Abstract
Giardiasis and cryptosporidiosis are common enteric parasitic diseases that have similar routes of transmission. In this work, we have identified epitopes within the Giardia variant-specific surface protein (VSP) sequences that are recognized by IgG antibodies from 13 of 14 (93%) sera from patients with stool-confirmed giardiasis. The conserved epitopes are shared among VSPs from both of the assemblages that commonly infect humans, and they are likely to be structural, as both sodium dodecyl sulfate treatment and dithiothreitol reduction decrease antibody recognition. In a multiplex bead assay (MBA), we used three VSP fragments from an assemblage A Giardia strain, three VSP fragments from assemblage B strains, and the α-1 giardin structural antigen to detect IgG antibodies to Giardia and used the recombinant 17- and 27-kDa antigens to simultaneously detect IgG antibodies to Cryptosporidium. The MBA differentiated between sera from Giardia and Cryptosporidium outbreaks and also identified a giardiasis outbreak that may have included cryptosporidiosis cases. Approximately 40% of cryptosporidiosis outbreak samples had high MBA responses for both the 27- and 17-kDa antigens, while <10% of nonoutbreak and giardiasis outbreak samples had high responses. At least 60% of giardiasis outbreak samples were positive for antibodies to multiple Giardia antigens, while ≤12% of nonoutbreak samples and samples from U.S. and British Columbia cryptosporidiosis outbreaks met our definition for Giardia seropositivity. A MBA using multiple parasite antigens may prove useful in the epidemiologic analysis of future waterborne or food-borne outbreaks of diarrheal disease.
Giardia intestinalis (syn. Giardia lamblia and Giardia duodenalis) and Cryptosporidium spp. (e.g., Cryptosporidium parvum, Cryptosporidium hominis, Cryptosporidium felis, and Cryptosporidium meleagridis) are enteric protozoan parasites with zoonotic potential that are commonly associated with diarrheal disease in humans (reviewed in reference 26). In the developing world where potential sources of fecal contamination are widespread, repeated and sometimes chronic infections occur at an early age (reviewed in reference 81). In the developed world, outbreaks are often associated with episodic events that result in the contamination of food, water, or recreational water with infectious organisms (15, 33; reviewed in reference 82). Because Giardia cysts and Cryptosporidium oocysts are resistant to commonly used disinfectants, such as chlorine, and have relatively low infectious doses (7, 25, 65), municipal water treatment failures in communities that draw from challenged raw water sources can result in widespread outbreaks of disease. The largest known community-wide, waterborne outbreak of cryptosporidiosis occurred in Milwaukee, WI, in 1993. Approximately 400,000 people (26% of residents) were symptomatic during the outbreak (42). A retrospective analysis of serum samples from Milwaukee children suggested that 37 to 70% of residents may actually have been infected (43). In addition to recognized outbreaks, low levels of community-acquired giardiasis and cryptosporidiosis have long been observed in the United States and Canada. Laboratory-based surveillance estimates (1999 to 2002) of the incidence of Giardia and Cryptosporidium infections in Calgary, Canada, were 19.6 and 6.0, respectively, per 100,000 residents per year (38). In the same general time frame, infection rates in the United States based upon case reports varied between 6.9 and 8.5 infections per 100,000 per year for Giardia and between 1.0 and 1.3 infections per 100,000 per year for Cryptosporidium (23, 24).
Giardia and Cryptosporidium infection estimates based on case surveillance or the detection of organisms in stool are likely to significantly underestimate the actual values in a population, given that asymptomatic infection is documented, shedding of organisms by infected individuals can be intermittent and low level, and detection by microscopy can be challenging, especially in asymptomatic individuals (6, 14, 59, 64, 92). Several groups have shown that serologic IgG antibodies against parasite surface antigens can serve as a useful indicator of the levels of infection in a community (reviewed in references 12 and 17). Assays to detect antibodies to Cryptosporidium have focused on the 17- and 27-kDa antigens (reviewed in reference 79), two low-molecular-weight proteins that are associated with a detergent-extractable portion of the parasite membrane by way of posttranslational glycolipid or lipid modifications (71, 74, 76). Because C. parvum protein-based assays can be used to detect antibody responses among patients infected with non-C. parvum species, the immunodominant 17- and 27-kDa epitopes must be conserved between species (20, 73, 75, 86, 87). In previous work, we demonstrated that recombinant 17- and 27-kDa proteins, when used in the enzyme-linked immunosorbent assay (ELISA) format, detected IgG antibodies with good sensitivity and specificity relative to the “gold standard” Western blot assay in both nonoutbreak and outbreak populations (50, 70, 74).
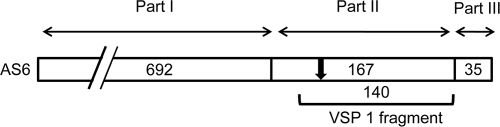
In contrast to the Cryptosporidium assays just described, most of the assays that detect antibodies to Giardia have used crude trophozoite or cyst antigens, and a sensitive and specific recombinant protein-based serologic assay has not yet been reported (12, 17). The immunodominant Giardia antigen is the variant-specific surface protein (VSP), a cysteine-rich (11 to 12% Cys) protein that covers the entire surface of the parasite (reviewed in reference 2). Although a trophozoite usually expresses only one VSP on its surface at a time, antigenic switching (perhaps using an RNA interference mechanism) occurs at a rate of one switch for every 6.5 to 13 generations (62, 77). Because of antigenic switching, the host immune system is exposed to many different VSP sequences during the course of an infection. The Giardia genome encodes a family of approximately 200 different VSPs, and the repertoire of genes found in the two main genotypes that infect humans (assemblages A and B) have been shown to be divergent (3, 18, 46, 47, 58, 61). Structurally, each VSP has a highly conserved, carboxy-terminal membrane anchor segment of 34 to 37 amino acids (part III), a moderately conserved segment of about 170 amino acids adjacent to the anchor (part II), and an amino-terminal region that varies greatly in both size and sequence (part I) (53). A diagram showing the positions and sizes of the three VSP regions for AS6 (CRP170) is shown in Fig. 1 (4, 47). Because of differences in the hypervariable amino-terminal region, VSPs can range in size from approximately 20 kDa to 200 kDa. In addition to the highly conserved transmembrane region, VSPs contain CXXC, GGCY (indicated by an arrow in Fig. 1), and Zn finger motifs as well as a CRGKY cytosolic tail that can be posttranslationally acylated on the cysteine with palmitate (3, 22, 69, 78, and reviewed in reference 55). VSP variation during infection and the antigenic divergence between the repertoires of the two Giardia assemblages may limit the effectiveness of the immune responses in animals and humans and may contribute to the frequent and often chronic nature of giardiasis (reviewed in reference 54).
FIG. 1.
Map showing the hypervariable (part I), semiconserved (part II), and highly conserved anchor (part III) regions of VSP AS6 (CRP170) (4, 47). The amino acid length of each region is indicated based on the conventions of Bienz et al. (8) and Muller et al. (53). The location of the 140-amino-acid VSP1 fragment targeted for amplification in this work is indicated beneath the map by a bracket. The location of the GGCY motif, 47 amino acids from the amino terminus of part II, is indicated by an arrow.
Epitope mapping of the antibody responses that result from infection in a mouse model has shown that the VSP contains two antigenic regions (52, 53). The hypervariable amino terminus (part I) of VSP H7 stimulated a low-level antibody response early in infection, while the semiconserved region (part II) stimulated a more-pronounced antibody response later in infection. Some of the new VSPs that resulted from antigenic switching cross-reacted with antibodies to the VSP H7 semiconserved region, but none of new hypervariable regions were recognized by anti-H7 sera. Muller et al. (52, 53) were unable to demonstrate cross-reactivity between VSP H7 immune sera from the GS strain infection (assemblage B) and CRP170, a VSP from the WB (assemblage A) strain of Giardia (85). Fine-level mapping of VSP H7 by Bienz et al. (9) identified a 130-amino-acid sequence within the semiconserved part II region that was recognized by mouse immune serum. These results suggested that a serologic antibody assay using multiple VSP semiconserved regions from both assemblages might be possible.
In this work, we used the multiplex bead assay (MBA) format to develop an assay that simultaneously detects specific human IgG antibodies to Cryptosporidium and Giardia surface antigens. Six VSP fragments, three from assemblage A and three from assemblage B, and the α-1 giardin (16, 67, 93) were used to detect antibodies to Giardia. In addition, the recombinant 17- and 27-kDa antigens were used to detect antibodies to Cryptosporidium. The assay provides new information about the serological response to two common waterborne protozoan parasites and offers a potential new tool for further study of the risks and prevalences of these infections at the community level.
MATERIALS AND METHODS
Growth of Giardia trophozoites and analysis of total proteins by Western blotting.
Giardia isolates WB (ATCC 30957; Afghanistan patient), EGY (Egyptian patient) (1), TH-1 (kindly provided by M. Wittner, Albert Einstein College of Medicine, Bronx, NY; New York patient), Portland-1 (ATCC 30888; Oregon patient) (44), GS (kindly provided by T. Nash, National Institutes of Health, Bethesda, MD) (Alaska patient) (63), VANC/90/UBC/44 (British Columbia [BC] patient) (29), KSU (Kansas patient) (49), BR-7 (CDC isolate, 1993; Brazil patient), and GM-1 (CDC isolate, 1988; Ethiopian patient) were grown axenically in modified TYI-S-33 medium at 37°C (34). Isolates WB, BR-7, TH-1, and Portland-1 are from assemblage A, while GS and GM-1 are from assemblage B (37; reviewed in reference 89; G. S. Visvesvara, unpublished observations). Trophozoites were collected by centrifugation and washed in buffer containing 0.85% NaCl-10 mM Na2HPO4 at pH 7.2 (PBS). Cell pellets were dissolved in 50 mM Tris (pH 7.5) with 1% sodium dodecyl sulfate (SDS), heated at 95°C for 5 min, and centrifuged to remove insoluble material. Protein concentrations were determined using the BCA microassay (Pierce, Rockford, IL). Proteins (5 μg/lane) were diluted in loading buffer and resolved on 8 to 16% SDS-polyacrylamide precast gels (Lonza, Rockland, ME) using the discontinuous electrophoresis buffer system described by Laemmli (35). Resolved proteins were then electrotransferred onto a polyvinylidene difluoride (PVDF) membrane (Immobilon P; Millipore Corp, Bedford, MA) and exposed to human or mouse sera at dilutions of 1:100 in 0.3% Tween 20-PBS. IgG Western blot analyses were conducted using the biotinylated mouse monoclonal anti-human IgG (clone HP6017; Zymed, South San Francisco, CA) and the streptavidin-alkaline phosphatase system previously described (74). Mouse IgG antibodies were detected using a biotinylated monoclonal rat anti-mouse IgG (Zymed).
Isolation of Giardia DNA and cloning of VSPs.
Genomic DNA was isolated from WB, GM-1, and GS trophozoites by lysis in buffer containing 10 mM Tris (pH 8.0), 10 mM EDTA, 0.5% SDS, and 1 mg/ml protease (Streptomyces griseus), followed by phenol-chloroform extraction, RNase A digestion, proteinase K digestion (Fisher, Fair Lawn, NJ), and ethanol precipitation (80). WB and GS isolates were chosen as representatives of assemblages A and B, respectively. Isolate GM-1 was included as a potential outlier from assemblage B: it is morphologically dissimilar from GS and was originally thought to be Giardia muris (hence the name GM-1) (G. S. Visvesvara, unpublished observation).
