Abstract
Staphylococcus aureus, a major pathogen for the mammary gland of dairy ruminants, elicits the recruitment of neutrophils into milk during mastitis, but the mechanisms are incompletely understood. We investigated the response of the bovine mammary gland to muramyl dipeptide (MDP), an elementary constituent of the bacterial peptidoglycan, alone or in combination with lipoteichoic acid (LTA), another staphylococcal microbial-associated molecular pattern (MAMP). MDP induced a prompt and marked influx of neutrophils in milk, and its combination with LTA elicited a more intense and prolonged influx than the responses to either stimulus alone. The concentrations of several chemoattractants for neutrophils (CXCL1, CXCL2, CXCL3, CXCL8, and C5a) increased in milk after challenge, and the highest increases followed challenge with the combination of MDP and LTA. MDP and LTA were also synergistic in inducing in vitro chemokine production by bovine mammary epithelial cells (bMEpC). Nucleotide-binding oligomerization domain 2 (NOD2), a major sensor of MDP, was expressed (mRNA) in bovine mammary tissue and by bMEpC in culture. The production of interleukin-8 (IL-8) following the stimulation of bMEpC by LTA and MDP was dependent on the activation of NF-κB. LTA-induced IL-8 production did not depend on platelet-activating factor receptor (PAFR), as the PAFR antagonist WEB2086 was without effect. In contrast, bMEpC and mammary tissue are known to express Toll-like receptor 2 (TLR2) and to respond to TLR2 agonists. Although the levels of expression of the inflammatory cytokines tumor necrosis factor alpha (TNF-α) and IL-1β were increased by LTA and MDP at the mRNA level, no protein could be detected in the bMEpC culture supernatant. The level of induction of IL-6 was low at both the mRNA and protein levels. These results indicate that MDP and LTA exert synergistic effects to induce neutrophilic inflammation in the mammary gland. These results also show that bMEpC could contribute to the inflammatory response by recognizing LTA and MDP and secreting chemokines but not proinflammatory cytokines. Overall, this study indicates that the TLR2 and NOD2 pathways could cooperate to trigger an innate immune response to S. aureus mastitis.
Staphylococcus aureus is a pathogen frequently isolated from mastitis milk or mammary abscesses in the lapine, ovine, bovine, and human species (4, 5, 23, 41, 53). The recognition of S. aureus by the mammary gland is not as well known as the recognition of Escherichia coli, another major pathogen for the mammary gland. The E. coli outer membrane lipopolysaccharide (LPS) has been shown to be the major pathogen-associated molecular pattern (MAMP) recognized by the mammary gland through interactions with Toll-like receptor 4 (TLR4), which is expressed by bovine mammary epithelial cells (MEpC) (bMEpC) (18, 24). LPS has been extensively used to mimic mastitis due to E. coli, and it was shown that the responses that it provokes are in many respects representative of those induced by live bacteria (7). The counterpart of LPS, as a proinflammatory bacterial agonist of the mammary gland innate immune system, has not yet been established for S. aureus. This pathogen releases various MAMPs during infection, and two of these have been the subject of many studies. Lipoteichoic acid (LTA) was shown to be an important pattern for immune recognition of S aureus (66). LTA signals through TLR2 (56), a receptor which is expressed in the mammary gland, in particular by bMEpC (18, 24). We have recently established the ability of LTA to elicit an intense inflammatory response in the bovine mammary gland (49). Another major staphylococcal MAMP is the minimal bioactive constituent of the bacterial peptidoglycan MurNac-l-Ala-d-iso-Gln (MDP). A major sensor of MDP is the nucleotide-binding oligomerization domain 2 (NOD2) protein, which is encoded by the caspase recruitment domain 15 gene (CARD15) (17). NOD2 is expressed mainly by two cell types that are exposed to microorganisms that produce peptidoglycan, i.e., antigen-presenting cells and epithelial cells (25, 63).
During infection, host-pathogen interactions are very complex and difficult to unravel. The use of isolated MAMPs makes it possible to decipher the components of the innate immune response at play in the mammary gland. Information gained from in vitro studies of the responses of relevant cells to the same bacterial agonists would increase our understanding of udder-pathogen interactions. The in vitro data can be compared with the immune response to experimentally induced infections. Similarities and differences are expected to point to important bacterial agonists of the host response and to important facets of this response. In turn, the new knowledge acquired can be used to devise new approaches with a view to modulating the inflammatory and the immune responses in the mammary gland. As information on the immune response of the mammary gland and mammary epithelial cells to staphylococcal MAMPs is relatively scarce, we decided to use two major staphylococcal MAMPs to induce mastitis and to stimulate MEpC. Epithelial cells, which line the lumen of the mammary gland, are the first and most abundant cells to be in contact with invading bacteria and their secreted products. Bovine MEpC have been shown to react to MAMPs such as LPS and LTA (62, 72).
We investigated the ability of MDP to trigger an inflammatory response in the bovine mammary gland. As it was reported previously that MDP and LTA exert synergistic effects on a variety of cells (30, 63), we sought to determine whether these NOD2 and TLR2 agonists could induce additive or synergistic effects in vivo in the mammary gland and in vitro on bMEpC. Our results indicate that MDP induces neutrophilic inflammation and synergizes with LTA in the mammary gland to recruit neutrophils. Moreover, MDP and LTA synergized to induce the secretion of neutrophil-oriented chemokines by bMEpC but did not induce the secretion of the major proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β). These new findings support the hypothesis that MDP and LTA are likely to play an important part in the initiation of inflammation during S. aureus mastitis. They contribute to an improved understanding of the detection of S. aureus by the mammary gland.
MATERIALS AND METHODS
Reagents.
The staphylococcal LTA used in this study was purified S. aureus lipoteichoic acid (PSLTA; InvivoGen, Toulouse, France), which was prepared according to the n-butanol procedure (42), followed by phenol treatment to remove lipoproteins. This commercial preparation contained <1.24 EU endotoxin/mg LTA. Endotoxin-free MDP (N-acetylmuramyl-l-alanyl-d-isoglutamine) was obtained from InvivoGen. The inactive d-d isoform (N-acetylmuramyl-d-alanyl-d-isoglutamine) from InvivoGen was used as a negative control. Lyophilized LTA and MDP were made soluble in sterile endotoxin-free water and then diluted in cell culture medium. ATP and Pefabloc SC were obtained from Sigma-Aldrich (St. Louis, MO), platelet-activating factor (PAF; C-16) was obtained from Cayman Chemical (Ann Arbor, MI), and recombinant human PAF-acetyl hydrolase (PAF-AH) was obtained from Peprotech (Rocky Hill, NJ). InSolution NF-κB activation inhibitor, phorbol-12-myristate-13-acetate (PMA) (an activator of protein kinase C), and ionomycin were obtained from Calbiochem (Merck Biosciences, Nottingham, United Kingdom), and the NF-κB inhibitor WEB2086 was obtained from Tocris Biosciences (Bristol, United Kingdom). A nuclear extract kit and the TransAM transcription factor NF-κB p65 activation assay kit were obtained from Active Motif (Carlsbad, CA).
Experimentally induced mastitis.
Inflammation of the mammary gland (mastitis) was induced by infusing MDP, LTA, or a combination of MDP and LTA in the lumen of the gland via the teat canal. Fourteen healthy midlactating Holstein cows of the experimental herd of the Institute at Nouzilly were selected on the basis of an absence of detectable bacterial growth from two weekly consecutive aseptically collected milk samples in their four mammary quarters and fewer than 100,000 cells/ml in milk. The cows were in their second or third lactation and between 2 and 6 months in lactation; they were milked twice a day, at 0800 and 1600 h. The use and care of the cows in this study were approved by the Regional Committee of Ethics for Animal Experimentation (CREEA) (approval CL2007-47).
A pilot experiment was designed to assess the doses of MDP active on the mammary gland. Doses of 1 μg, 10 μg, and 100 μg in 0.5 ml RPMI medium were infused in different mammary glands of each of three cows through the teat canal with aseptic precautions. In the major experiment, 11 cows were used. At time zero, each cow received 0.5 ml RPMI 1640 medium (Gibco) in one quarter, 20 μg MDP in another quarter, 5 μg LTA in the third quarter, and a mix of 20 μg MDP with 5 μg LTA in the fourth quarter. In both experiments, the infused quarters and the control quarter were aseptically sampled just after the morning milking, and infusion was carried out within 1 h postmilking. The quarters were then sampled at 4, 8, 12, 24, 32, 48, 72, and 96 h postinfusion (hpi).
Cells in milk were counted with an automated cell counter (Fossomatic model 90; Foss Food Technology, Hillerod, Denmark) as described previously (2). For the determination of the proportion of neutrophils among milk cells, cytospin slides were prepared and stained with May-Grünwald-Giemsa reagent as described previously (49).
Quantification of IFN-γ, TNF-α, IL-1β, IL-6, C5a, CXCL1, CXCL2, CXCL3, and CXCL8 by ELISA.
Gamma interferon (IFN-γ) concentrations were determined by using a bovine IFN-γ enzyme-linked immunosorbent assay (ELISA) kit (Mabtech AB, Sweden). The lowest level of quantification in milk was 15 pg/ml. To measure the concentrations of IL-1β, a sandwich ELISA using mouse anti-IL-1β monoclonal antibody (MAb 1D4; Serotec, Inc.), recombinant bovine IL-1β (Serotec, Inc.), and rabbit anti-bovine IL-1β (Serotec, Inc.) was performed as described previously (3). The lowest level of detection in milk was 0.10 ng/ml. Bovine IL-6 concentrations were measured by using a commercial ELISA (bovine IL-6 screening set; Thermo Scientific, Rockford, IL) as indicated by the manufacturer. ELISAs were performed as described previously for TNF-α (50), C5a (52), CXCL8 (IL-8), CXCL1 (GRO-α), CXCL2 (GRO-β), and CXCL3 (GRO-γ) (48, 51). The lowest levels of quantification were 0.2, 0.1, 0.15, 0.01, and 0.1 ng/ml for CXCL1, CXCL2, CXCL3, CXCL8, and TNF-α, respectively.
bMEpC.
bMEpC were isolated from five lactating cows as previously described and cryopreserved in liquid nitrogen (34). When needed, bMEpC were thawed and cultured without serum and antibiotics in Dulbecco's modified Eagle's medium (D-MEM)-F12 advanced medium (Gibco), which contains insulin (10 μg/ml), albumin (0.4 mg/ml), and transferrin (7.5 μg/ml), supplemented with 2 mM l-glutamine, 10 ng/ml insulin-like growth factor I (IGF-I) (Peprotech), 5 ng/ml fibroblast growth factor (FGF) (Peprotech), 5 ng/ml human recombinant epidermal growth factor (EGF) (Sigma), 1 μg/ml hydrocortisone (Sigma), and 20 mM HEPES (Cambrex Biowhittaker). Cells were used at their third passage. Cells were seeded into six-well tissue culture plates at a density of 4 × 105 cells/well and cultured until confluence, and the growth medium was then replaced with stimulation medium made up of D-MEM-F12 advanced medium with 2 mM l-glutamine, 20 mM HEPES, and 4 ng/ml hydrocortisone as additives. Stimulations with bacterial agonists were carried out 16 to 24 h later. The medium was removed, and agonists (LTA, MDP, LTA plus MDP, PMA, ATP, and PAF) were added in 4 ml of stimulation medium. Control wells were treated with stimulation medium only. At the indicated times after exposure to the MAMP, culture medium was aspirated and stored at −20°C. The cell monolayers were then washed twice with Hanks balanced salt solution (HBSS), and cells were harvested for RNA extraction (see below). For some experiments, LTA was inactivated by deacylation with PAF-AH as previously described (57). Briefly, 1 μg LTA was incubated with 1 μg PAF-AH for 2 h at 37°C. PAF-AH was inactivated by adding 100 μM Pefabloc SC (an inhibitor of PAF-AH) prior to the stimulation of bMEpC.
