Abstract
All reported cases of WA1 babesiosis have occurred in the Pacific coast region of the United States, suggesting that WA1 is limited to this geographic area. However, we detected WA1 IgG in 27% of clinical sera sent to our laboratory for WA1 IgG testing from across the United States over a 2-year period, suggesting that exposure to WA1 or a closely related organism occurs outside Pacific coast states. We sought to determine if this high WA1 IgG detection rate among clinical specimens merely reflects WA1 seroprevalence outside the Pacific region. WA1 IgG, as well as Babesia microti IgG, was measured in 900 blood donor specimens from 9 states. Overall seroprevalence was 2.0% for WA1 and 0.4% for B. microti; regional seroprevalences ranged from 0 to 4% and 0 to 2%, respectively. Additional studies were performed to determine if WA1 IgG reactivity was attributable to polyclonal B-cell activation associated with acute Epstein-Barr virus (EBV) infection; 40 WA1 IgG-positive clinical sera and the 18 WA1 IgG-positive blood donor specimens were all negative for EBV capsid antigen (EBVCA) IgM (a marker of acute EBV infection), and 40 EBVCA IgM-positive sera were all negative for WA1 IgG. These findings indicate that the high WA1 IgG detection rate among clinical specimens does not simply reflect the national WA1 seroprevalence among blood donors or nonspecific reactivity due to acute EBV infection. Rather, the findings suggest that infection with WA1 or a related organism is more common than indicated by the literature and is not limited to Pacific coast states.
The babesial piroplasm WA1 was first described in 1991 following its isolation from a Washington state resident with fever, chills, and myalgia (15). Morphological and ultrastructural studies revealed that WA1 is very similar to the well-characterized piroplasm Babesia microti; phylogenetic analysis, however, placed WA1 in a clade separate from B. microti and other Babesia species. Based on these findings, the recommended taxonomic designation for WA1 is Babesia duncani (1). Like other piroplasms, WA1 is transmitted to humans via a tick bite, but the animal reservoir has not been identified (8).
An additional 8 cases of WA1 infection have been described in the medical literature (4, 9, 13), all in males residing in Washington or California; two of these cases reflected transmission by transfusion of infected blood products from an asymptomatic donor. In serologic investigations, titers of WA1 IgG were markedly elevated (≥1:5,120) in convalescent-phase sera from all patients and in sera from the asymptomatic blood donors implicated in transfusion-transmitted cases (4, 9, 13). Consistent with the phylogenetic data, antibodies induced by WA1 infection did not cross-react with B. microti. Small seroprevalence studies conducted in California and Washington yielded wide variation in WA1 IgG detection rates, ranging from 1% to 20% (2, 4, 13, 15).
In response to the documented emergence of WA1 as a disease-causing agent, in 1997 our laboratory (Focus Diagnostics, Inc., Cypress, CA) developed an indirect immunofluorescence assay (IFA) for measuring WA1 IgG in clinical serum specimens (not for donor testing). During the intervening years, we have been surprised to observe that sera submitted for WA1 IgG testing arrive from diverse geographic areas of the United States (mostly from outside the Pacific region), and roughly a quarter of all specimens submitted for WA1 IgG are positive. One possible explanation for these findings is that WA1, or a closely related organism, is widely distributed throughout the United States and the unexpectedly high WA1 IgG detection rate among clinical specimens merely reflects the prevalence of WA1 IgG among the U.S. population residing outside Pacific coast states. We thus estimated the WA1 seroprevalence in the United States by measuring WA1 IgG in serum or plasma specimens from blood donors residing in different areas of the United States and compared it to our WA1 IgG results for patient samples submitted during a 2-year period (2008-2009). As an additional point of comparison, we also measured Babesia microti IgG, which is characterized by a detection rate of ≤1% among blood donors from diverse geographic areas of the United States (5, 6, 10, 11).