The deoxyoligonucleotide pairs listed in Table 1 were used to PCR amplify the target VSP coding sequence fragments from WB, GS, and GM-1 genomic DNA for directional cloning into the BamHI and EcoRI restriction sites of double-digested, shrimp alkaline phosphatase-treated (Roche Chemical, Indianapolis, IA) pGEX4T-2 vector (GE Healthcare, Piscataway, NJ). AmpliTaq gold DNA polymerase (Perkin-Elmer Cetus, Foster City, CA) was used as directed by the manufacturer with the PCR amplification protocol of Priest et al. (74). PCR products were isolated using the StrataPrep PCR purification kit (Stratagene, LaJolla, CA), digested with both restriction enzymes (New England Biolabs, Ipswich, MA), and ligated into the plasmid overnight at 16°C (5 U T4 DNA ligase HC; Roche). Ligated plasmids were transformed into E. coli HB101 cells (Promega Corp., Madison, WI). Plasmids from the resulting clones were purified (StrataPrep miniplasmid kit; Stratagene), sequenced using forward and reverse primers, and subcloned into E. coli BL21 Gold cells (Stratagene) for glutathione-S-transferase (GST) fusion protein expression.
TABLE 1.
Giardia strains and PCR primers used for amplification of VSP gene fragments
| VSP fragment | Strain | Target | Region (bp) | GenBank accession no. (reference) | Deoxyoligonucleotide primer sequencea |
|---|---|---|---|---|---|
| VSP1 | WB | AS6 | 978-1397 | M83933.1 (4) | for, 5′-CGCGGATCCAGCGTATGCACGGCGGCAGATG |
| rev, 5′-GCGGAATTCTTACTTGTTGACGCTTCCGCCGGTG | |||||
| VSP2 | WB | AS7 | 907-1326 | XM_001709256 (47) | for, 5′-CGCGGATCCTCATTCTGCACGAACGCAGCAG |
| rev, 5′-GCGGAATTCTTACTTGTTTGTGCTACCGCTATC | |||||
| VSP3 | WB | AS8 | 130-570 | XM_001707314 (47) | for, 5′-CGCGGATCCTGGGCAGCAACGACATGCACAG |
| rev, 5′-GCGGAATTCTTACCTGTTCGTGCTGTCTCCTCCAG | |||||
| VSP4 | GS | H7 | 1126-1557 | M80480.1 (58) | for, 5′-CGCGGATCCCCCGGGTCGACGCCGGATAAAAC |
| rev, 5′-GCGGAATTCTTAATCGCCGCCGGTGCTATTGTCGC | |||||
| VSP5 | GS | 42e | 19-432 | AF354538.1 (8) | for, 5′-CGCGGATCCGAGTGCAAAACACCAGCTAATAAG |
| rev, 5′-GCGGAATTCTTACTTGTTGACTGAGGGGTCCGTGG | |||||
| VSP6 | GM-1 | 42e | same as for VSP5 | Same as for VSP5 |
Underlining indicates linker sequences with restriction endonuclease sites. Reverse primers include an in-frame stop codon.
Purification of recombinant parasite antigens.
Bacterial cultures were grown, induced, and lysed as previously described (50, 74). Recombinant GST-linked VSP fusion proteins (rVSP/GST) were initially purified on a glutathione Sepharose 4B affinity column as directed by the manufacturer (GE Healthcare) using PBS buffer with 1 mM phenylmethylsulfonyl fluoride (PMSF) and 0.5 mM EDTA. Glutathione-eluted proteins were dialyzed overnight at 4°C against 300 volumes of PBS with 2 mM EDTA (Spectra/Por3; 3,500-Da cutoff; Spectrum Laboratories, Rancho Dominguez, CA) and then desalted by passage over a 50-ml G-25 fine column (GE Healthcare) equilibrated with 25 mM sodium acetate at pH 5.2. Proteins were bound on a Mono S HR 5/5 strong cation-exchange column (GE Healthcare) and eluted with a linear gradient from 0 to 1.0 M NaCl in 25 mM sodium acetate at pH 5.2. Protein-containing fractions were combined and dialyzed against 500 volumes of PBS overnight at 4°C (Spectra/Por3). Final samples were concentrated using Centricon-10 centrifugal filter devices as necessary (Millipore Corporation, Bedford, MA), and protein concentrations were determined using the BCA microassay (Pierce).
The recombinant VSP1 protein fragment without the GST fusion partner (rVSP1) was generated by thrombin cleavage while bound to a glutathione Sepharose 4B affinity column as directed by the manufacturer (GE Healthcare). Cleaved rVSP1 was eluted with PBS and desalted by passage over a 50 ml G-25 fine column (GE Healthcare) equilibrated with 25 mM Tris at pH 8.0. Residual contaminants were removed by passage over a Mono Q HR 5/5 strong anion-exchange column equilibrated with 25 mM Tris buffer at pH 8.0 (GE Healthcare). Highly purified rVSP1 was collected in the unbound Mono Q fraction.
Recombinant 6×His-tagged G. intestinalis α-1 giardin, GST-linked C. parvum 27-kDa antigen (rCp27/GST), GST-linked C. parvum 17-kDa antigen (rCp17/GST), and Schistosoma japonicum GST proteins were expressed and purified as previously described (16, 50, 74).
Polyclonal and monoclonal antibodies.
Mouse ascites fluid containing monoclonal antibodies 6E7 (recognizes the CRP170 VSP from isolate WB) and G10/4 (recognizes the H7 VSP from isolate GS) were kindly provided by Theodore Nash (NIH, Bethesda, Md.) (5, 57). A polyclonal antiserum to G. intestinalis rVSP1 was made using standard protocols (21) by immunizing BALB/c mice four times with 5 μg of purified, recombinant protein in TiterMax adjuvant (1:1 [vol/vol]; TiterMax USA, Inc., Norcross, GA). Anti-rVSP1 polyclonal antibodies were also purified from a serum sample from a giardiasis patient using Western blotted rVSP1 antigen and the MgCl2 elution method of Tsang and Wilkins (90). Eluted antibodies were desalted using G-25 M size exclusion columns (PD-10; GE Healthcare) that had been preequilibrated with buffer containing PBS with 0.3% Tween 20.
Measurement of antibody responses by ELISA.
Test sera were diluted 1:100 with PBS buffer containing 0.05% Tween 20, and assays were conducted in duplicate. Immulon 2HB flat-bottom microtiter plates (Thermo Electron Corp., Milford, MA) were coated overnight at 4°C with 50 μl of purified rVSP/GST or GST protein per well at a concentration of 2 μg/ml in 0.1 M sodium bicarbonate buffer (pH 9.6). Proteins were also incubated for 5 min at 1 mg/ml in PBS buffer with 0.5% or 1% SDS at the indicated temperature and then diluted to final concentrations of 2 μg/ml with 0.1 M sodium bicarbonate buffer (pH 9.6) for binding to the ELISA plate. For studies on the impact of reducing agents, ELISA wells coated with antigens were incubated for 1 h at 37°C with the indicated dithiothreitol (DTT) concentration in blocking buffer (0.3% Tween 20 in PBS, 100 μl/well) (see Tables 2 and 3). Plates were washed four times with 0.05% Tween 20 in PBS and incubated with 50 μl of the diluted positive serum per well for 2 h at room temperature. Bound antibodies were detected using the biotinylated secondary antibody (monoclonal mouse anti-human IgG clone HP6017 or rat anti-mouse IgG; Zymed, South San Francisco, CA) and alkaline phosphatase labeled-streptavidin (Invitrogen, Carlsbad, CA) system previously described (74). Absorbances were read at 405 nm using a Molecular Dynamics UVmax kinetic microplate reader (Sunnyvale, CA). Absorbances were expressed as a percentage of the value for untreated rVSP1/GST.
TABLE 2.
Effects of denaturing and reducing conditions on the serologic IgG antibody responses to Giardia VSPsb
| Pretreatment (Ta) | Blocking | Response to treatmentc |
||||||
|---|---|---|---|---|---|---|---|---|
| VSP1 | VSP2 | VSP3 | VSP4 | VSP5 | VSP6 | GST | ||
| None | Normal | 100c | 88 | 101 | 77 | 101 | 83 | 1 |
| 1% SDS (RTd) | Normal | 99 | 93 | 100 | 74 | 104 | 88 | 2 |
| 1% SDS (37) | Normal | 96 | 77 | 99 | 24 | 105 | 79 | 2 |
| 1% SDS (65) | Normal | 62 | 35 | 88 | 13 | 99 | 50 | 2 |
| 1% SDS (95) | Normal | 28 | 47 | 79 | 19 | 79 | 34 | 1 |
| None | 10 mM DTT | 1 | 4 | 2 | 1 | 73 | 6 | 2 |
| None | 50 mM DTT | 2 | 4 | 2 | 1 | 21 | 4 | 2 |
T, pretreatment temperature (°C).
All VSP protein fragments were assayed as GST fusion proteins.
Responses (OD405) are expressed as a percentage of the value obtained for rVSP1/GST under normal assay conditions (set at 100%).
RT, room temperature.
TABLE 3.
Effects of denaturing and reducing conditions on the mouse IgG antibody response to rVSP1b
| Pretreatment (Ta) | Blocking | Response to treatment |
||||||
|---|---|---|---|---|---|---|---|---|
| VSP1 | VSP2 | VSP3 | VSP4 | VSP5 | VSP6 | GST | ||
| None | Normal | 100c | 2 | 42 | 20 | 0 | 1 | 1 |
| 0.5% SDS (95) | Normal | 63 | 1 | 37 | 8 | 0 | 2 | 0 |
| None | 50 mM DTT | 77 | 0 | 5 | 1 | 0 | 0 | 1 |
T, pretreatment temperature (°C).
All VSP protein fragments were assayed as GST fusion proteins.
Responses (OD405) are expressed as a percentage of the value obtained for rVSP1/GST under normal assay conditions (set at 100%).
To characterize the antibodies that recognize rVSP1, 38 test sera from cyst-confirmed giardiasis patients were diluted 1:100 with 0.05% Tween 20 in PBS and assayed in duplicate using the standard ELISA protocol (100 ng antigen/well) (74). Each test serum was assayed for IgG, IgA, and IgM reactivity on the same ELISA plate by incubation with a mouse secondary antibody against human IgG (described above), human IgA (clone GA112; Zymed), or human IgM (clone HP6083; Zymed) at 1:1,000 dilutions. Each plate included both negative- and positive-control sera, and plates were allowed to develop until the positive-control serum absorbance value (405 nm) reached 2.0 for the IgG wells.
Antigen coupling to beads.