Monocytes and PBMC.
Peripheral blood mononuclear cells (PBMC) were prepared from the buffy coat after the centrifugation of peripheral blood anticoagulated with EDTA, followed by NH4Cl lysis of erythrocytes. Cells were washed with HBSS and resuspended in RPMI 1640 medium plus 10% fetal bovine serum (FBS) at a concentration of 1 × 106 cells/ml. Stimulation was performed with 200 ng/ml PMA and 4 μg/ml ionomycin for 4 h. Monocytes were isolated from PBMC by adherence to plastic in six-well dishes for 4 h at 37°C, followed by washing with HBSS. Stimulation was carried out with LTA plus MDP (1 μg/ml each) for 16 h in stimulation medium. After stimulation, cell supernatants were collected and stored at −20°C.
Tissue sample preparation.
In order to investigate the constitutive expression of the receptor to bacterial agonists of the innate immune system, RNA was prepared from mammary tissues of two lactating cows. For each cow, one uninfected, uninflamed quarter (<50,000 cells/ml milk) was sampled. Immediately after the cows were sacrificed, mammary tissue samples were taken at a depth of 2 to 5 cm from the cisterna (deep parenchyma). Samples of approximately 0.05 cm3 were dispensed in cryovials and immediately snap-frozen in liquid nitrogen.
Reverse transcription and PCR analysis.
Total RNA was extracted from bMEpC by using the NucleoSpin RNA II extraction kit (Macherey-Nagel, Düren, Germany), and the residual genomic DNA was removed by using DNase digestion with RNase-free DNase (Macherey-Nagel). The total RNA quantity and quality were assessed by using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). RNA quality was verified by agarose gel electrophoresis, and RNA integrity was analyzed by using the Agilent Bioanalyzer system (Agilent Technologies, Inc., Santa Clara, CA). Total RNA (1 μg) was then reverse transcribed to cDNA: 1 μg of RNA was incubated with 1 μg of random primers (Promega, Madison, WI) for 10 min at 65°C and then for 5 min on ice in a final volume of 10 μl. Reverse transcription (RT) was carried out by adding avian myeloblastosis virus (AMV) reverse transcriptase buffer (Promega), 4 mM deoxynucleoside triphosphate (dNTP) (Promega), 15 U of AMV reverse transcriptase (Promega), and 40 U of RNasin (Promega) to the mixture. The mixture was incubated for 1.5 h at 42°C and 5 min at 95°C. Diluted cDNA samples were stored at 4°C until use.
All primers (Table 1) used in this study were designed by using Clone Manager 9 (Scientific & Educational Software, Cary, NC) using publicly available bovine sequences and were purchased from Eurogentec (Liège, Belgium). Primers were designed to span an intron-exon boundary to prevent the amplification of genomic DNA.
TABLE 1.
Primers used in this study
| Gene | Oligonucleotide sequence (5′-3′)a | Amplicon size (bp) | Annealing temp (°C) | Annealing duration (s) | GenBank accession no. |
|---|---|---|---|---|---|
| 18S rRNA | CGGGGAGGTAGTGACGAAA (F) | 196 | 69 | 8 | AF176811 |
| CCGCTCCCAAGATCCAACTA (R) | |||||
| IL-1β | CTCTCACAGGAAATGAACCGA (F) | 152 | 67 | 4 | EU276067 |
| GCTGCAGGGTGGGCGTATCACC (R) | |||||
| TNF-α | TCTTCTCAAGCCTCAAGTAACAAGC (F) | 104 | 69 | 5 | EU276079 |
| CCATGAGGGCATTGGCATAC (R) | |||||
| IL-6 | TGCTGGTCTTCTGGAGTATC (F) | 153 | 62 | 5 | EU276071 |
| GTGGCTGGAGTGGTTATTAG (R) | |||||
| GAPDH | GGCATCGTGGAGGGACTTATG (F) | 186 | 62 | 30 | DQ403066 |
| GCCAGTGAGCTTCCCGTTGAG (R) | |||||
| NOD2 | CCCAGGGGCTCAGAACTAACA (F) | 238 | 62 | 30 | NM_001002889 |
| CCTTCATCCTGGACGTGGTTC (R) | |||||
| PAFR | CCTTTCGTGTGGACTCAGAGTTCCG (F) | 403 | 62 | 30 | NM_001040538 |
| ATCAGGGACAGAAGGATGCCACG (R) | |||||
| PEPT1/SLC15A1 | CTGGGCCTTGTTTGATCAGC (F) | 123 | 62 | 30 | NM_001099378 |
| ACGATCAGGATGGCGTTCAC (R) | |||||
| TLR2 | ACTGGGTGGAGAACCTCATGGTCC (F) | 307 | 62 | 30 | NM_174197 |
| ATCTTCCGCAGCTTACAGAAGC (R) | |||||
| TLR6 | CTCCGGGAGATAGTCACTTC (F) | 297 | 62 | 30 | NM_001001159 |
| GGCCCTGGATTCTATTATGG (R) |
F, forward; R, reverse.
PCR was performed using by specific primers for GAPDH, TLR2, TLR6, NOD2 (CARD15), PEPT1, and PAF-R (Table 1). Each amplification began with a 2-min denaturation step at 94°C followed by 30 cycles of denaturation at 94°C for 30 s, annealing at the gene-specific temperature (Table 1) for 30 s, extension at 72°C for 30 s, and a final cycle at 94°C for 10 min. Amplification was performed with a GeneAmp PCR System 2700 thermocycler (Applied Biosystems, Foster City, CA). PCR products (20 μl) were separated by electrophoresis on a 1.5% agarose gel containing ethidium bromide and analyzed by using Fluorchem 8900. The bands corresponding to target mRNAs were compared with the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) band. PCR products were sequenced in forward and reverse directions by Cogenics.
Relative quantities of gene transcripts were measured by RT-quantitative PCR (qPCR) by using the SYBR green I fluorophore (Roche Diagnostics, Mannheim, Germany). To establish standard curves, external standard DNA plasmids were prepared. cDNA obtained from peripheral blood mononuclear cells or bMEpC was amplified by PCR with specific primers. Each amplicon was cloned into the pDrive vector (Qiagen). Plasmids were purified by using a NucleoSpin plasmid kit (Macherey-Nagel) and sequenced to authenticate the cloned amplicon. Plasmid DNA concentrations were quantified by using a NanoDrop spectrophotometer, expressed in g/μl, and converted to copy numbers per μl of target DNA. A standard curve was established by using a 10-fold serial dilution from 5 × 105 to 5 final copies per μl of recombinant plasmid containing the gene of interest. PCR was performed with glass capillaries in a final volume of 10 μl containing 2 μl recombinant plasmid or cDNA sample (water was used as a negative control), 1 μl of Titanium Taq polymerase 10× buffer (Clontech, Le Pont de Claix, France), 2 mM MgCl2 (Promega), 0.2 mM dNTPs (Promega), 0.25 mM each primer pair (Eurogentec) (Table 1), 0.5 μl of SYBR green I diluted at 1/1,000, and 1 U of Titanium Taq polymerase (Clontech). Following an initial denaturation step for 1 min at 95°C, 45 cycles of 10 s at 95°C, 4 to 8 s at the annealing temperature (Table 1), and 10 s at 72°C were performed by using a LightCycler instrument (Roche Diagnostics). At the end of each run, a dissociation melt curve was determined to check for the presence of a single peak consistent with the presence of a single amplicon. The mRNA copy numbers were calculated for each sample using the standard curve to convert the obtained threshold cycle (CT) value into mRNA copy numbers. Results were then expressed in copy numbers after normalization against 18S rRNA (reference gene).
Assessment of NF-κB activation.
The detection and quantification of NF-κB p65 were carried out with the TransAM transcription factor NF-κB p65 activation assay kit (Active Motif). This assay, which involves the use of antibodies specific for the activated form of p65, has been shown to detect bovine p65 (43). bMEpC were cultured on six-well plates and stimulated for 2 h and 8 h with 1 μg/ml of LTA and MDP alone or in combination. The nuclear fraction was isolated according to the manufacturer's protocol and was stored at −80°C. Protein concentrations were determined by MicroBCA (Uptima, Montluçon, France). Equal amounts of nuclear proteins (7 μg) of the testing samples diluted in 20 μl of complete lysis buffer were added per well of a 96-well plate containing an immobilized NF-κB consensus oligonucleotide. Wells receiving 2.5 μg of the provided Jurkat nuclear extract were used as a positive control, and blank wells received 20 μl of Complete lysis buffer only. The plate was incubated for 1 h at room temperature, and the absorbance was read on a spectrophotometer at 450 nm. Results are expressed as optical density (OD) values.
The involvement of NF-κB activation in CXCL8 secretion was evaluated by use of a specific inhibitor. bMEpC (2 × 105 cells) cultivated in 12-well plates were preincubated with 15 μM InSolution NF-κB activation inhibitor (Calbiochem) for 1 h prior to 8 h of stimulation with 1 μg/ml of LTA and MDP alone or in combination. After stimulation, cell supernatants were collected and stored at −20°C before assessments of CXCL8 concentrations by ELISA.
Statistical analysis.