Another possible cause of the high rate of WA1 IgG detection among clinical specimens is nonspecific reactivity reflective of the polyclonal B-cell stimulation associated with acute Epstein-Barr virus (EBV) infection, as has been described for other IFAs measuring antibodies to parasites (7). We thus measured EBV capsid antigen (EBVCA) IgM, a marker of acute EBV infection (12), in selected WA1 IgG-positive samples, as well as WA1 IgG titers in EBVCA IgM-positive samples.
MATERIALS AND METHODS
Patient specimens.
Patient sera for WA1 IgG or B. microti IgG testing were submitted to Focus Diagnostics by national and regional laboratories located in diverse geographic areas of the United States. Most sera were accompanied by patient age and gender information, but no clinical data were provided for any of the specimens.
Blood donor specimen panels.
In response to our request for approximately 200 blood donor specimens from diverse geographic areas of the United States, Blood Systems, Inc., supplied a total of 1,000 serum specimens from 5 states (northern California, Arizona, North Dakota, southeastern Texas, and Alabama), and American Red Cross Blood Services supplied a total of 810 plasma specimens from 4 states (Oregon, Kansas, Maryland, and Massachusetts). All specimens were collected during the first quarter of 2009 and were shipped frozen to our facility. Donors supplying these specimens were stratified into 6 groups based on gender and age (females <30 years old, males <30 years old, females 30 to 50 years old, males 30 to 50 years old, females >50 years old, and males >50 years old); Table 1 shows the regional distribution of the 1,810 donors across these gender/age categories. Although the total numbers of donors per state were very similar (199 to 210), the number of donors within a given gender/age category showed wide regional variation; for example, the number of females 30 to 50 years old ranged from 8 of 200 (4%) for Texas to 46 of 210 (22%) for Oregon, and the number of males >50 years old ranged from 16 of 200 (8%) for Alabama to 58 of 199 (29%) for Massachusetts. Thus, rather than test all specimens from each state, we elected to build a 100-member specimen panel for each state that included specimens selected to produce, as closely as possible, the same donor gender/age distribution for each state. As a specimen selection guide, we used the proportions of all donors within the 6 gender/age categories (Table 1). For example, based on the finding that 15% of all donors were females <30 years of age, we targeted 15 specimens from females <30 years old from each state for inclusion in that state's 100-member panel. Table 2 shows the complete set of targeted specimen numbers and the actual numbers selected per state. Only two major deviations from the targeted numbers were noted; the Texas panel had fewer women and more men for the 30-to-50 age group, and the Alabama panel had more women and fewer men for the >50 age group.
TABLE 1.
Gender and age distribution of 1,810 blood donors from whom specimens were obtained
| State | No. of donors of age (yr): |
Total donors | |||||
|---|---|---|---|---|---|---|---|
| <30 |
30-50 |
>50 |
|||||
| Female | Male | Female | Male | Female | Male | ||
| Oregon | 20 | 15 | 46 | 36 | 42 | 51 | 210 |
| California | 26 | 28 | 22 | 40 | 39 | 45 | 200 |
| Arizona | 31 | 40 | 42 | 39 | 23 | 25 | 200 |
| North Dakota | 16 | 18 | 24 | 37 | 51 | 54 | 200 |
| Kansas | 16 | 14 | 38 | 41 | 38 | 53 | 200 |
| Texas | 61 | 65 | 8 | 26 | 18 | 22 | 200 |
| Alabama | 46 | 32 | 45 | 44 | 17 | 16 | 200 |
| Maryland | 25 | 36 | 39 | 47 | 20 | 34 | 201 |
| Massachusetts | 26 | 18 | 34 | 37 | 26 | 58 | 199 |
| Total (% of all donors) | 267 (15) | 266 (15) | 298 (16) | 347 (19) | 274 (15) | 358 (20) | 1,810 (100) |
TABLE 2.