Antigen coupling to SeroMap beads (Luminex Corporation, Austin, TX) has been previously described (50). Briefly, the carboxyl groups on each bead were chemically modified to an ester using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (Pierce) and then reacted with primary amine groups on the antigens to form covalent amide bonds. For most recombinant antigens, 120 μg of protein was used for coupling to 12.5 × 106 beads. Coupling of the rCp17/GST (150 μg) had to be empirically adjusted in order to increase the positive-control serum signal. We lowered the amount of rCP27/GST protein in the coupling to 95 μg with no loss of signal. A blank coupling with glycine (500 μl of a 1.0 M solution in PBS per reaction) and a control coupling with GST (120 μg) were also performed. To assess the efficiency of coupling, beads were assayed (procedure described below) using an anti-GST mouse monoclonal antibody (Zymed) and a biotinylated rat anti-mouse IgG monoclonal antibody (Zymed). All six of the VSPs, both Cryptosporidium antigens, and the GST-only control were coupled to the beads with similar efficiencies: median fluorescence intensity minus background (MFI-BG) values ranged from 25,020 to 27,224 (average, 25,978). After coupling, the beads were quantified by hemocytometer and stored at 4°C in PBS containing 1.0% bovine serum albumin (BSA), 0.05% Tween 20, and 0.02% sodium azide. For each milliliter of bead suspension, protease inhibitors were included at 200 μg Pefabloc (Roche Diagnostics, Indianapolis, IL), 200 μg EDTA, and 1 μg each of leupeptin and pepstatin A.
Multiplex bead assay.
A 1:200 dilution of serum in PBS containing 0.5% BSA, 0.05% Tween 20, 0.02% sodium azide, 0.5% polyvinyl alcohol, and 0.8% polyvinylpyrrolidone (buffer 1) was incubated for 1 h at 37°C and stored overnight at 4°C. Polyvinyl alcohol and polyvinylpyrrolidone have been shown to reduce background without loss of sensitivity (91). After centrifugation at 16,000 × g for 5 min, 25 μl of the clarified serum dilution was added to 25 μl of buffer 1 containing 2,500 beads from each spectrally classified bead in each well in a 96-well filtered-bottom plate (Millipore, Bedford, MA) to yield a 1:400 final serum dilution. All sera were assayed in duplicate. The beads were suspended and allowed to gently shake for 45 min at room temperature. Each well was washed three times with 100 μl PBS containing 0.05% Tween 20 using a vacuum device. For total IgG detection, a biotinylated mouse monoclonal antibody to human IgG (clone HP6017; Zymed) was diluted 1:500 in PBS containing 0.5% BSA, 0.05% Tween 20, and 0.02% sodium azide (buffer 2), and 50 μl was added to each well. The beads were suspended, allowed to gently shake for 45 min at room temperature, and then washed three times with PBS with 0.05% Tween 20 as described above. To each well, 50 μl of buffer 2 containing 250 ng R-phycoerythrin-labeled streptavidin (Invitrogen) was added, and the beads were suspended and allowed to gently shake for 30 min at room temperature. After being washed four times with PBS with 0.05% Tween 20 as described above, the beads in each well were suspended in 125 μl PBS, and data were acquired using a Luminex instrument (Luminex Corp., Austin, T) equipped with Bio-Plex Manager 4.1 software (Bio-Rad, Hercules, CA). Gated data were acquired on at least 100 monodispersed beads of each classification, and the median fluorescence intensity for each analyte was calculated. After subtraction of the background blank, the mean value for the duplicate wells was reported as the median fluorescence intensity minus background (MFI-BG). Observed values for human samples ranged from 0 to 27,147. A positive-control serum diluted to yield a mid-range fluorescence intensity was used on each plate along with a negative control. Except in cases of negative responses (values below the cutoffs), a sample assay was repeated if two or more standard deviation values for the duplicate wells were >15% of the respective mean values. Where indicated, assays were also run using biotinylated mouse monoclonal anti-human IgA (clone GA112; Zymed) and anti-human IgM (clone HP6083; Zymed) as the secondary antibodies at 1:500 dilutions.
Human serum specimens.
Blood samples were collected from donors by venipuncture, and the resulting sera were divided into aliquots for storage either at −80°C or at −20°C (British Columbia samples and sporadic U.S. Giardia samples). Sera from five stool-confirmed cases of cryptosporidiosis from a 1997 food-borne outbreak in Washington State were collected approximately 8 weeks after exposure (13, 50). Sera from patients with stool-confirmed cryptosporidiosis were also available from 10 U.S. Coast Guard cutter crew members who were infected during the 1993 Milwaukee waterborne outbreak (51) and from 14 Texas residents who were infected during a waterborne outbreak in 1998 (39). Written, informed consent was obtained from these U.S. study participants, and the protocol was approved by the Centers for Disease Control and Prevention Institutional Review Board. A panel of 35 anonymous, banked sera that were known to be negative for antibodies to the immunodominant Cryptosporidium antigens by large-format Western blotting was used to help define the MBA cutoffs for the rCp17/GST and rCP27/GST antigens. Anonymous, banked sera were also available from 41 U.S. citizens who had no history of foreign travel (74) and from 14 stool-confirmed giardiasis cases that were studied at the CDC between 1974 and 1994. For one giardiasis case, multiple samples spanning a 10-month period following diagnosis were available.
All of the Canadian samples were collected from exposed individuals during the course of outbreak investigations, and all personal identifiers were removed prior to use in this study. Sera were available from 67 oocyst-positive cryptosporidiosis patients from waterborne outbreaks in British Columbia, Canada, in 1996 (30, 66, 70). We were also able to locate residual serum specimens from three outbreaks of giardiasis that occurred in Canada between 15 and 25 years ago for use in our study; 21 of 53 samples from the 1985 Canadian student outbreak (32), 14 of 15 samples from the 1995 Revelstoke, British Columbia, waterborne outbreak (11), and 56 of 72 samples from the 1990 Creston, British Columbia, waterborne outbreak (29, 31) were available for testing. Although no clinical data were linked to these residual specimens, the tested sets must contain some samples from cyst-confirmed giardiasis patients because 81% of the original Canadian student outbreak samples (43/53), 87% of the samples from the 1995 Revelstoke outbreak (13/15), and 53% of the 1990 Creston outbreak samples (38/72) were donated by laboratory-confirmed (cyst-positive) giardiasis patients. The giardiasis outbreak sets may also include sera from exposed donors who were symptomatic but not stool confirmed and sera from exposed donors who did not meet the symptom-based case definition and who had no laboratory evidence of infection. The exact timing of serum collection relative to parasite exposure was unknown for the waterborne outbreaks.
Data and sequence analysis.
Statistical analyses were conducted using the Mann-Whitney rank sum test (SigmaStat for Windows version 2.03.0; SPSS, Inc., Chicago, IL). Statistical significance was set at an alpha level of 0.05. Protein sequences were aligned using ClustalW version 2 (36) and manually optimized.
Reagents.
Unless otherwise stated, reagents were purchased from Sigma Chemicals (St. Louis, MO).
Nucleotide sequence accession numbers.
DNA sequences for VSP2, VSP3, VSP5, and VSP6 have been deposited in GenBank under accession numbers HM036221, HM036222, HM036223, and HM036224, respectively.
RESULTS
Cloning of the VSP antigens.
VSP fragments from the semiconserved part II region (Fig. 1) (53) were amplified from assemblage A and assemblage B genomic DNAs based upon the epitope mapping work of Bienz et al. (9). A 5′ target sequence slightly upstream of the previously identified epitope was chosen in order to include the full CXXC motif at amino acids 387 to 390 of the VSP H7 sequence and to use a slightly more divergent region for the PCR primer sequence. We selected the CRP170 (AS6), AS7, and AS8 VSP sequences from the WB strain (4, 47) and the H7 and 42e sequences from the GS strain (8, 58) (Table 1). We attempted to amplify the sequences of both the H7 and 42e VSP fragments from strain GM-1 (assemblage B) but were successful only with the latter sequence. The DNA sequence of VSP1 was identical to the AS6 sequence (GenBank accession no. AY142135) but differed from the CRP170 sequence at four positions (one amino acid change). VSP2 differed from the reported AS7 sequence at 10 positions, resulting in one amino acid substitution, while VSP3 differed from AS8 at 16 positions, resulting in six amino acid substitutions. The sequence of VSP4 was identical to the previously reported H7 sequence. VSP5 differed from the reported 42e sequence at 37 positions, resulting in 17 amino acid substitutions. The VSP6 DNA fragment from the GM-1 Giardia strain diverged from the VSP 42e sequence at the 3′ end and was most similar to VSP 42b (GenBank AF354535) in that region.
The deduced amino acid sequences of the six VSP fragments used in our study are shown in Fig. 2. All of the sequences contain seven CXXC motifs, four of the fragments have the complete GGCY motif, and, like the full-length VSPs, all have a high Cys content (11.8 to 13.3%). Predicted isoelectric points ranged from a low of 5.2 (VSP4) to a high of 7.5 (VSP1). A total of 36 residues (25%) were invariant among the cloned VSP fragments, with another nine positions (6%) having conservative amino acid substitutions. VSP5 and VSP6, the two VSP 42e-like sequences that were amplified from different Giardia strains by use of the same deoxyoligonucleotide pair, were the most similar (65% sequence identity), while VSP1 and VSP3 were the most divergent (only 41% sequence identity).
FIG. 2.
Clustal sequence alignment of Giardia VSP fragments. VSP fragment coding sequences were PCR amplified from isolated genomic DNA using the primer pairs given in Table 1. Clones were sequenced, and predicted amino acid sequences were aligned using ClustalW2 with manual optimization. VSP1 is identical to AS6 (GenBank accession no. AY142135.1) of the WB strain, and VSP4 is identical to H7 (GenBank accession no. M80480.1) of the GS strain. VSP2 (HM036221), VSP3 (HM036222), VSP5 (HM036223), and VSP6 (HM036224) are similar to AS7, AS8, 42e, and 42a/42e, respectively. Identical residues in the alignment are indicated by asterisks, and conserved substitutions are indicated by colons. Carats indicate positions occupied by the same residue in 5 of 6 proteins.
Characterization of a shared VSP epitope.
The six VSP fragment proteins shown in Fig. 2 were expressed with a GST tag, purified, and used in an ELISA format to examine the stability of the shared epitopes recognized by a high-titer giardiasis patient serum. Treatment with 1% SDS at a protein concentration of 1 mg/ml decreased, but did not eliminate, IgG binding to the VSPs even after incubation at 95°C for 5 min (Table 2). We did note that VSP4 was sensitive to SDS treatment at a lower incubation temperature than the other VSP fragments. In contrast, treatment with DTT to reduce disulfide bonds completely eliminated IgG binding for all of the VSP fragments except VSP5. The epitopes on VSP5 could be destroyed only by incubation in the presence of 50 mM DTT for 1 h at 37°C (data not shown). For rVSP1, a series of DTT concentrations between 0.125 and 10 mM was used to identify the 50% inhibitory concentration (IC50) for IgG binding. At room temperature, the IC50 was 1.9 mM, while at 37°C it was noticeably lowered to 0.25 mM (data not shown). Under the same incubation conditions, IgG reactivity to a control protein with a nonstructural epitope was not diminished at any DTT concentration (data not shown).
Serum antibodies from a human giardiasis patient serum sample were eluted from PVDF-immobilized rVSP1 (lacking the GST fusion partner) and assayed by ELISA for reactivity to the VSP1/GST through VSP5/GST fusion proteins. Purified GST was included as a negative control. Absorbances (405 nm) of 1.236, 1.354, 1.282, 0.957, and 1.383 were observed for VSP1/GST through VSP5/GST, respectively, while the GST negative-control absorbance was only 0.055. Based on these results, we believe that we have identified a region of the VSP sequence where disulfide bond-stabilized secondary structure is required for IgG antibody recognition. Given that the CXXC motifs are a signature element of VSP structure among proteins from both assemblage A and assemblage B organisms (4, 58), the epitopes containing disulfide bond-stabilized structures are likely to be conserved among VSPs and are, therefore, an inviting target for a serologic antibody assay.