Statistical analyses of the concentrations of analytes in milk or cell culture supernatants were performed with the nonparametric Friedman test. When variations were significant, the significance of variations relative to control quarters (milk cell concentrations) or relative to time zero (chemokines and cytokines) was analyzed by multiple comparisons for each time postinfusion. Comparisons were also done between concentrations in quarters challenged with different stimuli. Multiple comparisons were done with Georgin and Gouet software (16), which applied Siegel and Castellan's nonparametric solution (60) with a Bonferroni correction. A probability level of a P value of <0.05 was considered significant. The same procedure was used to analyze the responses of bMEpC to MAMPs compared to unstimulated cells.
RESULTS
Induction of leukocyte recruitment in milk by MDP and LTA.
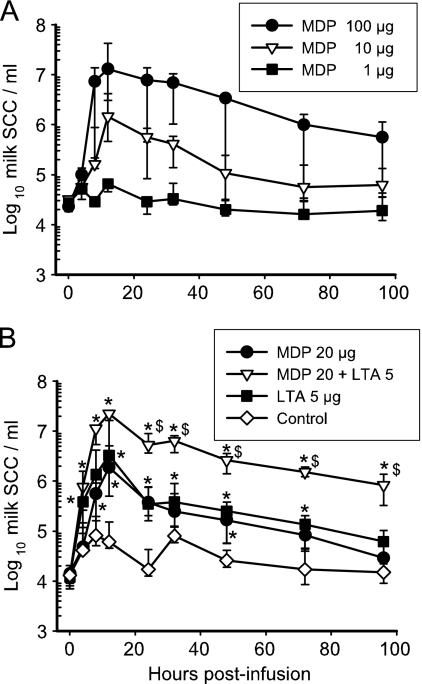
Owing to the absence of published information, a pilot experiment was necessary to assess the dose-response of the mammary gland to MDP. Three cows received 3 doses (1, 10, or 100 μg) of MDP simultaneously in three different quarters. An inflammatory response resulted from the intraluminal infusion of MDP via the teat canal. With the highest dose (100 μg), an intense cellular recruitment occurred as soon as 8 hpi and peaked at 12 hpi (Fig. 1A). A sizeable influx of cells in milk was also induced by 10 μg MDP, but 1 μg MDP had no effect (Fig. 1A). As the individual variability was high with 10 μg MDP, the dose of 20 μg was retained to investigate the inflammatory response of the mammary gland to the simultaneous challenge with LTA (TLR2 agonist) and MDP (NOD2 agonist). Previous experiments established that LTA is not active at 1 μg but that it induced mild mastitis at a dose of 10 μg per gland (49). An intermediate dose of 5 μg was chosen for the challenge in combination with MDP.
FIG. 1.
Cellular response induced by LTA and MDP in the mammary gland. Concentrations of cells in milk of mammary glands (quarters) infused at 0 h with different amounts of MDP or S. aureus LTA, alone or in combination, were monitored at 4, 8, 12, 24, 32, 48, 72, and 96 hpi. Data are median values and interquartile ranges. (A) Pilot experiment with three cows which received 1, 10, or 100 μg MDP in three different quarters. The fourth quarter served as a control. (B) Eleven cows received 20 μg MDP, 5 μg LTA, or the combination of 20 μg MDP and 5 μg LTA in three different quarters. *, statistical significance (P < 0.05, multiple comparisons) of values from challenged versus control quarters; $, significance (P < 0.05, multiple comparisons) of values from quarters challenged with MDP plus LTA versus either LTA or MDP. SCC, somatic cell count.
Intramammary infusion of 5 μg LTA or 20 μg MDP induced a prompt cell influx but of a relatively short duration, since cell concentrations in milk were not different from the values for control quarters at 96 hpi (Fig. 1B). The cell recruitment after infusion of the combination of MDP plus LTA tended to be more intense at the beginning of the inflammatory response (although not significantly) than with either agonist alone, but the main difference was the protracted cell influx. Differences between quarters that had received LTA plus MDP and quarters that had received either LTA or MDP were significant (P < 0.05) from 24 h to 96 hpi (Fig. 1B), showing that the cell response to the mixture was synergistic, with a more-than-1-log difference. There was a comparatively slight reaction in the control quarters, illustrating that mammary glands of the same udder are not completely independent with respect to inflammation.
Examination of milk cells on cytospin slides indicated that before challenge, mononuclear cells were the major population (60 to 75% of milk cells), but in all samples with cell concentrations above 1 million cells/ml, neutrophils were the dominant population (80 to 96%). Very few if any epithelial cells were shed in milk during the experiment.
Infusion of the pathogen recognition receptor (PRR) agonists induced a transient febrile episode: rectal temperatures significantly increased as soon as 8 hpi and peaked (39.6°C) at 12 hpi but had returned to prechallenge values (38.2°C versus 38.4°C; median values for the 11 cows) at 24 hpi. No systemic clinical sign was apparent, and local signs were mainly modified milk, such as clots and flakes, and yellowish coloration in three quarters that had received the mix of MDP plus LTA at 12 and 24 hpi.
Milk concentrations of chemoattractants for neutrophils.
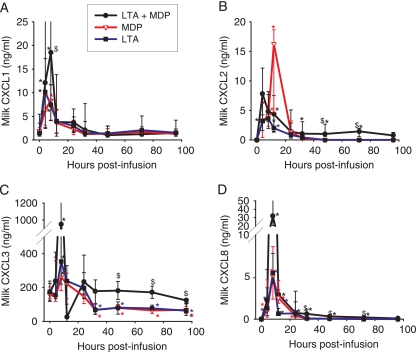
As neutrophils were massively recruited in milk following challenge with MDP and LTA, we measured the concentrations of several mediators that are chemotactic for this cell type. The ELR plus CXC (ELR+CXC) chemokines, which display the three amino acids glutamate-leucine-arginine (ELR) before the CXCL motif, are neutrophil chemoattractants (32). Variations in the concentrations of four of the ELR+CXC chemokines were monitored in milk during the inflammatory response: CXCL1, CXCL2, CXCL3, and CXCL8. Concen- trations of CXCL1 in milk increased significantly only at 4 and 8 hpi with the three stimuli (Fig. 2A). Concentrations of CXCL1 after infusion with LTA plus MDP were significantly above concentrations after infusion with LTA or MDP at 8 hpi. Concentrations of CXCL2 increased significantly at 4, 8, 12, and 24 hpi with the three stimuli (Fig. 2B). The increases were also significant at 32, 48, and 72 hpi with LTA plus MDP. Increases in CXCL2 concentrations were significantly higher with LTA plus MDP than with LTA or MDP at 4, 48, and 72 hpi but not at the peak of the response (8 to 24 hpi). Concentrations of CXCL3 were already high before infusion (about 170 ng/ml). We have recently established that CXCL3 is the constitutive bovine milk chemokine responsible for the chemotactic activity of normal milk (51). Concentrations of CXCL3 increased significantly only at 8 hpi with the three stimuli (Fig. 2C). Later on, concentrations significantly decreased at 32, 48, 72, and 96 hpi with the LTA and MDP stimuli, whereas with the LTA plus MDP stimulus, concentrations did not differ from the initial concentration. Concentrations with LTA plus MDP were significantly above concentrations with LTA or MDP at 8, 32, 48, 72, and 96 hpi. CXCL8 concentrations were significantly above prechallenge values at 4, 8, 12, and 24 hpi with the LTA and MDP stimuli and up to 96 hpi with the mix of LTA plus MDP (Fig. 2D). Concentrations after challenge with LTA plus MDP were significantly above concentrations with LTA or MDP at 8, 32, 48, and 72 hpi.
FIG. 2.
Concentrations of ELR+CXC neutrophil-oriented chemokines CXCL1 (A), CXCL2 (B), CXCL3 (C), and CXCL8 (D) in milk of quarters infused with either MDP, S. aureus LTA, or LTA plus MDP from the time of infusion (time zero) to 96 hpi. Data are from six cows (median values and interquartile ranges). *, significantly increased or decreased (CXCL3) concentrations relative to that at time zero (P < 0.05); $, significant difference between LTA plus MDP and LTA or MDP alone (P < 0.05).
Overall, the concentrations of the four ELR+CXC chemokines were augmented as soon as 4 hpi, peaked at 8 hpi (except for CXCL2 which peaked at 12 hpi), and fell abruptly at 24 hpi. This fall in concentrations may have been amplified by proteolysis or trapping in clots, because the 12-hpi samples were those which showed the strongest modifications (clots, flakes, and yellowish coloration). The challenge with LTA plus MDP induced significantly greater increases at the beginning of the inflammatory response (4 and 8 hpi) but also later on, at 32 to 96 hpi for CXCL3 and CXCL8, thus causing a protracted secretion.
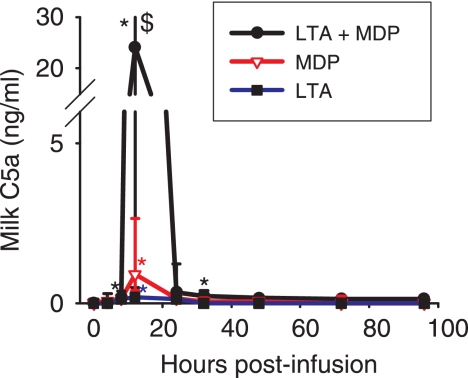
C5a is the main neutrophil-oriented chemotactic component derived from the activation of the complement system. After challenge with either LTA or MDP, C5a concentrations significantly increased only at 12 hpi, whereas with LTA plus MDP, increases in concentrations were significant from 8 to 32 hpi (Fig. 3). Concentrations after challenge with LTA plus MDP were significantly higher than concentrations with LTA or MDP at 12 hpi.
FIG. 3.
Concentrations of the complement-derived chemoattractant C5a in milk of quarters infused with either MDP, S. aureus LTA, or LTA plus MDP from the time of infusion (time zero) to 96 hpi. Data are from six cows (median values and interquartile ranges). *, significantly increased concentration relative to that at time zero (P < 0.05); $, significant difference between LTA plus MDP and LTA or MDP alone (P < 0.05).
Milk concentrations of inflammatory cytokines.