Number of blood donor samples per gender/age category selected for analysis
| State | No. of samples for age group (yr) |
|||||
|---|---|---|---|---|---|---|
| <30 |
30-50 |
>50 |
||||
| Female | Male | Female | Male | Female | Male | |
| Oregon | 15 | 15 | 16 | 19 | 15 | 20 |
| California | 15 | 15 | 16 | 19 | 15 | 20 |
| Arizona | 15 | 15 | 16 | 19 | 15 | 20 |
| North Dakota | 15 | 15 | 16 | 19 | 15 | 20 |
| Kansas | 16 | 14 | 16 | 19 | 15 | 20 |
| Texas | 15 | 16 | 8 | 26 | 15 | 20 |
| Alabama | 15 | 16 | 16 | 20 | 17 | 16 |
| Maryland | 15 | 15 | 16 | 19 | 15 | 20 |
| Massachusetts | 15 | 15 | 16 | 19 | 15 | 20 |
| Targeta | 15 | 15 | 16 | 19 | 15 | 20 |
Targeted specimen numbers.
Serologic assays.
Patient and blood donors specimens were tested for WA1 IgG using an IFA developed and validated in-house. The assay utilized glass slides spotted with washed WA1-infected gerbil erythrocytes; after spotting, the slides were fixed with acetone and held in a −20°C freezer until use. Serum or plasma specimens diluted 1:256 (4) in phosphate-buffered saline (PBS) were added to slide wells and incubated for 30 min at 35 to 39°C; after washing, fluorescein-tagged goat anti-human IgG was added to each well and incubated for 30 min at 35 to 39°C. Following washing, the slides were dried and mounted, and fluorescence was assessed by microscopic evaluation. Specimens demonstrating 1+ or greater fluorescence were serially diluted and retested to determine the titer, defined as the highest dilution demonstrating 1+ fluorescence. Validation studies demonstrated that when using the CDC-recommended screening dilution of 1:256 (4), the WA1 IgG detection rate using our assay was 6% (3/50) among sera submitted for other testing from laboratories located in Washington, Oregon, and northern California and 2% (1/50) among sera from southern California blood donors; all positive sera had titers of 1:256 or 1:512. Serum from patient WA2 (4) was also evaluated as part of WA1 IgG validation studies and exhibited a titer of 1:8,192; this value was similar to the titers of 1:4,096 obtained using WA1 as a substrate and 1:16,384 obtained using WA2 as a substrate when the specimen was tested at the CDC (4).
Patient and donor specimens were tested for B. microti IgG using an in-house IFA that employed slides spotted with washed B. microti-infected hamster erythrocytes. The screening dilution was 1:64 (5, 6, 10, 11); in all other aspects, the assay was performed as described for WA1 IgG.
Selected WA1 IgG-positive specimens were tested for IgM recognizing EBVCA using an IFA kit (Focus Diagnostics, Cypress, CA) cleared by the Food and Drug Administration. Specimens were diluted 1:20 in IgG-precipitating buffer, and after 15 min, diluted specimens were added to slide wells and incubated for 90 min at 37°C. After washing, goat anti-human IgM was added to each well and incubated for 30 min at 37°C. Following washing, the slides were dried and mounted, and fluorescence was assessed by microscopic evaluation.
RESULTS
Patient serum results for 2008 and 2009.
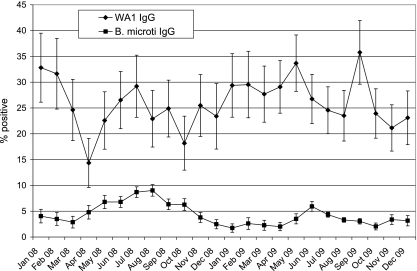
Fig. 1 presents the monthly positivity rate, with 95% confidence intervals, among sera submitted for WA1 IgG or B. microti IgG testing during 2008 and 2009. More than 100 specimens per month were submitted for each assay. The B. microti IgG detection rate showed seasonal variation, with higher values during summer months; in contrast, the WA1 IgG detection rate did not show clear-cut seasonal variation. The detection rate over the entire 2-year period was 5% for B. microti IgG and 27% for WA1 IgG. The geographic distribution of laboratories submitting B. microti IgG-positive sera by census region was 17% West, 2% Midwest, 12% South, and 69% Northeast; the geographic distribution of laboratories submitting WA1 IgG-positive sera was 1% West, 13% Midwest, 80% South, and 6% Northeast.
