Abstract
Tuberculosis (TB) remains a major cause of illness and death worldwide, making a new TB vaccine an urgent public health priority. Purified protein derivative (PPD)-negative adults (n = 50) were equally randomized to receive 3 doses at 1-month intervals (at 0, 1, and 2 months) of one of the following vaccines: Mtb72F/AS02A (10 or 40 μg antigen), Mtb72F/saline (10 or 40 μg antigen), or AS02A. Mtb72F/AS02A recipients received an additional dose 1 year after the first dose to evaluate if the elicited immune response could be boosted. Mtb72F/AS02A vaccines were locally reactogenic but clinically well tolerated, with transient adverse events (usually lasting between 1 and 4 days) that resolved without sequelae being observed. No vaccine-related serious adverse events were reported. Vaccination with Mtb72F/AS02A induced a strong Mtb72F-specific humoral response and a robust Mtb72F-specific CD4+ T-cell response, both of which persisted at 9 months after primary immunization and for 1 year after the booster immunization. There was no significant difference between the magnitude of the CD4+ T-cell response induced by the 10-μg and 40-μg Mtb72F/AS02A vaccines. The Mtb72F-specific CD4+ T cells predominantly expressed CD40L; CD40L and interleukin-2 (IL-2); CD40L and tumor necrosis factor alpha (TNF-α); CD40L, IL-2, and TNF-α; and CD40L, IL-2, TNF-α, and gamma interferon (IFN-γ). Serum IFN-γ, but not TNF-α, was detected 1 day after doses 2 and 3 for the Mtb72F/AS02A vaccine but did not persist. Vaccine-induced CD8+ T-cell responses were not detected, and no immune responses were elicited with AS02A alone. In conclusion, Mtb72F/AS02A is clinically well tolerated and is highly immunogenic in TB-naïve adults. The 10- and 40-μg Mtb72F/AS02A vaccines show comparable safety and immunogenicity profiles.
Tuberculosis (TB) is a major cause of illness and death worldwide, causing approximately 1.7 million deaths a year (43). Despite global efforts to control or eradicate the disease, the WHO estimates that in 2008 an estimated 8.9 million to 9.9 million people became infected with Mycobacterium tuberculosis. The situation is compounded by the emergence of multidrug-resistant TB. Since one-third of the world's population is estimated to be latently infected with M. tuberculosis and at possible risk of disease, TB prevention remains one of today's greatest public health challenges. An efficacious vaccination strategy is an essential tool to control TB.
M. bovis bacillus Calmette-Guérin (BCG), consisting of attenuated strains of M. bovis, is the only TB vaccine currently available. It effectively prevents meningeal and miliary TB in young children (8) but appears to be ineffective in preventing adult-onset TB (protection rates, 0 to 80%) (10) and pulmonary TB in children.
GlaxoSmithKline (GSK) Biologicals' candidate TB vaccine antigen, Mtb72F, is composed of a fusion protein derived from two highly immunogenic M. tuberculosis antigens: Mtb39A (Rv0125 encoding PepA) and Mtb32A (Rv1196 encoding PPE18) (34, 35). Mtb72F, formulated with GSK Biologicals' proprietary AS02A adjuvant system, was shown to be well tolerated in animal models and protected against M. tuberculosis challenge in nonhuman primates, where Mtb72F/AS02A was shown to be capable of inducing long-term protection against tuberculosis, as determined by protection against severe disease and death and by other clinical and histopathological parameters (6, 30, 34, 39). A first-time-in-human study evaluated Mtb72F/AS02A (10 μg) in purified protein derivative (PPD)-negative TB-naïve, healthy adults in the United States given according to a 0-, 1-, and 2-month schedule and was found to be clinically well tolerated and highly immunogenic (42).
This study assessed whether a larger amount of the Mtb72F/AS02A vaccine antigen (40 μg) could improve the elicited immune response compared with the response elicited by the previously tested 10-μg antigen dose (42). In addition, a fourth vaccine dose was given to the vaccine recipients to evaluate whether the immune response could be boosted approximately 1 year after the primary vaccination course.
MATERIALS AND METHODS
Study design and ethics.
This phase I open, randomized, controlled trial (NCT00291889) was conducted between July 2004 and May 2006 at the Center for Vaccinology, Ghent University Hospital, Ghent, Belgium. The protocol was approved by the Ethics Committee of the Ghent University Hospital and was undertaken in accordance with the Declaration of Helsinki and good clinical practices. Written informed consent was obtained from all participants before they entered the study.
The participants were equally randomized to one of five treatment groups, according to allocation using a central randomization system on the Internet. The groups were Mtb72F/AS02A (10 or 40 μg antigen, investigational vaccines), Mtb72F/saline (10 or 40 μg antigen, active comparators), and AS02A alone (control). All groups received a primary vaccination course at 0, 1, and 2 months. Participants receiving Mtb72F/AS02A were given a booster dose 9 months after completion of the primary vaccination course, approximately 1 year after dose 1, and were followed for one additional year.
Study population.
Healthy adults aged 18 to 45 years were enrolled if they were seronegative for human immunodeficiency virus (HIV) and hepatitis C virus (HCV) antibodies and for hepatitis B surface antigen (HBsAg). Participants were excluded if they had a positive PPD skin test, an abnormal chest X ray, a history of BCG vaccination, documented exposure to M. tuberculosis, or potential contact with TB patients. Female participants who were pregnant or who were planning to become pregnant or to discontinue contraceptive precautions were also excluded.
Study vaccines.