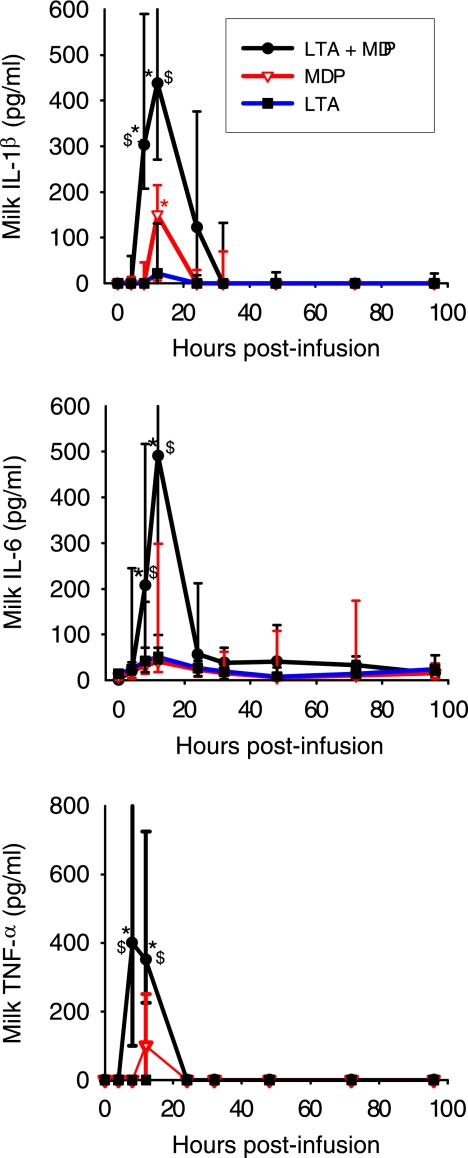
Inflammatory cytokines such as TNF-α, IL-1β, and IFN-γ are known to induce chemokines indirectly by stimulating myeloid and stromal cells. IFN-γ was not found in milk except in two quarters challenged with the mix of LTA plus MDP at 12 hpi (15 pg/ml). In contrast, IL-1β was found in all quarters infused with MDP or LTA plus MDP but in only six quarters infused with 5 μg LTA and at rather low concentrations. Only the peak value, which was reached at 12 hpi, differed significantly from the baseline for MDP, whereas the values at 8 and 12 hpi differed significantly for LTA plus MDP (Fig. 4). After challenge with LTA plus MDP, IL-1β concentrations were significantly higher than concentrations with LTA or MDP at 8 hpi, as a result of an earlier increase. Concentrations of IL-6 increased very slightly after the infusion of LTA or MDP, but increases after the infusion of LTA plus MDP were marked at 8 and 12 hpi (Fig. 4). Values returned to baseline as soon as 24 hpi. TNF-α was found in only 7 of the 11 quarters challenged with LTA plus MDP at 8 hpi (median value, 330 pg/ml) and 12 hpi (90 pg/ml) (Fig. 4). Overall, very small amounts of inflammatory cytokines were detected in milk, even when MDP was associated with LTA. Concentrations were sizeable only at the peak of the inflammatory response, when cell concentrations were of several millions per ml in milk (Fig. 1).
FIG. 4.
Concentrations of the proinflammatory cytokines IL-1β, IL-6, and TNF-α in milk of quarters infused with either MDP, S. aureus LTA, or LTA plus MDP from the time of infusion (time zero) to 96 hpi. Data are from 11 cows (median values and interquartile ranges). *, significantly increased concentration relative to that at time zero (P < 0.05); $, significant difference between LTA plus MDP and LTA or MDP alone (P < 0.05).
Chemokines and proinflammatory cytokines can be secreted by bMEpC stimulated with bacteria or bacterial components (31, 62, 70, 72). We thus made the assumption that MEpC could be important contributors of proinflammatory cytokines and of neutrophil-oriented chemokines and set out to investigate the response of these cells to stimulation with the PRR agonists used to challenge mammary glands.
Secretion of chemokines by bMEpC in response to MDP and LTA.
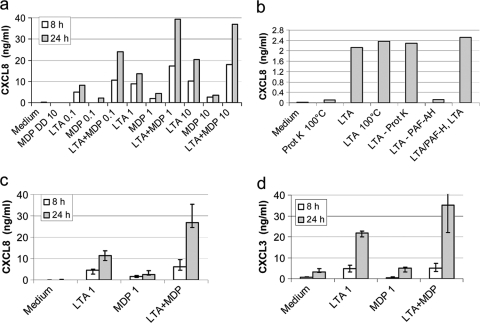
In order to check whether MDP stimulated bMEpC and to titrate the MDP and LTA preparations, we performed a pilot experiment with different concentrations of MDP, LTA, or MDP plus LTA to stimulate bMEpC of one cow. Both LTA and MDP induced the secretion of CXCL8 by MEpC, but LTA was a stronger inducer (Fig. 5a). It was shown previously that NOD2 does not detect MDP-dd, in which the second amino acid, d-Glx, is replaced by the enantiomer l-Glx (17). We therefore checked if MEpC reacted to MDP-dd. The isoform MDP-dd was inactive, showing the stereospecificity of the response to the active isoform MDP-ld (Fig. 5a). Purified S. aureus LTA induced bMEpC to secrete CXCL8 with a dose-response from 0.1 to 10 μg/ml. Synthetic MDP was less efficient than LTA, and increasing its concentration did not improve the CXCL8 response (Fig. 5a). To check the purity of the LTA preparation, LTA was submitted to heat treatment, proteinase K digestion, and PAF-AH. LTA activity resisted boiling at 95°C for 10 min and proteinase K but was inactivated by PAF-AH (Fig. 5b), suggesting that its activity was due to LTA and not to lipoproteins, which were shown to resist PAF-AH (58).
FIG. 5.
Secretion of the chemokines CXCL8 and CXCL3 by bMEpC stimulated with LTA and MDP alone or in combination. (a) Dose-response of bMEpC from one cow to LTA and MDP. (b) Susceptibility of LTA to PAF-AH or proteinase K. Purified LTA (200 ng/ml) was used to stimulate bMEpC; alternatively, LTA (200 ng/ml) was pretreated with the LTA-inactivating enzyme PAF-acetyl-hydrolase (LTA-PAF-AH) or with lipoproteins inactivating proteinase K (LTA-Prot K). LTA (200 ng/ml) was added to the treated LTA to check whether PAF-AH treatment had generated inhibitors of CXCL8 secretion (LTA-PAF-AH or LTA). (c) Secretion of CXCL8 by MEpC (median values from cells of five cows, first quartile [Q1] and Q3) in response to LTA and MDP at 8 h and 24 h poststimulation. Shown is the secretion of CXCL3 in response to LTA and MDP at 1 μg/ml. (d) Secretion of CXCL3 in response to LTA and MDP at 0.1 μg/ml. MDP-DD, inactive isoform of muramyl-dipeptide (MurNAc-d-Ala-d-iso-Gln); MDP, active isoform (MurNAc-l-Ala-d-iso-Gln).
As there was a trend for the potentiation of activity between the two agonists, they were tested in combination at suboptimal concentrations (1 μg/ml) to stimulate bMEpC of five cows. The association of LTA plus MDP induced concentrations of IL-8 that exceeded the concentrations induced by either component alone (Fig. 5c). The CXCL3 response was additive with the agonists at 1 μg/ml (Fig. 5d).
Secretion of inflammatory cytokines by bMEpC.
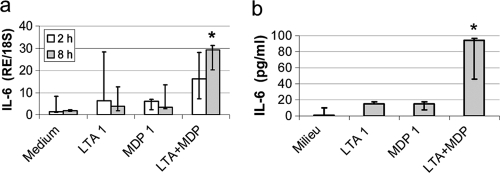
We investigated the capacity of bMEpC to produce proinflammatory cytokines in response to LTA and MDP. These agonists were used at the suboptimal concentration of 1 μg/ml with a view to revealing additive or synergistic effects. LTA or MDP did not significantly increase the expression of IL-6 at 2 h poststimulation, even when used in combination. At 8 h poststimulation, only the combination of the two agonists induced a significant increase of IL-6 expression (Fig. 6a). At the protein level, the secretion of IL-6 by bMEpC was significantly increased only by the combination of LTA and MDP (Fig. 6b).
FIG. 6.
Production of IL-6 by bMEpC stimulated with LTA or MDP at 1 μg/ml. (a) Production at the mRNA level at 2 h or 8 h poststimulation. (b) Production at the protein level at 24 h poststimulation. Shown are median values (Q1 and Q3) of bMEpC from five cows. There was a significant difference (P < 0.05 by Friedman test) only between medium only and LTA plus MDP at 8 h poststimulation for mRNA and at 24 h for protein secretion. RE/18S, expression relative to 18S rRNA.
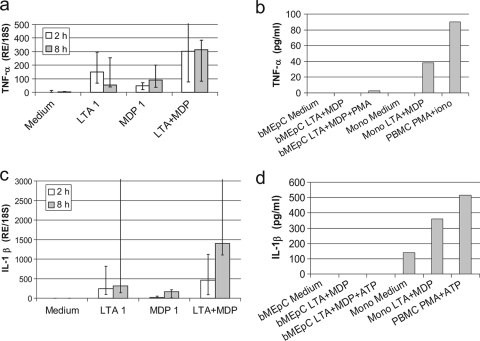
There was a significant increase in the level of expression of TNF-α mRNA at 2 and 8 h poststimulation (Fig. 7a), but TNF-α was not detectable in the cell culture supernatant at 16 h poststimulation (Fig. 7b). ELISA determinations were performed on the supernatants collected at 1 h and 6 h poststimulation to detect a possible early and transient secretion, but results were negative (result not shown). Monocytes and PBMC were stimulated with LTA plus MDP and PMA plus ionomycin, respectively, and TNF-α was detected in the culture supernatant (Fig. 7b), showing that the absence of detectable TNF-α in the bMEpC supernatants was not due to technical reasons. As PMA was shown to stimulate the protease TACE (TNF-α-converting enzyme), which is required to release TNF-α from the cell membrane (10), bMEpC were stimulated with LTA plus MDP and 200 ng/ml PMA, but TNF-α was not detected in the cell supernatant (Fig. 7b). Also, immunoblot analysis of whole-cell extracts with an antibody to bovine TNF-α (rabbit polyclonal antibody; LifeSpan Biosciences, Seattle, WA) after SDS-PAGE did not reveal a TNF-α band (results not shown).
FIG. 7.
Production of TNF-α and IL-1β by cells stimulated with LTA or MDP. (a) bMEpC stimulated with LTA (1 μg/ml), MDP (1 μg/ml), or a combination of LTA plus MDP (1 μg/ml) responded with an increased level of expression of TNF-α mRNA (P < 0.05 by Kruskal-Wallis test). Shown are median values (Q1 and Q3) from bMEpC of five cows. (b) TNF-α in cell culture supernatants after 16 h of culture; results are from a representative experiment with either bMEpC or leukocytes. (c) IL-1β mRNA levels in response to LTA or MDP. Shown are median values (Q1 an Q3) from bMEpC of five cows. (d) IL-1β in cell culture supernatants after 16 h of culture; results are from the bMEpC of five cows or a representative experiment with either bMEpC or leukocytes. RE/18S, expression relative to 18S rRNA; PMA, phorbol myristate acetate; Mono, monocytes; PBMC, peripheral blood mononuclear cells stimulated with PMA and ionomycin.