FIG. 1.
Rates of positive results among clinical specimens submitted for WA1 IgG or B. microti IgG testing over a 2-year period (2008-2009). Horizontal bars connected by a vertical line represent the 95% confidence interval.
The gender and age distribution of patients positive for WA1 IgG or B. microti IgG in relation to titer is shown in Table 3. The majority (63%) of sera positive for WA1 IgG exhibited an endpoint titer equal to the CDC-recommended (4) screening dilution of 1:256; in contrast, sera positive for B. microti IgG were evenly distributed among the 3 titer groupings (1:64, 1:128, and >1:128). For WA1 IgG-positive sera, there was a clear female predominance within all 3 age groups; indeed, 2 of the 7 gender/age groups—females 30 to 50 and females >50—accounted for over half (54%) of all WA1 IgG-positive sera. Although there was a slight male predominance within all 3 age groupings for B. microti IgG-positive specimens, the large proportion of specimens from patients of unknown gender and age precluded more-detailed analysis.
TABLE 3.
Gender and age distribution of patients whose sera were positive for WA1 IgG or B. microti IgGa
| Patient gender, age (yr) | % positive specimens with indicated titer of: |
|||||||
|---|---|---|---|---|---|---|---|---|
| WA1 IgG |
B. microti IgG |
|||||||
| 1:256 | 1:512 | >1:512 | Total | 1:64 | 1:128 | >1:128 | Total | |
| Female, <30 | 5 | 1 | 1 | 8 | 1 | 1 | 1 | 3 |
| Male, <30 | 4 | 1 | 1 | 6 | 2 | 1 | 1 | 4 |
| Female, 30-50 | 17 | 6 | 3 | 25 | 4 | 3 | 3 | 9 |
| Male, 30-50 | 6 | 3 | 3 | 12 | 3 | 3 | 4 | 11 |
| Female, >50 | 20 | 5 | 4 | 29 | 5 | 5 | 8 | 18 |
| Male, >50 | 10 | 4 | 4 | 18 | 5 | 6 | 11 | 22 |
| Gender/age unknown | 1 | 0 | 0 | 1 | 12 | 11 | 10 | 33 |
| Total | 63 | 20 | 17 | 100 | 32 | 30 | 39 | 100 |
Data are percentages of total specimens positive for WA1 IgG or B. microti IgG, respectively.
WA1 IgG and B. microti IgG detection rates among blood donor sera.
Table 4 presents the detection rate for WA1 IgG and B. microti IgG among the selected 100 blood donor specimens from each of the 9 states. Only one specimen among the 900 evaluated was positive for both analytes. B. microti IgG was detected in 4 of 900 (0.4%) samples; the regional detection rate ranged from 0% to 2%. All 4 samples positive for B. microti IgG had titers of 1:64; 2 samples were from female donors >50 years old, one sample was from a female donor <30 years old, and the dual-positive donor was a 43-year-old male.
TABLE 4.
Detection of WA1 IgG and B. microti IgG among blood donor specimensa
| State | No. of specimens with result |
|||
|---|---|---|---|---|
| B. microti IgG−, WA1 IgG− | B. microti IgG+, WA1 IgG− | B. microti IgG−, WA1 IgG+ | B. microti IgG+, WA1 IgG+ | |
| Oregon | 96 | 2 | 2 | 0 |
| California | 97 | 0 | 3 | 0 |
| Arizona | 100 | 0 | 0 | 0 |
| North Dakota | 99 | 0 | 1 | 0 |
| Kansas | 96 | 0 | 3 | 1 |
| Texas | 96 | 0 | 4 | 0 |
| Alabama | 99 | 0 | 1 | 0 |
| Maryland | 98 | 0 | 2 | 0 |
| Massachusetts | 98 | 1 | 1 | 0 |
| Total | 879 | 3 | 17 | 1 |
n = 100 for each state.