Different Giardia strains share VSP-like epitopes.
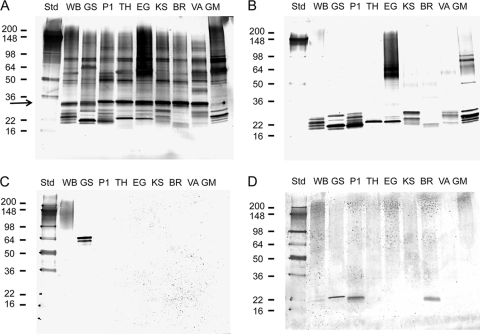
To determine whether the common VSP structural epitopes described above might be expressed among different Giardia strains, we grew trophozoites in axenic culture, resolved the proteins on SDS polyacrylamide gels under nonreducing conditions, and performed Western blot analyses. Figure 3 A shows a blot that was incubated with a high-titer serum sample from a giardiasis patient and then developed using an anti-human IgG secondary antibody. A common band was observed among all of the isolates except for GM-1 at approximately 30 kDa (Fig. 3A, arrow), and multiple low-molecular-mass bands were noted in the 22-kDa range. Using a monoclonal antibody that we developed, the 30-kDa protein was identified as the G. intestinalis α-1 giardin, a protein previously recognized as a significant parasite antigen (data not shown) (16, 67, 93). The reason for the absence of the α-1 giardin protein band in the GM-1 isolate lane is unknown, but treatment of the extracted proteins with a reducing agent prior to electrophoresis did yield a significant monoclonal antibody-reactive protein band at the appropriate apparent molecular weight in the GM-1 lane (data not shown).
FIG. 3.
Western blot comparisons of trophozoite extracts from human-derived Giardia strains. SDS-solubilized trophozoite proteins from axenically grown WB (WB), GS (GS), Portland-1 (P1), TH-1 (TH), EGY (EG), KSU (KS), BR-7 (BR), VANC/90/UBC/44 (VA), and GM-1 (GM) strains of Giardia were resolved on 8 to 16% gradient SDS-polyacrylamide gels and transferred to a PVDF membrane. Sources of the strains are given in Materials and Methods. Blotted proteins were incubated with serum from a giardiasis patient (1:100 dilution in 0.3% Tween 20-PBS) (A), antibodies against rVSP1 that were affinity-purified from the giardiasis patient serum shown in panel A (B), mouse monoclonal antibodies against VSP H7 (1:500) and CRP170 (1:500) (C), or a mouse serum raised against rVSP1 (1:100 dilution in 0.3% Tween 20-PBS) (D). Positions of the molecular weight standards (Std; in thousands) are indicated on the left. Bound IgG antibodies were detected using biotinylated mouse monoclonal anti-human IgG or biotinylated rat monoclonal anti-mouse IgG and the streptavidin-alkaline phosphatase system described in Materials and Methods. The location of α-1 giardin is indicated by an arrow.
In Fig. 3B, a Western blot was probed with monospecific polyclonal antibodies that were eluted from PVDF-immobilized rVSP1. The source of the antibodies was the same high-titer patient serum as shown in Fig. 3A. Bound IgG antibodies were detected in every Giardia isolate in the low-molecular-mass region around 22 kDa. Bands were also observed in the 50- to 100-kDa size ranges of the GM-1 and EGY isolate lanes. Notably absent were the CRP170 protein in the 95- to 200-kDa region of the WB isolate lane (56) and the H7 VSP at 54 to 72 kDa in the GS isolate lane (58). In Fig. 3C, a Western blot was probed with monoclonal antibodies 6E7 and G10/4 and the presence of VSPs CRP170 and H7 was confirmed in our WB and GS strain crude antigen preparations, respectively. These results would suggest that the semiconserved structural epitopes recognized by the eluted human IgG antibodies shown in Fig. 3B either are not present on larger VSPs like H7 and CRP170 (perhaps improperly folded) or are not exposed when an extensive, amino-terminal variable region I sequence is present on the VSP.
That rVSP1 can elicit both structural and linear epitope antibodies is shown in Fig. 3D and in Table 3. For the WB isolate (Fig. 3D, lane WB), anti-rVSP1 mouse antiserum recognized a protein smear in the 90- to 200-kDa size range similar to that recognized by the 6E7 monoclonal antibody in Fig. 3C as well as a 21-kDa protein (weakly). The serum also cross-reacted with proteins in the 22-kDa size range in the GS, Portland-1, and BR-7 isolates (Fig. 3D, lanes GS, P1, and BR). When the mouse serum was reacted with the six recombinant VSP fragments by ELISA (Table 3), we noted that VSP3 and VSP4 were weakly recognized and that cross-reactivity was eliminated by DTT reduction of the antigens. In contrast, recognition of rVSP1 was only moderately impacted by reduction. As previously observed with the human serum assays described in Table 2, SDS pretreatment of the antigens at 95°C did not completely eliminate antibody reactivity.
Preliminary analysis of the human immune response to the VSP fragments.
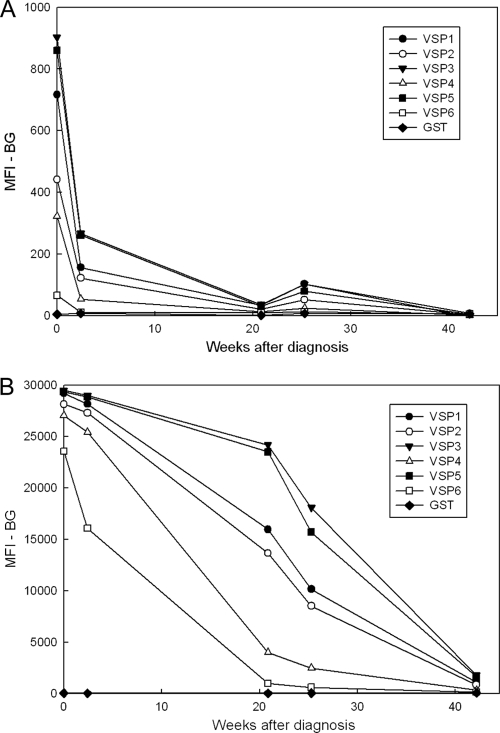
Although some researchers have reported good correlation between levels of Giardia-specific IgM or IgA antibodies and Giardia infection status (10, 59, 84, 88), we elected to focus solely on IgG responses for our MBA analysis of outbreak samples for two reasons. First, in an ELISA analysis of an abbreviated panel of 38 sera from outbreak patients (chosen from all the sample sets), levels of VSP1-specific IgG and IgM were much higher than levels of IgA (average optical densities at 450 nm [OD450] of 0.440 for IgG and 0.333 for IgM versus 0.014 for IgA), but high IgM values were also noted among five negative-control sera (OD450 range of 0.023 to 0.134 for IgM versus range of 0.006 to 0.011 for IgG). Second, in an MBA analysis of a set of five longitudinal samples collected from a single giardiasis patient over a 10-month period, the IgA responses were weak, even at the first time point, and decreased sharply to levels that were only slightly above the background at 22 weeks (Fig. 4A). In contrast, IgG responses for all the VSPs were well above their respective cutoff values at 22 weeks, and all would have been considered positive even at 43 weeks (Fig. 4B). IgM responses to the VSPs, although moderate in intensity at the first time point (range, 1,067 to 3,275 MFI-BG units), were considered unreliable because, unlike the IgG and IgA responses, the initial responses and the final responses at the end of the 10-month follow-up period were similar in magnitude (average VSP decline of 38%) (data not shown). In addition, the negative-control serum had moderate IgM responses to some VSPs (range, 37 to 1,071 MFI-BG units). IgM responses in the negative-control serum assays may reflect nonspecific antibody binding, since background IgM responses to the GST-only coupled beads were also consistently higher than the IgG responses (range of 128 to 274 MFI-BG units for IgM versus 29 to 59 MFI-BG units for IgG). The lower IgG background among individuals with no known history of Giardia infection and the longevity of the IgG response in the one longitudinal serum set from a giardiasis patient suggested that IgG might be a better choice for seroepidemiologic studies. We did not make further efforts to optimize the assay for IgM.
FIG. 4.
MBA detection of antibodies to VSP fragments in longitudinal specimens from a single giardiasis patient. Longitudinal samples were collected from a giardiasis patient 1 to 43 weeks after diagnosis and treatment. MBA was conducted as described in Materials and Methods using beads coated with rVSP1/GST, rVSP2/GST, rVSP3/GST, rVSP4/GST, rVSP5/GST, rVSP6/GST, or GST only. (A) Bound IgA antibodies were detected using a biotinylated mouse monoclonal anti-human IgA and R-phycoerythrin-labeled streptavidin; (B) bound IgG antibodies were detected using a biotinylated mouse monoclonal anti-human IgG and R-phycoerythrin-labeled streptavidin.
IgG MBA performance.
Each assay well included GST-only coupled beads to control for nonspecific antibody binding to the fusion partner and glycine-only coupled beads to control for nonspecific binding to the bead itself. MFI-BG values for the glycine-only beads were always below the cutoff values for the Giardia and Cryptosporidium antigens (average, 6 MFI-BG units; range, 0 to 52). For the GST-only beads, the 95th percentile for the U.S. nonoutbreak control serum set was 116 MFI-BG units (range 17 to 190), and only 3% of the GST responses among our test samples exceeded this threshold. In our experience, the background level of IgG binding to the GST-only bead appeared to be more related to the age of the reagents used for coupling than to the characteristics of the coupled antigen. Antibody responses in Giardia-positive patients were always >3-fold higher than the GST-only responses for at least one antigen.
To assess interassay variability, we ran the same set of 37 samples more than 2 weeks apart using the six VSP and two Cryptosporidium antigens (data not shown). Comparison of the paired values (n = 296) by linear regression analysis gave a correlation coefficient of 0.997. Only 17 of 242 (7%) samples in our study had to be repeatedly tested because of high well-to-well variability for multiple antibody responses.
MBA detection of IgG antibodies among nonoutbreak and cryptosporidiosis patients.