Upon stimulation with LTA, bMEpC increased the level of expression of IL-1β mRNA (P < 0.05 by a Friedman test). Increased levels of expression were noticeable at 2 and 8 h poststimulation with LTA but only at 8 h poststimulation with MDP (Fig. 7c). The combination of LTA plus MDP tended to induce a more-than-additive effect but not significantly due to the high individual response variability (Fig. 7c). At the protein level, IL-1β was not detected in bMEpC culture supernatants after stimulation with LTA plus MDP (Fig. 7d). Using the same ELISA, sizeable concentrations of IL-1β were found in monocyte or PBMC culture supernatants in response to LTA plus MDP or PMA plus ATP (Fig. 7d).
Overall, the capacity of bMEpC to produce major proinflammatory cytokines appeared to be limited to small concentrations of IL-6.
Expression of PRR targeted by MDP and LTA in bMEpC and mammary tissue.
We checked whether bMEpC expressed the receptors supposed to detect LTA and MDP under our culture conditions. In addition, we looked for the expression of these receptors in mammary tissue of uninflamed udders. Due to the lack of commercially available specific antibodies for bovine receptors, expression was investigated by RT-PCR only.
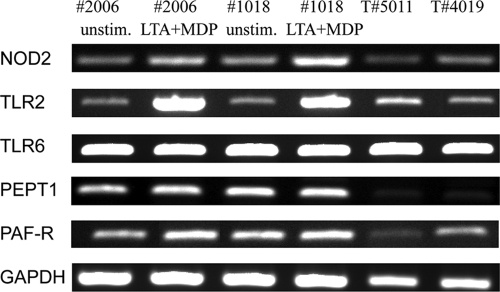
The main sensor of MDP is NOD2, a member of the NACHT-LRR receptor family (17). We found transcripts for NOD2 in cDNA prepared from unstimulated and stimulated bMEpC cultures as well as cDNA of healthy mammary tissue (Fig. 8). Both MEpC and mammary tissue yielded an amplicon of the expected size. The identity of NOD2 was checked by sequencing of the PCR product. Complete homology with the known sequence of bovine NOD2 was obtained. As NOD2 is in the cytosol and MDP is a hydrophilic molecule which cannot passively cross the cytoplasmic membrane, the response of bMEpC to extracellular MDP implies a translocation mechanism. In intestinal epithelial cells, MDP has been shown to gain entry by using the dipeptide/tripeptide transporter PEPT1, also known as SLC15A1 (solute carrier family 15 member 1) (68). By RT-PCR, bMEpC and mammary tissue were found to express SLC15A1 (Fig. 8). Complete homology of the amplicon sequence with the known sequence of bovine SLC15A1was obtained.
FIG. 8.
Expression of transcripts of genes encoding PRR (NOD2, TLR2, and TLR6), peptide transporter (PEPT1), or PAF receptor by MEpC or bovine mammary tissue. RNA was extracted from bMEpC of two cows (cows 2006 and 1018), which were left unstimulated or were stimulated with LTA plus MDP (1 μg/ml each) for 8 h. RNA was also obtained from the mammary tissue of uninfected uninflamed quarters of two lactating cows (cows 5011 and 4019). RT-PCR was performed as described in Materials and Methods. GAPDH expression was used as a positive control.
The main receptor for LTA is TLR2, in association with TLR6 and CD14 (1, 56), and bovine TLR2 has been shown to sense staphylococcal LTA (13). It was shown previously that bMEpC express TLR2 in culture and in vivo although in small amounts in unstimulated cells or uninflamed mammary tissue (47). We checked by RT-PCR that bMEpC expressed TLR2 and TLR6 mRNAs under our culture conditions. Transcripts were detected (Fig. 8), and the sequences of the amplicons corresponded to the expected bovine sequences.
Another receptor for staphylococcal LTA was hypothesized to be at the surface of epithelial cells, i.e., PAF receptor (PAFR), the receptor for platelet-activating factor (36). The generation of NO by macrophages was shown previously to depend on the indirect stimulation of PAFR by LTA through the generation of PAF (21), and mucin production by human epithelial cells in response to LTA was previously stated to result from the direct interaction of LTA with PAFR (36). Consequently, we investigated whether CXCL8 secretion by bMEpC in response to LTA could involve PAFR stimulation. First, we looked for the expression of PAFR by MEpC and found mRNA expression in bMEpC (Fig. 8). We then checked whether bMEpC responded to PAF. Culture for 8 h or 16 h with PAF-C16 (10, 50, or 100 nM) did not induce CXCL8 secretion (results not shown). We also used WEB2086, an inhibitor of PAFR which was proven previously to be efficient on bovine PAFR (6), to test whether PAFR contributed to CXCL8 secretion. The inhibitor WEB2086 did not significantly reduce CXCL8 secretion by bMEpC in response to LTA (Fig. 9). Overall, these results do not substantiate a role for PAFR in the stimulation of bMEpC by staphylococcal LTA.
FIG. 9.
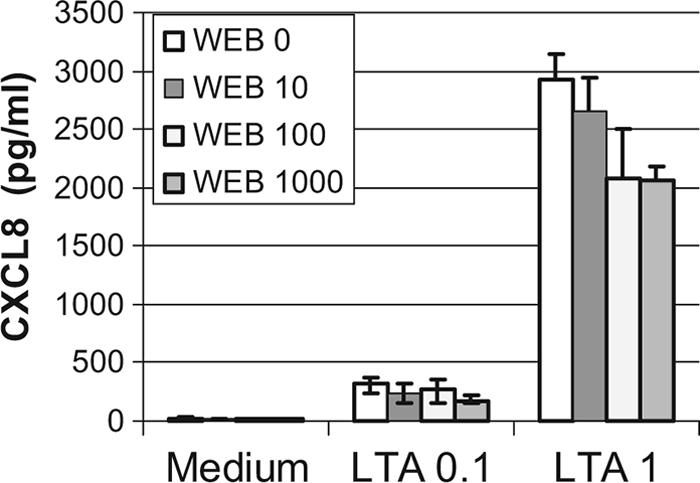
Effect of the PAFR inhibitor WEB2086 on the secretion of CXCL8 by bMEpC stimulated with LTA. Bovine MEpC were preincubated without (WEB 0) or with 10, 100, or 1,000 nM WEB2086 and then stimulated with 0.1 or 1 μg/ml LTA or not stimulated (medium). The cell culture supernatant was collected 16 h later, and CXCL8 concentrations were determined by ELISA. Results are median values (Q1 and Q3) from bMEpC of four cows. Reductions of CXCL8 concentrations by WEB2086 were not statistically significant (Friedman test).
Activation of NF-κB in bMEpC by LTA, MDP, and LTA plus MDP.
TLR2 and NOD2 signaling pathways converge on NF-κB activation, which is a major effector of increased transcription for these two PRRs (63). We checked whether NF-κB was involved in the stimulated secretion of CXCL8 by measuring the activation of NF-κB p65 in nuclear cell extracts of bMEpC unstimulated or stimulated for 2 h or 8 h with LTA or MDP by using the TransAM transcription factor NF-κB p65 ELISA. The activation of p65 occurred with LTA as soon as 2 h poststimulation and with LTA or MDP at 8 h poststimulation (Fig. 10). The combination of LTA and MDP did not activate more NF-κB p65 than did LTA alone (Fig. 10). Also, the contribution of the NF-κB activation pathway was tested by using a pharmacological inhibitor of NF-κB. The production of CXCL8 by bMEpC stimulated with LTA, MDP, or LTA plus MDP was significantly reduced by the NF-κB inhibitor (Fig. 11). Overall, these results indicate that NF-κB was involved in the CXCL8 response of bMEpC to LTA and MDP.
FIG. 10.
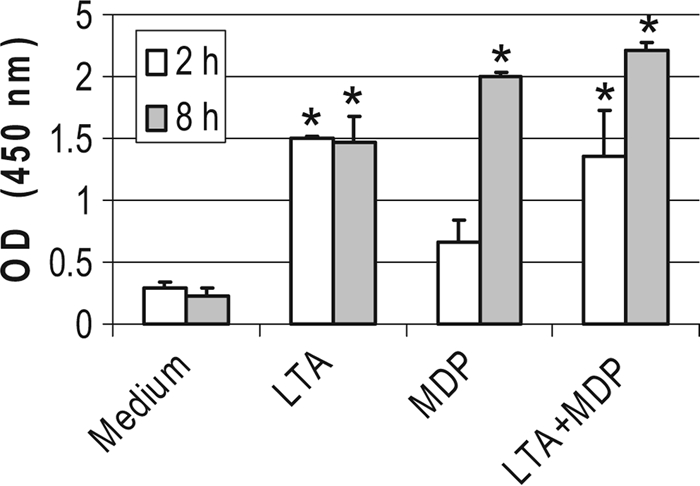
Activation of NF-κB p65 in bMEpC after 2 h or 8 h of stimulation with LTA (1 μg/ml) or MDP (1 μg/ml) used alone or in combination. Activation was measured in nuclear cell extracts of bMEpC by ELISA. Results are median values (Q1 and Q3) from bMEpC of four cows. *, significant differences compared to the unstimulated (medium) bMEpC (P < 0.05 by Friedman test and Bonferroni's multiple comparisons).
FIG. 11.
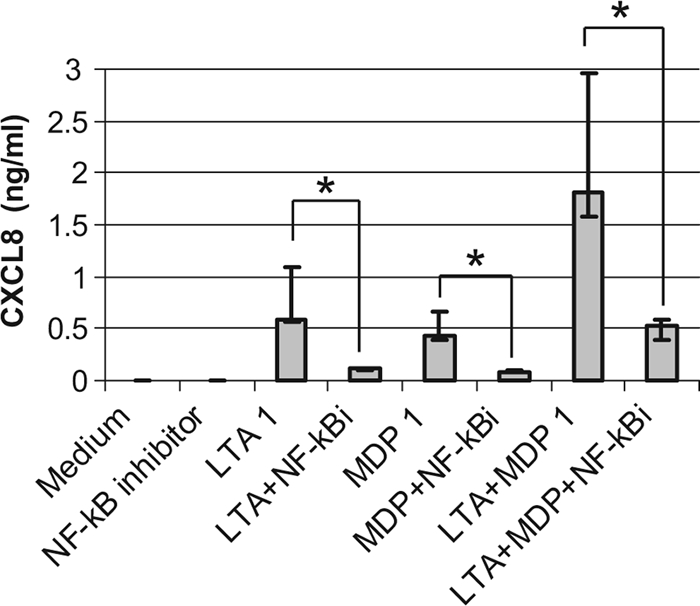
Effect of a pharmacological inhibitor of NF-κB on CXCL8 secretion by bMEpC incubated with LTA or MDP. Bovine MEpC were preincubated for 1 h at 37°C with 15 μM NF-κB activation inhibitor (Calbiochem) before the addition of MAMPs (1 μg/ml). Cell culture supernatants were collected 8 h poststimulation, and CXCL8 concentrations were determined by ELISA. Results are median values (Q1 and Q3) from bMEpC of five cows *, reductions of CXCL8 concentrations by NF-κB inhibition are significant (P < 0.05 by Friedman test and Bonferroni's multiple comparisons).