WA1 IgG was detected in 18 of 900 (2.0%) samples (Table 4); the regional detection rate ranged from 0% to 4%, with 8 of the 9 states characterized by a detection rate of at least 1%. None of the 18 WA1 IgG-positive sera had titers of >1:512; 7 of 18 (39%) positive specimens were from women, and 10 of 18 (56%) were from persons >50 years old.
Assessment of possible nonspecific reactivity associated with EBV infection.
Based on a published report (7) that sera from patients with acute EBV infection exhibit nonspecific reactivity in parasitic antibody IFAs, we tested 40 WA1 IgG-positive clinical sera (25 with titers of 1:256, 12 with titers of 1:512, and 3 with titers of ≥1:1,024) and the 18 WA1 IgG-positive blood donor specimens for EBVCA IgM. Similarly, we tested 40 EBVCA IgM-positive sera for WA1 IgG. All 58 WA1 IgG-positive specimens were negative for EBVCA IgM (i.e., titers < 1:20), and all 40 EBVCA IgM-positive sera were negative for WA1 IgG (titers < 1:256). Thus, WA1 IgG reactivity did not appear to reflect acute EBV infection.
DISCUSSION
Our finding of increased WA1 IgG titers in 27% of clinical specimens submitted from diverse geographic areas suggests that infection with WA1, or a closely related organism, occurs throughout the United States. It is possible that this high rate of WA1 IgG detection among clinical specimens merely reflects the prevalence of WA1 IgG in the general U.S. population; although small studies have assessed WA1 seroprevalence in Pacific coast states (3, 10, 12), similar studies have not been performed in other geographic areas. As a first step in testing this hypothesis, we measured WA1 IgG in serum or plasma specimens from blood donors residing in 9 states located in different geographic areas of the United States. The results provide no support for the stated hypothesis; WA1 IgG was detected in only 2% of blood donor specimens, with similar detection rates (0 to 4%) in all geographic regions.
The WA1 seroprevalence rates we found among blood donors are consistent with the rates of 1 to 5% reported for Washington and northern California residents by 3 different groups of investigators (4, 13, 15). In contrast, a fourth group (2) reported much higher WA1 IgG detection rates of 18% and 20% in two different donor groups. Similar to our approach, all investigators defined WA1 IgG detection as a serum titer of either ≥1:256 or ≥1:320. It remains unclear what factors contributed to the higher seroprevalence values in one of the four published studies.
As a point of comparison, we evaluated the monthly B. microti IgG detection rate among patient sera submitted for this test and also measured B. microti IgG in the panel of 900 blood donor specimens. As expected, B. microti IgG detection among patient specimens showed a seasonal peak during the Northern Hemisphere summer months (14) in both years covered by the evaluation. Among the 900-member blood donor panel, only 0.4% of specimens were positive for B. microti IgG, consistent with published reports of B. microti seroprevalence rates of ≤1% (5, 6, 10, 11).
There are a number of limitations to our study. First, we cannot assume that the geographic location of the laboratory submitting a given WA1 IgG-positive clinical specimen is the same geographic region where the patient resides. At least 2 laboratories in the Western region, 2 laboratories in the South, and one laboratory in the Northeast are large reference laboratories with a national client base. However, the possibility that all the WA1 IgG-positive clinical specimens we identified reflect tick exposure or transfusion in Pacific coast states appears highly unlikely.