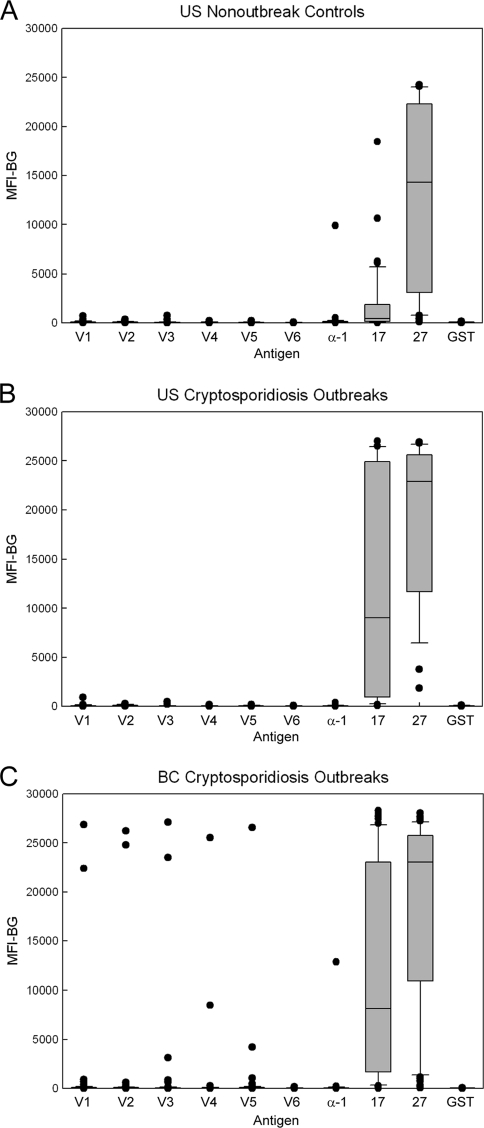
Frost et al. (19) have demonstrated that a large proportion of the U.S. population has antibodies to the two immunodominant Cryptosporidium antigens. Large numbers of individuals with antibodies to these two antigens were also seen in the U.S. nonoutbreak control sample set by the MBA (Fig. 5A). Because a negative population that could be used to help establish cutoff values likely does not exist, positive cutoffs for the two C. parvum antigen responses were determined by comparing MBA results from cryptosporidiosis patients, U.S. nonoutbreak controls, and a panel of confirmed Western blot-negative samples (n = 172) to results obtained using the “gold standard” large-format Western blot (70, 74). The J-index (94) was maximized at a cutoff value of 400 MFI-BG units for the rCp17/GST antigen (91% sensitivity, 87% specificity; 89% correct; J-index = 0.78) and at a cutoff of 1,860 MFI-BG units for the rCp27/GST antigen (95% sensitivity, 100% specificity; 96% correct; J-index = 0.95). Using these values, 56% of the U.S. nonoutbreak samples were positive for antibodies to both Cryptosporidium antigens, while 29% were positive for antibodies only to the 27-kDa antigen.
FIG. 5.
MBA detection of IgG antibodies among nonoutbreak controls and cryptosporidiosis outbreak cases. The MBA was conducted as described in Materials and Methods using beads coated with rVSP1/GST through rVSP6/GST (V1 to V6), α-1 giardin (α-1), rCp17/GST (17), rCp27/GST (27), and GST alone (GST). Bound IgG antibodies were detected using a biotinylated mouse monoclonal anti-human IgG and R-phycoerythrin-labeled streptavidin. Distributions for the U.S. nonoutbreak sample set (n = 41) (A), the U.S. cryptosporidiosis outbreak sample set (n = 29) (B), and the BC cryptosporidiosis outbreak sample set (n = 67) (C) are shown. Boxes include values between the 25th and 75th percentiles, whiskers include values between the 10th and 90th percentile, and outliers are indicated by data points. The median values are indicated within the box by a horizontal line.
In contrast to the Cryptosporidium results, it appeared that most of the U.S. nonoutbreak serum donors lacked a Giardia-specific antibody response (Fig. 5A), and we used this serum set to establish a cutoff that could be applied to the Giardia outbreak specimens. We chose the 95th percentile values for each antigen from the nonoutbreak controls as the respective positive cutoffs and required the presence of two positive antibody responses to define a serologically positive Giardia patient. Cutoff values were set at 207, 219, 126, 156, 196, and 61 MFI-BG units for rVSP1/GST through rVSP6/GST, respectively, and 243 MFI-BG units for α-1 giardin. Using these cutoffs and the two-antigen response definition, 5 of 41 (12%) nonoutbreak serum donors were considered positive for antibodies to Giardia. In this sample set, we did not find anyone who had only one positive antibody response to a recombinant Giardia antigen.
Results from an analysis of sera from U.S. and Canadian cryptosporidiosis outbreaks are shown in Fig. 5B and C, respectively. For the Cryptosporidium antigens, 23 of 29 U.S. samples (79%) and 57 of 67 BC samples (85%) were positive for antibodies to both antigens. Thirteen of the U.S. samples (45%) and 28 of the BC samples (42%) had very high responses (>10,000 MFI-BG units) to both antigens. Six additional U.S. patients (21%) and 5 additional BC patients (7%) were positive for only one of the Cryptosporidium antigens. Giardia seroprevalence was low in both sample sets (6% for the U.S. set and 12% for the BC set), but two high responders (3%) with MFI-BG values of >10,000 were noted in the BC sample set. Values for the rCp17/GST and rCp27/GST antibody responses from both outbreak sample sets were significantly higher than those of the U.S. nonoutbreak sample set (Mann-Whitney rank sum test; P ≤ 0.003).
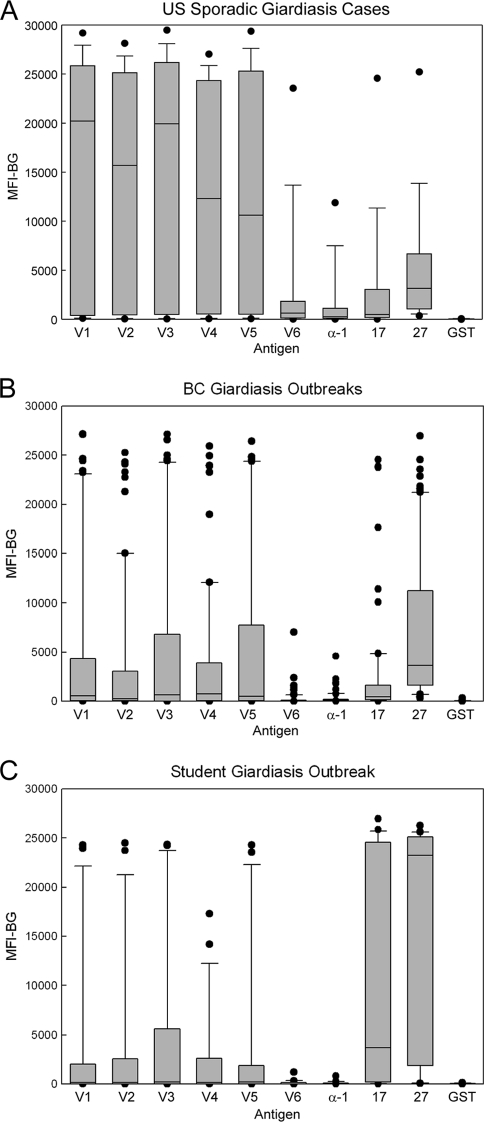
MBA detection of IgG antibodies among giardiasis sample sets.
The IgG antibody response distributions observed for the giardiasis sample sets (Fig. 6) were quite different from those of the cryptosporidiosis and nonoutbreak sample sets shown in Fig. 5. Thirteen of 14 (93%) sporadic U.S. giardiasis patient sera demonstrated IgG antibodies to multiple recombinant antigens (Fig. 6A), while 42 of 70 (60%) BC outbreak sera (Fig. 6B) and 13 of 21 (62%) sera from the Canadian student outbreak (Fig. 6C) had multiple responses. Six of 70 BC outbreak sera (9%) were positive for antibodies to only one of the recombinant antigens and thus did not meet the two-antigen response requirement of our serologic definition. Interestingly, four of these six patients had responses only to the rVSP4/GST antigen, and all four of these responses were well above the 153 MFI-BG unit cutoff value (range, 609 to 23,904 MFI-BG units). VSP3 and VSP5 were the most commonly recognized recombinant antigens (60% of sera), while VSP6 (45%) and α-1 giardin (24%) were the least commonly recognized. In summary, a total of 68 of 105 (65%) sera from giardiasis patients and giardiasis outbreak donors met our serologic definition and were considered positive for Giardia antibodies, and half of the sera (n = 52) reacted with five or more rVSPs.
FIG. 6.
MBA detection of IgG antibodies among giardiasis serum sets. The MBA was conducted as described in Materials and Methods using beads coated with rVSP1/GST through rVSP6/GST (V1 to V6), α-1 giardin (α-1), rCp17/GST (17), rCp27/GST (27), and GST alone (GST). Bound IgG antibodies were detected using a biotinylated mouse monoclonal anti-human IgG and R-phycoerythrin-labeled streptavidin. IgG antibody response distributions for the U.S. sporadic giardiasis confirmed case patient set (n = 14) (A), the BC giardiasis outbreak sample set (n = 70) (B), and the Canadian student giardiasis outbreak sample set (n = 21) (C) are shown. Boxes include values between the 25th and 75th percentiles, whiskers include values between the 10th and 90th percentile, and outliers are indicated by data points. The median values are indicated within the box by a horizontal line.
Statistical analysis showed that the antibody responses of the U.S. patients with sporadic giardiasis (Fig. 6A) to rVSP1/GST through rVSP6/GST were significantly higher than those observed among the U.S. nonoutbreak controls shown in Fig. 5A (P < 0.001). The differences between the α-1 giardin responses were also significant but were not as pronounced (P = 0.04). Similarly, the BC giardiasis outbreak donors (Fig. 6B) had statistically higher responses to rVSP1/GST through rVSP6/GST than the U.S. nonoutbreak controls (P ≤ 0.003), but the differences between the α-1 giardin responses were not significant. Among the Canadian student giardiasis outbreak donors (Fig. 6C), responses to all of the antigens except rVSP6/GST and α-1 giardin were significantly higher than those of the U.S. nonoutbreak patients (P < 0.05).
Although the sample sets are likely not representative of the populations from which they were drawn, we decided to compare the cryptosporidiosis responses of the U.S. nonoutbreak controls (Fig. 5A) to those of the Canadian student giardiasis outbreak donors (Fig. 6C). Responses to both of the Cryptosporidium antigens were observed for 57% of the Canadian student outbreak samples. We noted that 9 of the 21 (43%) students had responses to both Cryptosporidium antigens in excess of 10,000 MFI-BG units. As previously noted, high responders were common among cryptosporidiosis outbreak cases (>40%) but represented only 4% (3/70) of the BC giardiasis donors, 5% (2/41) of the U.S. nonoutbreak controls, and 7% (1/14) of the U.S. sporadic-giardiasis case patients. Although statistically significant differences were not evident when antibody responses to the rCp17/GST (P = 0.056) and rCp23/GST (P = 0.145) antigens were compared between the Canadian student giardiasis sample set and U.S. nonoutbreak controls, the responses clearly trended higher among the Canadian student samples (Fig. 6C). These results strongly suggest that some of the Canadian students had been recently infected with Cryptosporidium.
DISCUSSION
The presence of conserved motifs within the variant surface proteins of Giardia was first recognized by Nash et al. (61), and the role of these sequences in the immune responses of experimentally infected mice has been investigated thoroughly (8, 9, 52, 53, 85). Bienz et al. (9) and Stager et al. (85) reported that antibody recognition of their recombinant VSP antigen fragments by mouse antibodies was unaffected by pretreatment of the antigen with either a denaturing agent (urea) or a reducing agent (DTT). Antibodies to the H7 semiconserved part II region were shown to recognize some of the newly expressed VSPs after antigenic variation from the original H7 clone (53), but neither Muller et al. (53) nor Stager et al. (85) were able to demonstrate any antigenic similarities between the VSP H7 from assemblage B Giardia strain GS and the CRP170 (AS6) VSP from the assemblage A Giardia strain WB. In contrast, IgG antibody binding to our VSP fragments was largely abolished by antigen pretreatment with DTT and was also diminished by SDS denaturation. In addition, we observed that IgG antibodies eluted from the rVSP1 fragment of CRP170 reacted strongly with the VSP4 protein sequence from VSP H7. It seems likely that our VSP constructs and expression system favor the formation of disulfide bonds and promote the formation of structural epitopes that are recognized by sera from infected patients better than those previously reported in the literature (9, 85). To our knowledge, ours is the first report describing the recognition of the part II semiconserved sequences near the carboxy terminus of the VSP by serum IgG antibodies from human giardiasis patients.