DISCUSSION
S. aureus is a leading cause of mastitis and one of the most successful pathogens for the mammary gland. In spite of numerous studies on the pathogenesis of S. aureus mastitis, the bacterial molecular patterns causing inflammatory and innate immune responses to S. aureus in the bovine mammary gland are not well defined. Recently, we have shown that staphylococcal LTA is recognized by the mammary gland and induces a massive influx of neutrophils (49). Peptidoglycan fragments are other major agonists of the innate immune system. MDP is the minimal peptidoglycan structure recognized by NOD2 (17), but it does not seem to be produced in significant amounts naturally by S. aureus or host enzymes (11). Recently, it was reported that a natural S. aureus peptidoglycan fragment, including the MDP structure, activates NOD2 (69). Here, we show that MDP is another MAMP sensed by the mammary gland and that the combination of LTA and MDP exerts a synergistic effect on the recruitment of leukocytes in milk. More than 80% of these leukocytes were neutrophils, indicating that the local response to the intramammary infusion of LTA, MDP, or both was a neutrophilic inflammation. In line with the preferential recruitment of neutrophils was the synergistic effect of LTA plus MDP on the production of ELR+CXC chemokines (CXCL1, CXCL2, CXCL3, and CXCL8) and of C5a, chemoattractants which target mainly neutrophils (Fig. 2 and 3). With the suboptimal amounts of LTA and MDP used in this study to favor the demonstration of synergy, the inflammatory cytokines TNF-α, IL-1β, and IL-6 were detected in milk only with the combination of LTA and MDP (Fig. 4), indicating that the synergy extends to the generation of proinflammatory cytokines in the mammary gland. Because MEpC are considered sentinels of the mammary gland, we investigated the response of these cells to the MAMPs that we had used in vivo.
In our hands, LTA proved to be an efficient inducer of the secretion of chemokines by bMEpC. This somewhat contradicts a previous report showing that LTA was a poor stimulator of bovine MEpC compared to LPS (62). This may be because in the latter study, streptococcal LTA was used, as the response to Streptococcus pneumoniae LTA was only 1% compared to that of S. aureus LTA (20). The commercial LTA preparation that we used was prepared by the n-butanol extraction method, which preserves its activity (42), and is devoid of TLR4-stimulating capacity. The possible contamination of staphylococcal LTA preparations with lipoproteins has fuelled controversies as to the real activity of LTA (22). This is why we subjected the LTA preparation to enzymatic treatment with PAF-AH, as this enzyme was shown previously to inactivate LTA but not lipopeptides (58). The almost complete loss of CXCL8-stimulating activity after treatment with PAF-AH indicates that the activity was indeed due to LTA and not to lipoproteins. Both staphylococcal LTA and diacylated lipopeptides signal through the dimeric receptor TLR2/TLR6. We checked that under our culture conditions, bMEpC expressed transcripts of genes encoding TLR2 and TLR6. Our positive result is in line with data from previously published reports (18, 47, 62). Cofactors like CD14 and CD36 are known to increase responses to LTA (37, 56). CD36 is expressed by MEpC (61), and although CD14 may not be expressed at the membrane by bMEpC, soluble CD14 is found in milk and renders CD14-negative cells responsive to LPS (3, 33). Overall, it is not surprising that bMEpC are able to respond to LTA.
Besides TLR2, PAFR has been shown to act as an activating receptor for LTA on epithelial cells and macrophages (21, 36). The infusion of PAF in the teat cistern of bovine mammary gland induces the recruitment of leukocytes in the lumen, and this response is reduced by WEB2086, an inhibitor of PAFR (46). This finding suggests that PAFR is expressed in the mammary gland by cells that have access to the luminal compartment. We found transcripts of the gene encoding PAFR in bMEpC and healthy mammary tissue, but under our conditions, bMEpC did not secrete CXCL8 in response to PAF. Also, there was only a slight, insignificant reduction in the level of secretion of CXCL8 by bMEpC stimulated by LTA in the presence of high concentrations of WEB2086, an inhibitor of PAFR. These results suggest that PAFR did not contribute significantly to CXCL8 production in response to LTA.
Another finding of this study is that bMEpC are responsive to MDP. When used alone, MDP was not a strong inducer of chemokines compared to LTA, but it was able to potentiate responses to LTA: at suboptimal concentrations, there was a clear synergy between the two agonists for the secretion of IL-8 and IL-6 (Fig. 5 and 6). Previous studies have shown that bMEpC respond to streptococcal LTA (62) or staphylococcal LTA (72), but to our knowledge, MDP had never been used to stimulate MEpC. It was demonstrated previously that the NOD-like receptor (NLR) NOD2 is a major MDP sensor (17, 31). As MDP induced inflammation when infused in the lumen of the mammary gland, we searched for the expression of NOD2 in mammary tissue and bMEpC. We found transcripts of NOD2 in uninflamed mammary tissue and in unstimulated MEpC under our culture conditions (Fig. 8). The unavailability of antibodies cross-reacting with bovine NOD2 prevented us from checking that bMEpC expressed this PRR at the protein level. Although there is no previous report on the expression of NOD2 by MEpC in the bovine species, it was shown previously that various human epithelial cell lines, including a breast epithelial cell line, express functional NOD2 (65). MDP has been shown to signal via other NLRs such as Nalp1 and Nalp3, components of inflammasomes triggering the processing of pro-IL-1β (14, 40). Specifically, IL-1β was secreted by human monocytes or macrophages following stimulation with MDP, and this secretion was dependent on the processing of pro-IL-1β by caspase-1 (40). In our study, MDP did not induce the secretion of IL-1β, although IL-1β transcripts were produced. This may be because mammary epithelial cells do not use inflammasomes as macrophages or a reconstituted cellular system does. Whatever it may be, the absence of the IL-β protein in spite of the presence of IL-1β mRNA is not in favor of inflammasome activation by MDP in bMEpC.
Under our culture conditions, bMEpC responded to MDP when exposed to this hydrophilic molecule in solution, although all known sensors of MDP are cytosolic. It was reported previously that the extracellular presentation of MDP failed to induce a response from macrophages, which could result from the incapacity of MDP to cross the hydrophobic cytoplasmic membrane and reach the cytosolic NOD2 (17). The hypothesis of a membrane transporter allowing MDP to cross the cytoplasmic or endosomal membranes has been put forward but not yet substantiated (28), even though the cytosolic delivery of diverse bacterial products by the hemichannel pannexin-1 was recently described (27). In colonic epithelial cells, MDP is taken up by the apical dipeptide/tripeptide transporter PEPT1, and the uptake of MDP activates NF-κB by a mechanism involving NOD2 (68). We found transcripts of the PEPT1 transporter in bMEpC in culture and in mammary tissue, and thus, this could be an explanation for the responsiveness of bMEpC to extracellular MDP. Recently, it was shown that MDP is internalized and traffics via the endocytic machinery, involving the clathrin-dependent coated-pit pathway, probably in conjunction with a membrane receptor (35). Whatever the mechanism, the fact that MDP induced an inflammatory response after infusion in the lumen of the lactating mammary gland indicates that this mechanism may operate in vivo. Alternatively to MEpC, mammary macrophages may be responsible for the response to MDP.
We found that the combination of LTA and MDP resulted in much stronger neutrophilic inflammation after infusion in the mammary gland and induced a stronger chemokine response by bMEpC than did the use of LTA or MDP alone. Synergistic activity between LTA and MDP in vivo and in vitro on macrophages, monocytes, or dendritic cells was reported previously (30, 56, 64). Our results indicate that NF-κB was involved in the response of bMEpC to LTA and MDP. A pharmacological inhibitor of NF-κB significantly reduced CXCL8 secretion (Fig. 11), and activated NF-κB p65 was found in increased amounts in nuclear extracts of stimulated cells (Fig. 10). These results are at odds with a previous report showing that whole killed S. aureus or staphylococcal LTA failed to activate NF-κB in bMEpC (72). We have no explanation for this discrepancy. One biological consequence of this synergy would be that the mammary gland is able to detect lower concentrations of S. aureus MAMPs, thus triggering an innate immune response early during the infection process.
The milk chemokine and cytokine profile of the mammary inflammatory response to the combination of LTA plus MDP was comparable to the profile induced by S. aureus experimental infections, in that low concentrations of proinflammatory cytokines are found in milk of S. aureus-infected glands compared to responses induced by Escherichia coli (3, 54). In particular, TNF-α is not found in milk from S. aureus-infected quarters (2). The inability of bMEpC to secrete TNF-α and IL-1β in response to LTA and MDP is in keeping with these in vivo observations. However, several studies have reported increases in TNF-α or IL-1β gene expression levels in bMEpC stimulated with S. aureus or LTA (19, 62, 70, 72). It is noteworthy that in these studies, the secretion of TNF-α or IL-1β was not investigated. In our study, we found a divergence between TNF-α mRNA expression and protein secretion. The dissociation between transcription and translation (or secretion) is not uncommon, because the production of these two potent proinflammatory cytokines is tightly regulated (55). At present, we have no explanation for the absence of secretion of TNF-α by bMEpC stimulated by LTA and MDP. The divergence between the increase in numbers of TNF-α transcripts and protein secretion may depend on the cell type and species. For the human monocytic Monomac-6 cell line, MDP was reported previously to induce a weak induction of TNF-α mRNA but not protein (71), and there was a previously reported example of dissociation between mRNA and the protein for TNF-α by bMEpC (44). In a previous study we found transient and low levels of TNF-α secretion in the supernatant of bMEpC cocultured with live S. aureus (34). This finding suggests that even whole and metabolically active staphylococci are not good inducers of TNF-α for bMEpC.