A second limitation is that the very small number of WA1 IgG-positive sera obtained from documented cases of WA1 infection precludes the accurate determination of sensitivity, specificity, positive predictive value, and negative predictive value for the WA1 IgG IFA. The low WA1 IgG detection rate we observed among blood donor specimens suggests good assay specificity; however, blood donors are presumed to be afebrile, whereas patients whose sera are submitted for WA1 IgG testing are presumed to be febrile following tick exposure. A thorough assessment of WA1 IgG reactivity in sera from patients with fever caused by other infections is thus required to define the specificity of the WA1 IgG assay. As a first step in the process, we measured WA1 IgG in sera positive for EBVCA IgM and found that all were negative for WA1 IgG. We also measured EBVCA IgM in WA1 IgG-positive specimens from patients and blood donors and similarly found that all were negative for EBVCA IgM. In addition to supporting the hypothesis that the WA1 IgG IFA exhibits good specificity, these data indicate that the previously documented (7) nonspecific reactivity in parasitic antibody IFAs linked to EBV-induced polyclonal B-cell activation does not characterize the WA1 IgG IFA. Additional studies utilizing sera from patients with other febrile illnesses are needed for a complete assessment of the specificity of the WA1 IgG IFA.
A third limitation is the lack of clinical information for patients whose clinical specimens were positive for WA1 IgG; no information was available regarding travel history, transfusion history, symptoms, or tick exposure status. Without such data, it is difficult to interpret some of our findings. For example, the female predominance among WA1 IgG-positive patients stands in stark contrast to the observation that all 9 documented WA1 cases occurred in males (4, 9, 13, 15) and to the male predominance observed for tick-borne infections in general (3, 16, 17, 18). Similarly, the lack of a seasonal increase in WA1 IgG detection during North American summer months is atypical for a tick-borne infection (14). Finally, the lack of clinical data precludes establishment of a link between our evidence of exposure to WA1 or a related organism and clinical disease. A thorough clinical study is thus needed to determine the organism triggering the production of WA1-reactive IgG, where and how the exposure occurred, and possible links between WA1 IgG detection and the symptoms prompting these patients to seek medical attention.
Acknowledgments
We thank Marianna Wilson from the CDC Parasitic Serology Laboratory for the generous gift of serum from patient WA2, Michael Busch and Valerie Winkelman from Blood Services Inc. and Susan Stramer from American Red Cross Blood Services for supplying blood donor serum and plasma specimens, and Jay Lieberman for critical review of the manuscript.
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Conrad, P. A., A. M. Kjemtrup, R. A. Carreno, J. Thomford, K. Wainwright, M. Eberhard, R. Quick, S. R. Telford, and B. L. Herwaldt. 2006. Description of Babesia duncani n. sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int. J. Parasitol. 36:779-789. [DOI] [PubMed] [Google Scholar]
- 2.Fritz, C. L., A. M. Kjemtrup, P. A. Conrad, G. R. Flores, G. L. Campbell, M. E. Schriefer, D. Gallo, and D. J. Vugia. 1997. Seroepidemiology of emerging tickborne infectious diseases in a northern California community. J. Infect. Dis. 175:1432-1439. [DOI] [PubMed] [Google Scholar]
- 3.Gardner, S. L., R. C. Holman, J. W. Krebs, R. Berkelman, and J. E. Childs. 2003. National surveillance for the human ehrlichiosis in the United States, 1997-2001, and proposed methods for evaluation of data quality. Ann. N. Y. Acad. Sci. 990:80-89. [DOI] [PubMed] [Google Scholar]