Even though some antibody reactivity may have been lost because of the SDS treatment, we were able to use affinity-purified polyclonal IgG antibodies against rVSP1 to document the presence of cross-reactive proteins in trophozoite extracts from multiple axenic strains of Giardia. The tested strains were derived from infected patients and represented both of the assemblages that infect humans. We did not detect either the CRP170 or the H7 VSPs, but we did see multiple, low-molecular-weight antigens in most extracts. Unfortunately, clinical information on the relative timing of Giardia infection and serum collection was limited to a single patient in our study, and we were unable to determine with certainty whether human responses to the VSP fragments were early, late, or both. We also do not know whether the 22-kDa antigens represent cleaved VSPs (68) or are short VSPs with the membrane-spanning regions intact (47). In the future, we plan to extract Giardia membrane proteins with Triton X-114 in an effort to differentiate between these two possibilities and to better characterize these low-molecular-weight proteins.
Serologic IgG antibody levels have been reported by some researchers to correlate better with Giardia infection status than IgA levels (31, 41), and we obtained a similar result using our rVSP1 fragment in an ELISA format. The more transient nature of the IgA responses than of the IgG responses in our analysis of a longitudinal serum set from a giardiasis patient is also similar to the situation reported for IgA and IgG antibody responses to the 17- and 27-kDa antigens among cryptosporidiosis patients. IgG responses to the Cryptosporidium antigens are known to remain elevated for 2 to 12 months postinfection, while IgA responses are weak and more transient (30, 48, 51, 70). In volunteers who were infected with Giardia, Nash et al. (59, 60) found that most patients developed parasite-specific IgM, IgA, and IgG responses within 2 to 4 weeks of infection but that IgG responses were somewhat delayed. Because the Giardia-infected volunteers were monitored for only 4 to 8 weeks postinoculation, information on the long-term longevity of the IgG response is lacking. We are currently collecting longitudinal serum samples from giardiasis patients in order to more fully examine the timing, longevity, targets, and relative magnitudes of the human IgG, IgM, and IgA antibody responses to the VSP fragments.
The sensitivity of the IgG MBA using multiple VSP fragments was 93% for samples collected from 14 cyst-confirmed giardiasis case patients. Because the sporadic patient samples were collected from acutely ill individuals who sought out medical treatment and were collected in a time frame that maximized the likelihood of IgG antibody detection, this sensitivity value is likely an upper limit of the performance of the MBA. The sporadic sample set probably does not represent the true spectrum of giardiasis antibody responses that would be observed in an infected population. The median VSP response values and the seropositivity results (60 to 62%) observed for the BC and Canadian student giardiasis outbreak sample sets were much lower than those of the sporadic cases. Because we were not able to link the individual samples to the clinical results, the giardiasis outbreak sample sets we tested may have included samples from symptomatic but unconfirmed donors (presumed giardiasis cases) as well as from exposed individuals who did not meet the case definition and had no laboratory evidence of infection. For example, 38 of the 72 Creston giardiasis outbreak samples originally collected in 1990 were from stool-positive cases, 13 were from unconfirmed cases, and 21 were from donors who were defined as noncases (31). The residual set of 56 Creston samples that we tested in this study likely included sera from all three categories of donors. Thus, our BC giardiasis serum sets have some of the characteristics of a waterborne outbreak community serosurvey and would be expected to have lower overall values than a stool-confirmed patient serum set. The lower median responses observed among outbreak sample sets may also be a function of the timing of sample collection, as the samples were collected weeks after the outbreaks began and likely included both acute- and convalescent-phase patient sera (31, 32).
Despite these limitations, half of all the sporadic giardiasis and giardiasis outbreak samples in our study (52 of 105 samples) were positive for antibodies to five or more VSPs, and 67 of our 68 serologically positive patients reacted with VSPs from both Giardia assemblages. Although they did not meet our serologic definition and were not included in the final sensitivity calculations, strong positive reactions only to VSP4 were also found in four donors. We have not characterized the antibody responses among these four donors, and therefore we do not yet know if they recognize linear or structural epitopes on the VSP4 fragment. VSP6 from the GM-1 Giardia strain and α-1 giardin both performed poorly in the MBA relative to the other VSP fragments. In fact, we could have eliminated both of these antigens from the analysis and would have lost only one serologically positive response. Poor VSP6 performance might be related to amino acid substitutions at positions 52 and 86 that eliminate a conserved Gln and a conserved Tyr, respectively. In order to continue using our conservative, two-response definition for seropositivity, we may need to search for additional assemblage B VSP fragments that are better conserved than the VSP6 and perhaps more closely related to VSP4 than is VSP5.
The MBA easily distinguished samples collected from cryptosporidiosis outbreak patients from those collected from giardiasis outbreaks. We did, however, note several patients in the BC cryptosporidiosis outbreak set who likely had concurrent infections with Giardia. Concurrent infection are not surprising given that water supplies in this region of British Columbia were found to contain Giardia cysts and that nearby giardiasis outbreaks had previously been recognized (11, 27, 28, 31). Elevated responses to the 17-kDa antigen, the most recognizable feature of the Cryptosporidium outbreak specimens, were also observed among the Canadian student giardiasis outbreak specimens. In fact, high responses to both Cryptosporidium antigens were found among 43% of the students. Our data strongly suggest that, even though the distributions of student responses to the two Cryptosporidium antigens did not statistically differ from those of the nonoutbreak controls, some of the students likely acquired cryptosporidiosis during their travels in the USSR (32). Those Cryptosporidium infections probably went unrecognized since this organism was not commonly considered in the differential diagnosis of diarrheal disease in the mid-1980s and many of the students had already been diagnosed with giardiasis. Our results demonstrate the potential power of the MBA in a retrospective analysis of outbreak samples.
The most obvious weakness of the MBA is that some patients with stool-confirmed Giardia or Cryptosporidium infections do not mount detectable serologic IgG responses to the assay antigens. We have previously demonstrated that 66% of stool-confirmed Cryptosporidium infections in children were associated with increases in antigen-specific IgG antibody responses (72) and that the ability to detect an antibody response increased with subsequent infections (73). In the case of Giardia, Ljungstrom and Castor (41) found that 32% of cyst-positive outbreak patients had no detectable IgG or IgA antibodies to trophozoite antigens, and Nash et al. (59) found that fewer patient volunteers developed IgG (70%) and IgA (60%) responses than developed IgM responses (100%). Using a whole trophozoite cell-antigen IgG ELISA, Smith et al. (83) reported a sensitivity of 81% for symptomatic, cyst-confirmed giardiasis patients (83). A weak or absent antibody response might be related to the timing of sample collection relative to infection, differences in the infectious doses, the immune status and previous infection experience of the host, the duration of the infection, or the severity of symptoms. The ability of the MBA to detect multiple infections among both symptomatic and asymptomatic individuals even after cessation of (oo)cyst shedding might be considered a reasonable trade-off for the weakness cited above. In future work, we hope to determine the number of serologically positive Giardia infections that are missed by stool microscopy and to more accurately determine the sensitivity of the MBA for cyst-positive case patients so that a more robust comparison of the two assays can be made. A fully validated MBA would be a useful new tool for studies of the prevalence and risks of waterborne infections at the community level.
Finally, we expressed all of our recombinant proteins as fusions with S. japonicum GST so that the efficiency of covalent coupling to the beads could be easily monitored and to increase the size of our low-molecular-weight antigens for more efficient coupling. We have three lines of evidence to suggest that GST does not significantly impact MBA performance. First, we included GST-only coupled beads in each of our assays as a control for low-avidity background GST responses like those observed by Lightowlers and Mitchell (40). Elevated responses among our serum sets were rarely detected (3% of MFI-BG values were >116; maximum value, 348) and did not interfere with the interpretation of the parasite-specific antibody responses. Second, we have preliminary evidence showing that background can be further decreased without loss of signal intensity by preincubation of the serum dilutions with a GST/E. coli particulate extract. The particulate material along with absorbed antibodies can easily be removed during the centrifugation of the serum dilution (J. W. Priest and D. M. Moss, unpublished observation). This step may be particularly useful when highly purified recombinant proteins are difficult to obtain for coupling. Third, we performed a preliminary analysis on a panel of 92 sera from confirmed S. japonicum-infected patients (kindly provided by V. C. W. Tsang, CDC, Atlanta, GA) and found that ELISA responses to purified GST protein were generally quite low (median OD405, 0.016; range, 0 to 0.214) (data not shown). Because the prevalence of anti-GST antibodies among schistosomiasis patients has not been firmly established (45), additional studies using the MBA to analyze defined schistosomiasis patient sera are warranted before using the assay in regions of S. japonicum endemicity around the world.
Acknowledgments
G.S.V. thanks M. Wittner, Albert Einstein College of Medicine, for sharing Giardia strain TH-1. We thank T. E. Nash, NIH, for sharing VSP-specific monoclonal antibodies and the GS parasite isolate and V. C. W. Tsang (CDC) for access to his defined schistosomiasis serum bank.
Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 28 September 2010.
REFERENCES
- 1.Abaza, S. M., J. J. Sullivan, and G. S. Visvesvara. 1991. Isoenzyme profile of four strains of Giardia lamblia and their infectivity to jirds. Am. J. Trop. Med. Hyg. 44:63-68. [DOI] [PubMed] [Google Scholar]
- 2.Adam, R. D. 2001. Biology of Giardia lamblia. Clin. Microbiol. Rev. 14:447-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam, R. D., A. Nigam, V. Seshadri, C. A. Martens, G. A. Farneth, H. G. Morrison, T. E. Nash, S. F. Porcella, and R. Patel. 2010. The Giradia lamblia vsp gene repertoire: characteristics, genomic organization, and evolution. BMC Genomics 11:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam, R. D., Y. M. Yang, and T. E. Nash. 1992. The cysteine-rich protein gene family of Giardia lamblia: loss of the CRP170 gene in an antigenic determinant. Mol. Cell. Biol. 12:1194-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal, A., J. W. Merritt, Jr., and T. E. Nash. 1989. Cysteine-rich variant surface proteins of Giardia lamblia. Mol. Biochem. Parasitol. 32:39-47. [DOI] [PubMed] [Google Scholar]
- 6.Bern, C., Y. Ortega, W. Checkley, J. M. Roberts, A. G. Lescano, L. Cabrera, M. Verastegui, R. E. Black, C. Sterling, and R. H. Gilman. 2002. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg. Infect. Dis. 8:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betancourt, W. Q., and J. B. Rose. 2004. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet. Parasitol. 126:219-234. [DOI] [PubMed] [Google Scholar]
- 8.Bienz, M., A. Siles-Lucas, P. Wittwer, and N. Muller. 2001. vsp gene expression by Giardia lamblia clone GS/M-83-H7 during antigenic variation in vivo and in vitro. Infect. Immun. 69:5278-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bienz, M., P. Wittwer, V. Zimmermann, and N. Muller. 2001. Molecular characterization of a predominant antigenic region of Giardia lamblia variant surface protein H7. Int. J. Parasitol. 31:827-832. [DOI] [PubMed] [Google Scholar]
- 10.Birkhead, G., E. N. Janoff, R. L. Vogt, and P. D. Smith. 1989. Elevated levels of immunoglobulin A to Giardia lamblia during a waterborne outbreak of gastroenteritis. J. Clin. Microbiol. 27:1707-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.British Columbia Ministry of Health Planning. 2001. A report on the health of British Columbians. Drinking water quality in British Columbia: the public health perspective. Provincial Health Officer's annual report 2000. Ministry of Health Planning, Victoria, British Columbia.