Contrary to TNF-α, the absence of production of IL-1β by bMEpC is not unexpected. After the transcription of the coding gene, IL-1β is produced by monocytes and macrophages as an inactive cytoplasmic precursor (pro-IL-1β) whose maturation and secretion are mediated by caspase-1, a protease that processes pro-IL-1β into biologically active IL-1β (9). The activation of caspase-1 is controlled by inflammasomes, multiprotein complexes which play a prominent role in inflammation (15). Agonists of TLR may trigger the transcription of the gene encoding IL-1β, but this is not sufficient for inflammasome activation. The case of MDP/NOD2 is less clear. Depending on the cell type and stimulation conditions, MDP was unable to stimulate IL-1β production (29, 39) or, on the contrary, was supposed to activate caspase-1 and release IL-1β (38, 67). It was recently reported that no combination of pure TLR2 and NOD2 agonists is sufficient for inflammasome activation and secretion of IL-1β by murine macrophages (59). In macrophages and monocytes, the induction of IL-1β secretion by TLR agonists requires a second signal provided by extracellular ATP to be efficient (45). ATP activates the purinergic receptor P2X7R, thus promoting an efflux of K+, which is implicated in IL-1β secretion (26). We tested whether the addition of ATP to LTA and MDP induced IL-1β secretion by bMEpC, but the attempt was unsuccessful, although ATP augmented the secretion of IL-1β by bovine monocytes stimulated under the same conditions (Fig. 7). In relation to the induction of IL-1β production by S. aureus, it was shown previously that although TLR agonists or MDP (NOD2 agonist) did not induce the activation of caspase-1, living S. aureus cells induced the activation and secretion of IL-1β (39). Recently, it was shown that for S. aureus to activate the macrophage Nalp3 inflammasome, the combined action of TLR agonists, MDP, and a pore-forming toxin was necessary (8). To our knowledge, there is no published report on the inflammasomes of MEpC. In fact, the production of IL-1β by bMEpC is not documented. The lack of production of TNF-α in response to LTA and MDP and of IL-1β in the additional presence of ATP indicates that bMEpC do not respond to bacterial agonists like monocytes or macrophages do. As IL-1β was found in milk after the infusion of MDP or LTA plus MDP, but not in the culture supernatant of bMEpC stimulated with these MAMPs, the question arises of the contribution of MEpC to the production of milk IL-1β. It is possible that cells other than MEpC were responsible for the presence of IL-1β in milk or that a cooperation between different cell types is required. Much remains to be done to investigate the production of IL-1β by MEpC and to assess the role of the inflammasomes in the defense of the mammary gland against its common bacterial pathogens. In particular, the expression by bMEpC of functional proteins of the NOD-like receptor family, comprising NOD1, NOD2, and inflammasomes, remains to be established. In this study, LTA and MDP were considered agonists of TLR2 and NOD2, respectively. Although the reactivity of bovine TLR2 to LTA and expression at the protein level were verified previously (12, 47, 72), the case for NOD2 is not settled. We found an expression of transcripts by bMEpC, but protein production was not checked for the lack of proper reagents. Also, we did not establish that bMEpC reacted to MDP through the NOD2 activation pathway. Although this is plausible, because there is at the moment no described alternative pathway leading to NF-κB activation and the production of chemokines such as CXCL8, the role of NOD2 remains to be proven.
Whereas this study focused on the effect of two individual staphylococcal agonists of PRR, the infection pathophysiology is more complex, because S. aureus produces an array of MAMPs and toxins that are sensed by cells of the immune system and because the end response results from the integration of multiple incoming signals. Individual or combined purified agonists and complex and only partially defined stimuli like bacterial culture supernatants or whole dead or living bacteria can be used to progressively unravel the network of PRR signaling pathways that shape the response of MEC and mammary tissue to bacterial pathogens.
In conclusion, this study shows that the mammary gland recognizes MDP and responds by mounting neutrophilic inflammation and that MDP synergizes with LTA. Our results strongly suggest that MEpC contribute to this response. A new finding was that bMEpC have the capacity to sense MDP. Another finding was that the bMEpC response was characterized by the secretion of chemokines but not proinflammatory cytokines. We are now investigating whether this characteristic still applies when MEpC are confronted with staphylococcal supernatants or whole bacteria. This study confirms that bovine MEpC are geared to react to MAMPs, a feature which is in conformity with an important role of sentinels of the lumen of the mammary gland.
Acknowledgments
We are grateful for the excellent services provided by the Experimental Infectiology Platform (PFIE) Unit at Nouzilly.
This work was supported in part by a grant from the French government (MRNT) and from APIS-GENE within the framework of the national Genanimal program and by the European Community Eadgene Network of Excellence.
Footnotes
Published ahead of print on 8 September 2010.
REFERENCES
- 1.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman, D. D. 2009. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J. Anim. Sci. 87:10-25. [DOI] [PubMed] [Google Scholar]
- 3.Bannerman, D. D., M. J. Paape, J. W. Lee, X. Zhao, J. C. Hope, and P. Rainard. 2004. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clin. Diagn. Lab. Immunol. 11:463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergonier, D., and X. Berthelot. 2003. New advances in epizootiology and control of ewe mastitis. Livestock Production Sci. 79:1-16. [Google Scholar]
- 5.Botrel, M. A., M. Haenni, E. Morignat, P. Sulpice, J. Y. Madec, and D. Calavas. 2010. Distribution and antimicrobial resistance of clinical and subclinical mastitis pathogens in dairy cows in Rhone-Alpes, France. Foodborne Pathog. Dis. 7:479-487. [DOI] [PubMed] [Google Scholar]
- 6.Burgos, R. A., M. A. Hidalgo, S. M. Matthei, R. Hermosilla, H. Folch, and J. L. Hancke. 2004. Determination of specific receptor sites for platelet activating factor in bovine neutrophils. Am. J. Vet. Res. 65:628-636. [DOI] [PubMed] [Google Scholar]
- 7.Burvenich, C., V. Van Merris, J. Mehrzad, A. Diez-Fraile, and L. Duchateau. 2003. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 34:521-564. [DOI] [PubMed] [Google Scholar]
- 8.Craven, R. R., X. Gao, I. C. Allen, D. Gris, J. Bubeck Wardenburg, E. McElvania-Tekippe, J. P. Ting, and J. A. Duncan. 2009. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4:e7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello, C. A. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27:519-550. [DOI] [PubMed] [Google Scholar]
- 10.Doedens, J. R., R. M. Mahimkar, and R. A. Black. 2003. TACE/ADAM-17 enzymatic activity is increased in response to cellular stimulation. Biochem. Biophys. Res. Commun. 308:331-338. [DOI] [PubMed] [Google Scholar]
- 11.Dziarski, R. 2003. Recognition of bacterial peptidoglycan by the innate immune system. Cell. Mol. Life Sci. 60:1793-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farhat, K., S. Riekenberg, H. Heine, J. Debarry, R. Lang, J. Mages, U. Buwitt-Beckmann, K. Roschmann, G. Jung, K. H. Wiesmuller, and A. J. Ulmer. 2008. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J. Leukoc. Biol. 83:692-701. [DOI] [PubMed] [Google Scholar]
- 13.Farhat, K., K. S. Sauter, M. Brcic, J. Frey, A. J. Ulmer, and T. W. Jungi. 2008. The response of HEK293 cells transfected with bovine TLR2 to established pathogen-associated molecular patterns and to bacteria causing mastitis in cattle. Vet. Immunol. Immunopathol. 125:326-336. [DOI] [PubMed] [Google Scholar]
- 14.Faustin, B., L. Lartigue, J. M. Bruey, F. Luciano, E. Sergienko, B. Bailly-Maitre, N. Volkmann, D. Hanein, I. Rouiller, and J. C. Reed. 2007. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25:713-724. [DOI] [PubMed] [Google Scholar]
- 15.Franchi, L., T. Eigenbrod, R. Munoz-Planillo, and G. Nunez. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10:241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgin, P., and M. Gouet. 2000. Statistiques avec Excel 2000. Eyrolles, Paris, France.
- 17.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 18.Goldammer, T., H. Zerbe, A. Molenaar, H. J. Schuberth, R. M. Brunner, S. R. Kata, and H. M. Seyfert. 2004. Mastitis increases mammary mRNA abundance of beta-defensin 5, Toll-like-receptor 2 (TLR2), and TLR4 but not TLR9 in cattle. Clin. Diagn. Lab. Immunol. 11:174-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez-Barroso, A., J. L. Anaya-Lopez, L. Lara-Zarate, P. D. Loeza-Lara, J. E. Lopez-Meza, and A. Ochoa-Zarzosa. 2008. Prolactin stimulates the internalization of Staphylococcus aureus and modulates the expression of inflammatory response genes in bovine mammary epithelial cells. Vet. Immunol. Immunopathol. 121:113-122. [DOI] [PubMed] [Google Scholar]
- 20.Han, S. H., J. H. Kim, M. Martin, S. M. Michalek, and M. H. Nahm. 2003. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 71:5541-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han, S. H., J. H. Kim, H. S. Seo, M. H. Martin, G. H. Chung, S. M. Michalek, and M. H. Nahm. 2006. Lipoteichoic acid-induced nitric oxide production depends on the activation of platelet-activating factor receptor and Jak2. J. Immunol. 176:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto, M., K. Tawaratsumida, H. Kariya, A. Kiyohara, Y. Suda, F. Krikae, T. Kirikae, and F. Gotz. 2006. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J. Immunol. 177:3162-3169. [DOI] [PubMed] [Google Scholar]
- 23.Hermans, K., L. A. Devriese, and F. Haesebrouck. 2003. Rabbit staphylococcosis: difficult solutions for serious problems. Vet. Microbiol. 91:57-64. [DOI] [PubMed] [Google Scholar]
- 24.Ibeagha-Awemu, E. M., J. W. Lee, A. E. Ibeagha, D. D. Bannerman, M. J. Paape, and X. Zhao. 2008. Bacterial lipopolysaccharide induces increased expression of Toll-like receptor (TLR) 4 and downstream TLR signaling molecules in bovine mammary epithelial cells. Vet. Res. 39:11. [DOI] [PubMed] [Google Scholar]