- 4.Herwaldt, B. L., A. M. Kjemtrup, P. A. Conrad, R. C. Barnes, M. Wilson, M. G. McCarthy, M. H. Sayers, and M. L. Eberhard. 1997. Transfusion-transmitted babesiosis in Washington state: first reported case caused by a WA1-type parasite. J. Infect. Dis. 175:1259-1262. [DOI] [PubMed] [Google Scholar]
- 5.Hilton, E., J. DeVoti, J. L. Benach, M. L. Halluska, D. J. White, H. Paxton, and S. Dumler. 1999. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the Northeast United States. Am. J. Med. 106:404-409. [DOI] [PubMed] [Google Scholar]
- 6.Johnson, S. T., R. G. Cable, L. Tonnetti, B. Spencer, J. Rios, and D. A. Leiby. 2009. Seroprevalence of Babesia microti in blood donors from Babesia-endemic areas of the northeastern United States: 2000 through 2007. Transfusion 49:2574-2582. [DOI] [PubMed] [Google Scholar]
- 7.Kern, W., C. Kirsten, P. Forster, H. J. Diesfeld, and E. Vanek. 1987. Specificity of routine parasite serological tests in autoimmune disorders, neoplastic disease, EBV-infected mononucleosis, and HIV infection. Klin. Wochenschr. 65:898-905. [DOI] [PubMed] [Google Scholar]
- 8.Kjemtrup, A. M., and P. A. Conrad. 2000. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30:1323-1337. [DOI] [PubMed] [Google Scholar]
- 9.Kjemtrup, A. M., B. Lee, C. L. Fritz, C. Evans, M. Chervanak, and P. A. Conrad. 2002. Investigation of transfusion transmission of a WA1-type babesial parasite to a premature infant in California. Transfusion 42:1482-1487. [DOI] [PubMed] [Google Scholar]
- 10.Leiby, D. A., A. P. S. Chung, R. G. Cable, J. Trouern-Trend, J. McCullough, M. J. Homer, L. D. Reynolds, R. L. Houghton, M. J. Lodes, and D. H. Persing. 2002. Relationship between tick bites and the seroprevalence of Babesia microti and Anaplasma phagocytophila (previously Ehrlichia sp) in blood donors. Transfusion 42:1585-1591. [DOI] [PubMed] [Google Scholar]
- 11.Leiby, D. A., A. P. S. Chung, J. E. Gill, R. L. Houghton, D. H. Persing, S. Badon, and R. G. Cable. 2005. Demonstrable parasitemia among Connecticut blood donors with antibodies to Babesia microti. Transfusion 45:1804-1810. [DOI] [PubMed] [Google Scholar]
- 12.Okano, M., G. M. Thiele, J. R. Davis, H. L. Grierson, and D. T. Purtilo. 1988. Epstein-Barr virus and human diseases: recent advances in diagnosis. Clin. Microbiol. Rev. 1:300-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persing, D. H., B. L. Herwaldt, C. Glaser, R. S. Lane, J. W. Thomford, D. Mathiesen, P. J. Krause, D. F. Phillip, and P. A. Conrad. 1995. Infection with a Bebesia-like organism in northern California. N. Engl. J. Med. 332:298-303. [DOI] [PubMed] [Google Scholar]
- 14.Piesman, J., T. N. Mather, G. J. Dammin, S. R. Telford, C. C. Lastavica, and A. Spielman. 1987. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am. J. Epidemiol. 126:1187-1189. [DOI] [PubMed] [Google Scholar]
- 15.Quick, R. E., B. L. Herwaldt, J. W. Thomford, M. E. Garnett, M. L. Eberhard, M. Wilson, D. H. Spach, J. W. Dickerson, S. R. Telford, K. R. Steingart, R. Pollack, D. H. Persing, J. M. Kobayashi, D. D. Juranek, and P. A. Conrad. 1993. Babesiosis in Washington state: a new species of Babesia? Ann. Int. Med. 119:284-290. [DOI] [PubMed] [Google Scholar]
- 16.Reimann, C. A., E. B. Hayes, C. DiGuiseppi, R. Hoffman, J. A. Lehman, N. P. Lindsey, G. L. Campbell, and M. Fischer. 2008. Epidemiology of neuroinvasive arboviral disease in the United States, 1999-2007. Am. J. Trop. Med. Hyg. 79:974-979. [PubMed] [Google Scholar]
- 17.Shapiro, E. D. 2008. Lyme disease. Adv. Exp. Med. Biol. 609:185-195. [DOI] [PubMed] [Google Scholar]
- 18.Treadwell, T. A., R. C. Holman, M. J. Clarke, J. W. Krebs, C. D. Paddock, and J. E. Childs. 2000. Rocky mountain spotted fever in the United States, 1993-1996. Am. J. Trop. Med. Hyg. 63:21-26. [DOI] [PubMed] [Google Scholar]