- 12.Casemore, D. 2006. Towards a U.S. national estimate of the risk of endemic waterborne disease—sero-epidemiologic studies. J. Water Health 4(Suppl. 2):121-163. [DOI] [PubMed] [Google Scholar]
- 13.CDC. 1998. Foodborne outbreak of cryptosporidiosis—Spokane, Washington, 1997. MMWR Morb. Mortal. Wkly. Rep. 227:565-567. [PubMed] [Google Scholar]
- 14.Chappell, C. L., P. C. Okhuysen, C. R. Sterling, and H. L. DuPont. 1996. Cryptosporidium parvum: intensity of infection and oocyst excretion patterns in healthy volunteers. J. Infect. Dis. 173:232-236. [DOI] [PubMed] [Google Scholar]
- 15.Craun, G. F., R. L. Calderon, and M. F. Craun. 2005. Outbreaks associated with recreational water in the United States. Int. J. Environ. Health Res. 15:243-262. [DOI] [PubMed] [Google Scholar]
- 16.Crump, J. A., C. E. Mendoza, J. W. Priest, R. I. Glass, S. S. Monroe, L. A. Dauphin, W. F. Bibb, M. B. Lopez, M. Alvarez, E. D. Mintz, and S. P. Luby. 2007. Comparing serologic response against enteric pathogens with reported diarrhea to assess the impact of improved household drinking water quality. Am. J. Trop. Med. Hyg. 77:136-141. [PubMed] [Google Scholar]
- 17.Faubert, G. 2000. Immune response to Giardia duodenalis. Clin. Microbiol. Rev. 13:35-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franzen, O., J. Jerlstrom-Hultqvist, E. Castro, E. Sherwood, J. Ankarklev, D. S. Reiner, D. Palm, J. O. Andersson, B. Andersson, and S. G. Svard. 2009. Draft genome sequencing of Giardia intestinalis assemblage B isolate GS: is human giardiasis caused by two different species. PLoS Pathog. 5:e1000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost, F. J., T. B. Muller, R. L. Calderon, and G. F. Craun. 2004. Analysis of serological responses to Cryptosporidium antigen among NHANES III participants. Ann. Epidemiol. 14:473-478. [DOI] [PubMed] [Google Scholar]
- 20.Glaberman, S., I. M. Sulaiman, C. Bern, J. Limor, M. M. Peng, U. Morgan, R. Gilman, A. A. Lal, and L. Xiao. 2001. A multilocus genotypic analysis of Cryptosporidium meleagridis. J. Eukaryot. Microbiol. Suppl.:19S-31S. [DOI] [PubMed]
- 21.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 22.Hiltpold, A., M. Frey, A. Hulsmeier, and P. Kohler. 2000. Glycosylation and palmitoylation are common modifications of Giardia variant surface proteins. Mol. Biochem. Parasitol. 109:61-65. [DOI] [PubMed] [Google Scholar]
- 23.Hlavsa, M. C., J. C. Watson, and M. J. Beach. 2005. Cryptosporidiosis surveillance—United States 1999-2002. MMWR Surveill. Summ. 54:1-8. [PubMed] [Google Scholar]
- 24.Hlavsa, M. C., J. C. Watson, and M. J. Beach. 2005. Giardiasis surveillance—United States 1999-2002. MMWR Surveill. Summ. 54:9-16. [PubMed] [Google Scholar]
- 25.Hoff, J. C., E. W. Rice, and F. W. Schaefer III. 1985. Comparison of animal infectivity and excystation as measures of Giardia muris cyst inactivation by chlorine. Appl. Environ. Microbiol. 50:1115-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang, D. B., and A. C. White. 2006. An updated review on Cryptosporidium and Giardia. Gastroenterol. Clin. N. Am. 35:291-314. [DOI] [PubMed] [Google Scholar]
- 27.Isaac-Renton, J., J. Blatherwick, W. R. Bowie, M. Fyfe, M. Khan, A. Li, A. King, M. McLean, L. Medd, W. Moorehead, C. S. Ong, and W. Robertson. 1999. Epidemic and endemic seroprevalence of antibodies to Cryptosporidium and Giardia in residents of three communities with different drinking water supplies. Am. J. Trop. Med. Hyg. 60:578-583. [DOI] [PubMed] [Google Scholar]
- 28.Isaac-Renton, J., W. Moorehead, and A. Ross. 1996. Longitudinal studies of Giardia contamination in two community drinking water supplies: cyst levels, parasite viability, and health impact. Appl. Environ. Microbiol. 62:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isaac-Renton, J. L., C. Cordeiro, K. Sarafis, and H. Shahriari. 1993. Characterization of Giardia duodenalis isolates from a waterborne outbreak. J. Infect. Dis. 167:431-440. [DOI] [PubMed] [Google Scholar]
- 30.Isaac-Renton, J. L., C. S. L. Ong, W. R. Bowie, P. J. Lammie, and J. W. Priest. 2003. Cryptosporidium serology in human populations. AWWA Research Foundation, Denver, CO.
- 31.Isaac-Renton, J. L., L. F. Lewis, C. S. L. Ong, and M. F. Nulsen. 1994. A second community outbreak of waterborne giardiasis in Canada and serologic investigation of patients. Trans. R. Soc. Trop. Med. Hyg. 88:395-399. [DOI] [PubMed] [Google Scholar]
- 32.Isaac-Renton, J. L., W. A. Black, R. G. Mathis, E. M. Proctor, and C. H. Sherlock. 1986. Giardiasis in a group of travelers—attempted use of a serologic test. Can. J. Public Health 77:86-88. [PubMed] [Google Scholar]
- 33.Karanis, P., C. Kourenti, and H. Smith. 2007. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health 5:1-38. [DOI] [PubMed] [Google Scholar]
- 34.Keister, D. B. 1983. Axenic cultivation of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 77:487-488. [DOI] [PubMed] [Google Scholar]
- 35.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 36.Larkin, M. A., G. Blackshields. N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. ClustalW and ClustalX version 2. Bioinformatics 23:2947-2948. [DOI] [PubMed] [Google Scholar]
- 37.Lasek-Nesselquist, E., D. M. Welch, R. C. A. Thompson, R. F. Steuart, and M. L. Sogin. 2009. Genetic exchange within and between assemblages of Giardia duodenalis. J. Eukaryot. Microbiol. 56:504-518. [DOI] [PubMed] [Google Scholar]
- 38.Laupland, K. B., and D. L. Church. 2005. Population-based laboratory surveillance for Giardia sp. and Cryptosporidium sp. infections in a large Canadian health region. BMC Infect. Dis. 5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, Y.-M., P. W. Johnson, J. L. Call, M. J. Arrowood, B. W. Furness, S. C. Pichette, K. W. Grady, P. Reeh, L. Mitchell, D. Bergmire-Sweat, W. R. MacKenzie, and V. C. W. Tsang. 2001. Development and application of a quantitative, specific assay for Cryptosporidium parvum oocyst detection in high-turbidity environmental water samples. Am. J. Trop. Med. Hyg. 65:1-9. [DOI] [PubMed] [Google Scholar]
- 40.Lightowlers, M. W., and G. F. Mitchell. 1989. Assessment of the prevalence and titer of antibodies to a candidate schistosomiasis vaccine molecule, Sj26, in several human serum banks. Acta Trop. 46:229-238. [DOI] [PubMed] [Google Scholar]
- 41.Ljungstrom, I., and B. Castor. 1992. Immune response to Giardia lamblia in a water- borne outbreak of giardiasis in Sweden. J. Med. Microbiol. 36:347-352. [DOI] [PubMed] [Google Scholar]
- 42.MacKenzie, W. R., N. J. Hoxie, M. E. Proctor, M. S. Gradus, K. A. Blair, D. E. Peterson, J. J. Kazmierczak, D. G. Addiss, K. R. Fox, J. B. Rose, and J. P. Davis. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331:161-167. [DOI] [PubMed] [Google Scholar]
- 43.McDonald, A. C., W. R. MacKenzie, D. G. Addiss, M. S. Gradus, G. Linke, E. Zembrowski, M. R. Hurd, M. J. Arrowood, P. J. Lammie, and J. W. Priest. 2001. Cryptosporidium parvum-specific antibody responses among children residing in Milwaukee during the 1993 waterborne outbreak. J. Infect. Dis. 183:1373-1379. [DOI] [PubMed] [Google Scholar]
- 44.Meyer, E. A. 1976. Giardia lamblia: isolation and axenic culture. Exp. Parasitol. 39:101-105. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell, G. F. 1989. Glutathione S-transferases—potential components of anti-schistosome vaccines. Parasitol. Today 5:34-37. [DOI] [PubMed] [Google Scholar]
- 46.Monis, P. T., S. M. Caccio, and R. C. A. Thompson. 2009. Variation in Giardia: towards a taxonomic revision of the genus. Trends Parasitol. 25:93-100. [DOI] [PubMed] [Google Scholar]
- 47.Morrison, H. G., A. G. McArthur, F. D. Hillen, S. B. Aley, R. D. Adam, G. J. Olsen, A. A. Best, et al. 2007. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science 317:1921-1926. [DOI] [PubMed] [Google Scholar]
- 48.Moss, D. M., C. L. Chappell, P. C. Okhuysen, H. L. DuPont, M. J. Arrowood, A. W. Hightower, and P. J. Lammie. 1998. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J. Infect. Dis. 178:827-833. [DOI] [PubMed] [Google Scholar]
- 49.Moss, D. M., G. S. Visvesvara, H. M. Mathews, and D. A. Ware. 1992. Isoenzyme comparison of axenic Giardia lamblia strains. J. Protozool. 39:559-564. [DOI] [PubMed] [Google Scholar]
- 50.Moss, D. M., J. M. Montgomery, S. V. Newland, J. W. Priest, and P. J. Lammie. 2004. Detection of Cryptosporidium antibodies in sera and oral fluids using multiplex bead assay. J. Parasitol. 90:397-404. [DOI] [PubMed] [Google Scholar]
- 51.Moss, D. M., S. N. Bennett, M. J. Arrowood, S. P. Wahlquist, and P. J. Lammie. 1998. Enzyme-linked immunoelectrotransfer blot analysis of a cryptosporidiosis outbreak on a United States Coast Guard cutter. Am. J. Trop. Med. Hyg. 58:110-118. [DOI] [PubMed] [Google Scholar]
- 52.Muller, N., and S. Stager. 1999. Periodic appearance of a predominant variant antigen type during chronic Giardia lamblia infection in a mouse model. Int. J. Parasitol. 29:1917-1923. [DOI] [PubMed] [Google Scholar]