- 25.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 26.Kahlenberg, J. M., and G. R. Dubyak. 2004. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. Cell Physiol. 286:C1100-C1108. [DOI] [PubMed] [Google Scholar]
- 27.Kanneganti, T. D., M. Lamkanfi, Y. G. Kim, G. Chen, J. H. Park, L. Franchi, P. Vandenabeele, and G. Nunez. 2007. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26:433-443. [DOI] [PubMed] [Google Scholar]
- 28.Kanneganti, T. D., M. Lamkanfi, and G. Nunez. 2007. Intracellular NOD-like receptors in host defense and disease. Immunity 27:549-559. [DOI] [PubMed] [Google Scholar]
- 29.Kanneganti, T. D., N. Ozoren, M. Body-Malapel, A. Amer, J. H. Park, L. Franchi, J. Whitfield, W. Barchet, M. Colonna, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, S. Akira, and G. Nunez. 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440:233-236. [DOI] [PubMed] [Google Scholar]
- 30.Kim, H. J., J. S. Yang, S. S. Woo, S. K. Kim, C. H. Yun, K. K. Kim, and S. H. Han. 2007. Lipoteichoic acid and muramyl dipeptide synergistically induce maturation of human dendritic cells and concurrent expression of proinflammatory cytokines. J. Leukoc. Biol. 81:983-989. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi, K. S., M. Chamaillard, Y. Ogura, O. Henegariu, N. Inohara, G. Nunez, and R. A. Flavell. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731-734. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi, Y. 2006. Neutrophil infiltration and chemokines. Crit. Rev. Immunol. 26:307-316. [DOI] [PubMed] [Google Scholar]
- 33.Labeta, M. O., K. Vidal, J. E. Nores, M. Arias, N. Vita, B. P. Morgan, J. C. Guillemot, D. Loyaux, P. Ferrara, D. Schmid, M. Affolter, L. K. Borysiewicz, A. Donnet-Hughes, and E. J. Schiffrin. 2000. Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. J. Exp. Med. 191:1807-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahouassa, H., E. Moussay, P. Rainard, and C. Riollet. 2007. Differential cytokine and chemokine responses of bovine mammary epithelial cells to Staphylococcus aureus and Escherichia coli. Cytokine 38:12-21. [DOI] [PubMed] [Google Scholar]
- 35.Lee, J., I. Tattoli, K. A. Wojtal, S. R. Vavricka, D. J. Philpott, and S. E. Girardin. 2009. pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J. Biol. Chem. 284:23818-23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemjabbar, H., and C. Basbaum. 2002. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8:41-46. [DOI] [PubMed] [Google Scholar]
- 37.Lubick, K., and M. A. Jutila. 2006. LTA recognition by bovine gammadelta T cells involves CD36. J. Leukoc. Biol. 79:1268-1270. [DOI] [PubMed] [Google Scholar]
- 38.Maeda, S., L. C. Hsu, H. Liu, L. A. Bankston, M. Iimura, M. F. Kagnoff, L. Eckmann, and M. Karin. 2005. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science 307:734-738. [DOI] [PubMed] [Google Scholar]
- 39.Mariathasan, S., D. S. Weiss, K. Newton, J. McBride, K. O'Rourke, M. Roose-Girma, W. P. Lee, Y. Weinrauch, D. M. Monack, and V. M. Dixit. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228-232. [DOI] [PubMed] [Google Scholar]
- 40.Martinon, F., L. Agostini, E. Meylan, and J. Tschopp. 2004. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 14:1929-1934. [DOI] [PubMed] [Google Scholar]
- 41.Moazzez, A., R. L. Kelso, S. Towfigh, H. Sohn, T. V. Berne, and R. J. Mason. 2007. Breast abscess bacteriologic features in the era of community-acquired methicillin-resistant Staphylococcus aureus epidemics. Arch. Surg. 142:881-884. [DOI] [PubMed] [Google Scholar]
- 42.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Notebaert, S., L. Duchateau, and E. Meyer. 2005. NF-kappaB inhibition accelerates apoptosis of bovine neutrophils. Vet. Res. 36:229-240. [DOI] [PubMed] [Google Scholar]
- 44.Okada, H., T. Ito, H. Ohtsuka, R. Kirisawa, H. Iwai, K. Yamashita, T. Yoshino, and T. J. Rosol. 1997. Detection of interleukin-1 and interleukin-6 on cryopreserved bovine mammary epithelial cells in vitro. J. Vet. Med. Sci. 59:503-507. [DOI] [PubMed] [Google Scholar]
- 45.Perregaux, D. G., P. McNiff, R. Laliberte, M. Conklyn, and C. A. Gabel. 2000. ATP acts as an agonist to promote stimulus-induced secretion of IL-1 beta and IL-18 in human blood. J. Immunol. 165:4615-4623. [DOI] [PubMed] [Google Scholar]
- 46.Persson-Waller, K. 1997. Modulation of endotoxin-induced inflammation in the bovine teat using antagonists/inhibitors to leukotrienes, platelet activating factor and interleukin 1 beta. Vet. Immunol. Immunopathol. 57:239-251. [DOI] [PubMed] [Google Scholar]
- 47.Petzl, W., H. Zerbe, J. Gunther, W. Yang, H. M. Seyfert, G. Nurnberg, and H. J. Schuberth. 2008. Escherichia coli, but not Staphylococcus aureus triggers an early increased expression of factors contributing to the innate immune defense in the udder of the cow. Vet. Res. 39:18. [DOI] [PubMed] [Google Scholar]
- 48.Rainard, P. 2010. Consequences of interference of milk with chemoattractants for enzyme-linked immunosorbent assay quantifications. Clin. Vaccine Immunol. 17:848-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rainard, P., A. Fromageau, P. Cunha, and F. B. Gilbert. 2008. Staphylococcus aureus lipoteichoic acid triggers inflammation in the lactating bovine mammary gland. Vet. Res. 39:52. [DOI] [PubMed] [Google Scholar]
- 50.Rainard, P., and M. J. Paape. 1997. Sensitization of the bovine mammary gland to Escherichia coli endotoxin. Vet. Res. 28:231-238. [PubMed] [Google Scholar]
- 51.Rainard, P., C. Riollet, P. Berthon, P. Cunha, A. Fromageau, C. Rossignol, and F. B. Gilbert. 2008. The chemokine CXCL3 is responsible for the constitutive chemotactic activity of bovine milk for neutrophils. Mol. Immunol. 45:4020-4027. [DOI] [PubMed] [Google Scholar]
- 52.Rainard, P., P. Sarradin, M. J. Paape, and B. Poutrel. 1998. Quantification of C5a/C5a(desArg) in bovine plasma, serum and milk. Vet. Res. 29:73-88. [PubMed] [Google Scholar]
- 53.Riekerink, R. G. O., H. W. Barkema, D. F. Kelton, and D. T. Scholl. 2008. Incidence rate of clinical mastitis on Canadian dairy farms. J. Dairy Sci. 91:1366-1377. [DOI] [PubMed] [Google Scholar]
- 54.Riollet, C., P. Rainard, and B. Poutrel. 2000. Differential induction of complement fragment C5a and inflammatory cytokines during intramammary infections with Escherichia coli and Staphylococcus aureus. Clin. Diagn. Lab. Immunol. 7:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schindler, R., B. D. Clark, and C. A. Dinarello. 1990. Dissociation between interleukin-1 beta mRNA and protein synthesis in human peripheral blood mononuclear cells. J. Biol. Chem. 265:10232-10237. [PubMed] [Google Scholar]
- 56.Schröder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 57.Seo, H. S., J. H. Kim, and M. H. Nahm. 2006. Platelet-activating factor-acetylhydrolase can monodeacylate and inactivate lipoteichoic acid. Clin. Vaccine Immunol. 13:452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seo, H. S., and M. H. Nahm. 2009. Lipoprotein lipase and hydrofluoric acid deactivate both bacterial lipoproteins and lipoteichoic acids, but platelet-activating factor-acetylhydrolase degrades only lipoteichoic acids. Clin. Vaccine Immunol. 16:1187-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimada, T., B. G. Park, A. J. Wolf, C. Brikos, H. S. Goodridge, C. A. Becker, C. N. Reyes, E. A. Miao, A. Aderem, F. Gotz, G. Y. Liu, and D. M. Underhill. 2010. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe 7:38-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegel, S., and N. J. Castellan. 1988. Non-parametric statistics for the behavioral sciences. McGraw-Hill, New York, NY.
- 61.Spitsberg, V. L., E. Matitashvili, and R. C. Gorewit. 1995. Association and coexpression of fatty-acid-binding protein and glycoprotein CD36 in the bovine mammary gland. Eur. J. Biochem. 230:872-878. [DOI] [PubMed] [Google Scholar]
- 62.Strandberg, Y., C. Gray, T. Vuocolo, L. Donaldson, M. Broadway, and R. Tellam. 2005. Lipopolysaccharide and lipoteichoic acid induce different innate immune responses in bovine mammary epithelial cells. Cytokine 31:72-86. [DOI] [PubMed] [Google Scholar]
- 63.Strober, W., P. J. Murray, A. Kitani, and T. Watanabe. 2006. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 6:9-20. [DOI] [PubMed] [Google Scholar]
- 64.Thiemermann, C. 2002. Interactions between lipoteichoic acid and peptidoglycan from Staphylococcus aureus: a structural and functional analysis. Microbes Infect. 4:927-935. [DOI] [PubMed] [Google Scholar]
- 65.Uehara, A., Y. Fujimoto, K. Fukase, and H. Takada. 2007. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol. Immunol. 44:3100-3111. [DOI] [PubMed] [Google Scholar]
- 66.Van Amersfoort, E. S., T. J. Van Berkel, and J. Kuiper. 2003. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 16:379-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Beelen, A. J., Z. Zelinkova, E. W. Taanman-Kueter, F. J. Muller, D. W. Hommes, S. A. Zaat, M. L. Kapsenberg, and E. C. de Jong. 2007. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27:660-669. [DOI] [PubMed] [Google Scholar]
- 68.Vavricka, S. R., M. W. Musch, J. E. Chang, Y. Nakagawa, K. Phanvijhitsiri, T. S. Waypa, D. Merlin, O. Schneewind, and E. B. Chang. 2004. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology 127:1401-1409. [DOI] [PubMed] [Google Scholar]
- 69.Volz, T., M. Nega, J. Buschmann, S. Kaesler, E. Guenova, A. Peschel, M. Rocken, F. Gotz, and T. Biedermann. 3 June 2010, posting date. Natural Staphylococcus aureus-derived peptidoglycan fragments activate NOD2 and act as potent costimulators of the innate immune system exclusively in the presence of TLR signals. FASEB J. [Epub ahead of print.] doi: 10.1096/fj.09-151001. [DOI] [PubMed]
- 70.Wellnitz, O., and D. E. Kerr. 2004. Cryopreserved bovine mammary cells to model epithelial response to infection. Vet. Immunol. Immunopathol. 101:191-202. [DOI] [PubMed] [Google Scholar]
- 71.Wolfert, M. A., T. F. Murray, G. J. Boons, and J. N. Moore. 2002. The origin of the synergistic effect of muramyl dipeptide with endotoxin and peptidoglycan. J. Biol. Chem. 277:39179-39186. [DOI] [PubMed] [Google Scholar]
- 72.Yang, W., H. Zerbe, W. Petzl, R. M. Brunner, J. Gunther, C. Draing, S. von Aulock, H. J. Schuberth, and H. M. Seyfert. 2008. Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E. coli, but S. aureus fails to both activate NF-kappaB in mammary epithelial cells and to quickly induce TNFalpha and interleukin-8 (CXCL8) expression in the udder. Mol. Immunol. 45:1385-1397. [DOI] [PubMed] [Google Scholar]