenesis and control of bovine mastitis. A review. Vet. Q. 28:2-13. [DOI] [PubMed] [Google Scholar]
- 19.Nour El-Din, A. N., L. Shkreta, B. G. Talbot, M. S. Diarra, and P. Lacasse. 2006. DNA immunization of dairy cows with the clumping factor A of Staphylococcus aureus. Vaccine 24:1997-2006. [DOI] [PubMed] [Google Scholar]
- 20.Oldenburg, H. S., M. A. Rogy, D. D. Lazarus, K. J. Van Zee, B. P. Keeler, R. A. Chizzonite, S. F. Lowry, and L. L. Moldawer. 1993. Cachexia and the acute-phase protein response in inflammation are regulated by interleukin-6. Eur. J. Immunol. 23:1889-1894. [DOI] [PubMed] [Google Scholar]
- 21.Rennermalm, A., Y. H. Li, L. Bohaufs, C. Jarstrand, A. Brauner, F. R. Brennan, and J. I. Flock. 2001. Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine 19:3376-3383. [DOI] [PubMed] [Google Scholar]
- 22.Riollet, C., P. Rainard, and B. Poutrel. 2001. Cell subpopulations and cytokine expression in cow milk in response to chronic Staphylococcus aureus infection. J. Dairy Sci. 84:1077-1084. [DOI] [PubMed] [Google Scholar]
- 23.Scarpa, M., R. Piccinini, P. Brun, A. Grillo, G. Palù, C. Mengoli, V. Daprà, I. Castagliuolo, and A. Zecconi. 2009. Relationship between virulence factor genes in bovine Staphylococcus aureus subclinical mastitis isolates and binding to anti-adhesin antibodies. J. Dairy Res. 24:1-9. [DOI] [PubMed] [Google Scholar]
- 24.Schlageter, A. M., and T. R. Kozel. 1990. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect. Immun. 58:1914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shkreta, L., B. G. Talbot, M. S. Diarra, and P. Lacasse. 2004. Immune responses to a DNA/protein vaccination strategy against Staphylococcus aureus induced mastitis in dairy cows. Vaccine 23:114-126. [DOI] [PubMed] [Google Scholar]
- 26.Stutzmann, M. P., J. M. Entenza, P. Vaudaux, P. Francioli, M. P. Glauser, and P. Moreillon. 2001. Study of Staphylococcus aureus pathogenic genes by transfer and expression in the less virulent organism Streptococcus gordonii. Infect. Immun. 69:657-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talbot, B. G., and P. Lacasse. 2005. Progress in the development of mastitis vaccines. Livestock Production Sci. 98:101-113. [Google Scholar]
- 28.Tollersrud, T., L. Zernichow, S. R. Andersen, K. Kenny, and A. Lund. 2001. Staphylococcus aureus capsular polysaccharide type 5 conjugate and whole cell vaccines stimulate antibody responses in cattle. Vaccine 19:3896-3903. [DOI] [PubMed] [Google Scholar]
- 29.Tuchscherr, L. P., F. R. Buzzola, L. P. Alvarez, R. L. Caccuri, J. C. Lee, and D. O. Sordelli. 2005. Capsule-negative Staphylococcus aureus induces chronic experimental mastitis in mice. Infect. Immun. 73:7932-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuchscherr, L. P., F. R. Buzzola, L. P. Alvarez, J. C. Lee, and D. O. Sordelli. 2008. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infect. Immun. 76:5738-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tunney, M. M., S. Patrick, M. D. Curran, G. Ramage, D. Hanna, J. R. Nixon, S. P. Gorman, R. I. Davis, and N. Anderson. 1999. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J. Clin. Microbiol. 37:3281-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin, R. L., C. Li, Z. T. Yang, Y. J. Zhang, W. L. Bai, X. Li, R. H. Yin, H. Liu, S. Liu, Q. Yang, Y. G. Cao, and N. S. Zhang. 2009. Construction and immunogenicity of a DNA vaccine containing clumping factor A of Staphylococcus aureus and bovine IL18. Vet. Immunol. Immunopathol. 132:270-274. [DOI] [PubMed] [Google Scholar]
- 33.Zhou, H., Z. Y. Xiong, H. P. Li, Y. L. Zheng, and Y. Q. Jiang. 2006. An immunogenicity study of a newly fusion protein Cna-FnBP vaccinated against Staphylococcus aureus infections in a mice model. Vaccine 24:4830-4837. [DOI] [PubMed] [Google Scholar]