- 53.Muller, N., S. Stager, and B. Gottstein. 1996. Serologic analysis of antigenic heterogeneity of Giardia lamblia variant surface proteins. Infect. Immun. 64:1385-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nash, T. E. 1997. Antigenic variation in Giardia lamblia and the host's immune response. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352:1369-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nash, T. E. 2002. Surface antigenic variation in Giardia lamblia. Mol. Microbiol. 45:585-590. [DOI] [PubMed] [Google Scholar]
- 56.Nash, T. E., A. Aggarwal, R. D. Adam, J. T. Conrad, and J. W. Merritt, Jr. 1988. Antigenic variation in Giardia lamblia. J. Immunol. 141:636-641. [PubMed] [Google Scholar]
- 57.Nash, T. E., and A. Aggarwal. 1986. Cytotoxicity of monoclonal antibodies to a subset of Giardia isolates. J. Immunol. 136:2628-2632. [PubMed] [Google Scholar]
- 58.Nash, T. E., and M. R. Mowatt. 1992. Characterization of a Giardia lamblia variant-specific surface protein (VSP) gene from isolate GS/M and estimation of the VSP gene repertoire size. Mol. Biochem. Parasitol. 51:219-228. [DOI] [PubMed] [Google Scholar]
- 59.Nash, T. E., D. A. Herrington, G. A. Losonsky, and M. M. Levine. 1987. Experimental infections with Giardia lamblia. J. Infect. Dis. 156:974-984. [DOI] [PubMed] [Google Scholar]
- 60.Nash, T. E., D. A. Herrington, M. W. Levine, J. T. Conrad, and J. W. Merritt, Jr. 1990. Antigenic variation of Giardia lamblia in experimental human infections. J. Immunol. 144:4362-4369. [PubMed] [Google Scholar]
- 61.Nash, T. E., J. T. Conrad, and M. R. Mowatt. 1995. Giardia lamblia: identification and characterization of a variant-specific surface protein gene family. J. Eukaryot. Microbiol. 42:604-609. [DOI] [PubMed] [Google Scholar]
- 62.Nash, T. E., S. M. Banks, D. Alling, J. W. Merritt, Jr., and J. T. Conrad. 1990. Frequency of variant antigens in Giardia lamblia. Exp. Parasitol. 71:415-421. [DOI] [PubMed] [Google Scholar]
- 63.Nash, T. E., T. McCutchan, D. Keister, J. B. Dame, J. D. Conrad, and F. D. Gillin. 1985. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J. Infect. Dis. 152:64-73. [DOI] [PubMed] [Google Scholar]
- 64.Newman, R. D., S. R. Moore, A. A. M. Lima, J. P. Nataro, R. L. Guerrant, and C. L. Sears. 2001. A longitudinal study of Giardia lamblia infection in north-east Brazilian children. Trop. Med. Int. Health 6:624-634. [DOI] [PubMed] [Google Scholar]
- 65.Okhuysen, P. C., C. L. Chappell, J. H. Crabb, C. R. Sterling, and H. L. DuPont. 1999. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 180:1275-1281. [DOI] [PubMed] [Google Scholar]
- 66.Ong, C. S. L., D. L. Eisler, S. H. Goh, J. Tomblin, F. M. Awad-el-Kariem, C. B. Beard, L. Xiao, I. Sulaiman, A. Lal, M. Fyfe, A. King, W. R. Bowie, and J. L. Isaac-Renton. 1999. Molecular epidemiology of cryptosporidiosis outbreaks and transmission in British Columbia, Canada. Am. J. Trop. Med. Hyg. 61:63-69. [DOI] [PubMed] [Google Scholar]
- 67.Palm, J. E. D., M. E.-L. Wieland, W. J. Griffiths, I. Ljungstrom, and S. G. Svard. 2003. Identification of immunoreactive proteins during acute human giardiasis. J. Infect. Dis. 187:1849-1859. [DOI] [PubMed] [Google Scholar]
- 68.Papanastasiou, P., A. Hiltpold, C. Bommeli, and P. Kohler. 1996. The release of the variant surface protein of Giardia to its soluble isoform is mediated by the selective cleavage of the conserved carboxy-terminal domain. Biochemistry 35:10143-10148. [DOI] [PubMed] [Google Scholar]
- 69.Papanastasiou, P., M. J. McConville, J. Ralton, and P. Kohler. 1997. The variant-specific surface glycoprotein of Giardia, VSP4A1, is a glycosylated and palmitoylated protein. Biochem. J. 322:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Priest, J. W., A. Li, M. D. Khan, M. J. Arrowood, P. J. Lammie, C. S. Ong, J. M. Roberts, and J. Isaac-Renton. 2001. Enzyme immunoassay detection of antigen-specific immunoglobulin G antibodies in longitudinal serum samples from patients with cryptosporidiosis. Clin. Diagn. Lab. Immunol. 8:415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Priest, J. W., A. Mehlert, D. M. Moss, M. J. Arrowood, and M. A. J. Ferguson. 2006. Characterization of the glycosylphosphatidylinositol anchor of the immunodominant Cryptosporidium parvum 17-kDa antigen. Mol. Biochem. Parasitol. 149:108-112. [DOI] [PubMed] [Google Scholar]
- 72.Priest, J. W., C. Bern, J. M. Roberts, J. P. Kwon, A. G. Lescano, W. Checkley, L. Cabrera, D. M. Moss, M. J. Arrowood, C. R. Sterling, R. H. Gilman, and P. J. Lammie. 2005. Changes in serum immunoglobulin G levels as a marker for Cryptosporidium sp. infection in Peruvian children. J. Clin. Microbiol. 43:5298-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Priest, J. W., C. Bern, L. Xiao, J. M. Roberts, J. P. Kwon, A. G. Lescano, W. Checkley, L. Cabrera, D. M. Moss, M. J. Arrowood, C. R. Sterling, R. H. Gilman, and P. J. Lammie. 2006. Longitudinal analysis of Cryptosporidium species-specific immunoglobulin G antibody responses in Peruvian children. Clin. Vaccine Immunol. 13:123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Priest, J. W., J. P. Kwon, D. M. Moss, J. M. Roberts, M. J. Arrowood, M. S. Dworkin, D. D. Juranek, and P. J. Lammie. 1999. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigen. J. Clin. Microbiol. 37:1385-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Priest, J. W., J. P. Kwon, M. J. Arrowood, and P. J. Lammie. 2000. Cloning of the immunodominant 17-kDa antigen from Cryptosporidium parvum. Mol. Biochem. Parastiol. 106:261-271. [DOI] [PubMed] [Google Scholar]
- 76.Priest, J. W., L.-T. Xie, M. J. Arrowood, and P. J. Lammie. 2001. The immunodominant 17-kDa antigen from Cryptosporidium parvum is glycosylphosphatidylinositol-anchored. Mol. Biochem. Parasitol. 113:117-126. [DOI] [PubMed] [Google Scholar]
- 77.Prucca, C. G., I. Slavin, R. Quiroga, E. V. Elias, F. D. Rivero, A. Saura, P. G. Carranza, and H. D. Lujan. 2008. Antigenic variation in Giardia lamblia is regulated by RNA interference. Nature 456:750-754. [DOI] [PubMed] [Google Scholar]
- 78.Prucca, C. G., and H. D. Lujan. 2009. Antigenic variation in Giardia lamblia. Cell. Microbiol. 11:1706-1715. [DOI] [PubMed] [Google Scholar]
- 79.Riggs, M. W. 2002. Recent advances in cryptosporidiosis: the immune response. Microbes Infect. 4:1067-1080. [DOI] [PubMed] [Google Scholar]
- 80.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 81.Savioli, L., H. Smith, and A. Thompson. 2006. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 22:203-208. [DOI] [PubMed] [Google Scholar]
- 82.Smith, H. V., S. M. Caccio, N. Cook, R. A. B. Nichols, and A. Tait. 2007. Cryptosporidium and Giardia as foodborne zoonoses. Vet. Parasitol. 149:29-40. [DOI] [PubMed] [Google Scholar]
- 83.Smith, P. D., F. D. Gillin, W. R. Brown, and T. E. Nash. 1981. IgG antibody to Giardia lamblia detected by enzyme-linked immunosorbent assay. Gastroenterology 80:1476-1480. [PubMed] [Google Scholar]
- 84.Soliman, M. M., R. Taghi-Kilani, A. F. A. Abou-Shady, A. A. El-Mageid, A. A. Handousa, M. M. Hegazi, and M. Belosevic. 1998. Comparison of serum antibody responses to Giardia lamblia of symptomatic and asymptomatic patients. Am. J. Trop. Med. Hyg. 58:232-239. [DOI] [PubMed] [Google Scholar]
- 85.Stager, S., R. Felleisen, B. Gottstein, and N. Muller. 1997. Giardia lamblia variant surface protein H7 stimulates a heterogeneous repertoire of antibodies displaying differential cytological effects on the parasite. Mol. Biochem. Parasitol. 85:113-124. [DOI] [PubMed] [Google Scholar]
- 86.Strong, W. B., J. Gut, and R. G. Nelson. 2000. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton surface antigen products. Infect. Immun. 68:4117-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sturbaum, G. D., B. H. Jost, and C. R. Sterling. 2003. Nucleotide changes within three Cryptosporidium parvum surface protein encoding genes differentiate genotype I from genotype II isolates. Mol. Biochem. Parasitol. 128:87-90. [DOI] [PubMed] [Google Scholar]
- 88.Sullivan, P. B., G. Neale, A. M. Cevallos, and M. J. G. Farthing. 1991. Evaluation of specific serum anti-Giardia IgM antibody response in diagnosis of giardiasis in children. Trans. R. Soc. Trop. Med. Hyg. 85:748-749. [DOI] [PubMed] [Google Scholar]
- 89.Thompson, R. C. A., R. M. Hopkins, and W. L. Homan. 2000. Nomenclature and genetic groupings of Giardia infecting mammals. Parasitol. Today 16:210-213. [DOI] [PubMed] [Google Scholar]
- 90.Tsang, V. C. W., and P. P. Wilkins. 1991. Optimum dissociating condition for immunoaffinity and preferential isolation of antibodies with high specific activity. J. Immunol. Methods 138:291-299. [DOI] [PubMed] [Google Scholar]
- 91.Waterboer, T., P. Sehr, and M. Pawlita. 2006. Suppression of non-specific binding in serological Luminex assays. J. Immunol. Methods 309:200-204. [DOI] [PubMed] [Google Scholar]
- 92.Weber, R., R. T. Bryan, H. S. Bishop, S. P. Wahlquist, J. J. Sullivan, and D. D. Juranek. 1991. Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J. Clin. Microbiol. 29:1323-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weiland, M. E.-L., J. E. D. Palm, W. J. Griffiths, J. M. McCaffery, and S. G. Svard. 2003. Characterization of alpha-1 giardin: an immunodominant Giardia lamblia annexin with glycosaminoglycan-binding activity. Int. J. Parasitol. 33:1341-1351. [DOI] [PubMed] [Google Scholar]
- 94.Zweig, M. H., and G. Campbell. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561-577. [PubMed] [Google Scholar]