Mtb72F was manufactured at Corixa Corporation (now GSK Biologicals). Physiological saline and AS02A were manufactured at GSK Biologicals, Rixensart, Belgium. AS02A is a proprietary oil-in-water emulsion with two immunostimulants, monophosphoryl lipid A (MPL) and Quillaja saponaria fraction 21 (QS21) (14). The vaccines were reconstituted by adding saline or AS02A to the Mtb72F lyophilized pellet. After gentle shaking of the mixture, a 0.5-ml dose was administered by intramuscular injection into the deltoid muscle of the nondominant arm. Each 0.5-ml dose of Mtb72F/AS02A and AS02A contained approximately 50 μg MPL and 50 μg QS21.
Safety and reactogenicity evaluation.
Solicited local and general adverse events (AEs) were recorded on diary cards during a 7-day (including the day of vaccination) follow-up period after each vaccination and were checked by a study physician at scheduled visits. Local AEs recorded were injection-site pain, swelling, and redness, as measured by the longest surface diameter. Solicited general AEs were fatigue, fever (axillary temperature ≥ 37.5°C), gastrointestinal symptoms (nausea, diarrhea, vomiting, abdominal pain), and headache. Unsolicited AEs were recorded over a 30-day follow-up period after each vaccination, and serious AEs (SAEs) were collected throughout the study.
The intensities of the AEs were scored, and grade 3 AEs were defined as AEs preventing normal daily activity, a temperature of >39.5°C, and swelling or redness of >50 mm. All local AEs were considered to be related to vaccination. The relationship of all other AEs to vaccination was determined by the investigator on the basis of best clinical judgment.
Blood hematology and biochemistry parameters (complete blood count, renal and liver function tests) were measured before and 1 week and 1 month after each vaccine dose, 6 months after dose 3, and 1 year after the booster.
Immunogenicity evaluation.
The cell-mediated immune (CMI) response was assessed at specific time points by measuring (i) cytokines in serum by enzyme-linked immunosorbent assay (ELISA) and (ii) immune markers on isolated peripheral blood mononuclear cells (PBMCs) by an intracellular cytokine staining (ICS) assay. Anti-Mtb72F antibody concentrations were measured using ELISA at specific time points.
Intracellular cytokine staining.
Mtb72F-specific CD4+ and CD8+ T cells were detected using ICS and flow cytometry, based on a previously described method (24, 25, 42). Briefly, whole-blood samples were collected and PBMCs were isolated by standard Ficoll-Isopaque density gradient centrifugation and cryopreserved in liquid nitrogen. After they were thawed, PBMCs were stimulated in vitro using individual Mtb32A and Mtb39A 15-mer peptide pools (1.25 μg/ml) of each peptide, covering the entire sequence of the Mtb72F antigen, in the presence of anti-CD28 and anti-CD49d antibodies (BD Biosciences, Belgium). After 2 h of incubation at 37°C, brefeldin A (BD Biosciences) was added, followed by an overnight incubation. Cells were stained using peridinin chlorophyll protein (PerCP)-conjugated anti-CD4 and allophycocyanin (APC) H7-conjugated anti-CD8 antibodies (BD Biosciences) and were fixed with a Cytofix/Cytoperm buffer kit (Pharmingen). The fixed cells were permeabilized and stained with the following fluorochrome-conjugated monoclonal antibodies from Pharmingen: APC-conjugated anti-interleukin-2 (anti-IL-2), fluorescein isothiocyanate (FITC)-conjugated anti-gamma interferon (anti-IFN-γ), phycoerythrin (PE) Cy7-conjugated anti-tumor necrosis factor alpha (anti-TNF-α), and PE-conjugated anti-CD154 (CD40L). The cells were then washed, resuspended in fetal calf serum-containing phosphate-buffered saline, and analyzed on a BD LSR II flow cytometer. The data were analyzed using Diva software (BD Biosciences). Specific time points were then selected for further analyses of the data with FlowJo software (Tree Star Inc.).
The in vitro CD4+/CD8+ T-cell responses to the Mtb72F antigen were plotted as the sum of the background-subtracted individual Mtb32A and Mtb39A-specific T-cell responses. The correlation between the in vitro T-cell responses to a pool of peptides covering the entire Mtb72F antigen and the sum of the individual responses to Mtb32A and Mtb39A has been validated in-house (R2 = 0.9907).
Cytokines in serum.
Commercial ELISA kits were used to measure the levels of serum IFN-γ (assay cutoff, 1.0 pg/ml; Becton Dickinson) and TNF-α (assay cutoff, 0.12 pg/ml; R&D Systems Europe Ltd).
Anti-Mtb72F antibody response.
The Mtb72F-specific humoral response was determined by evaluating Mtb72F-specific IgG levels using a previously described ELISA (42) with a cutoff 1.0 ELISA units (EU)/ml. Anti-Mtb72F seropositivity is described as an antibody concentration of ≥1 EU/ml. Anti-Mtb72F antibody concentrations were represented using the geometric mean concentration (GMC) with 95% confidence intervals (CIs).
Intradermal PPD skin test.
At 1 to 3 weeks before the first vaccination and 1 month after completion of the primary vaccination course (day 86), an intradermal PPD skin test (2 tuberculin units/0.1 ml; tuberculin PPD RT 23 SSI; Statens Serum Institute) was performed on the dominant arm to assess the effect of vaccination on PPD status. One dose of the test (0.1 ml) contained 0.04 μg tuberculin PPD. The criterion for negative skin reactivity was 0 mm induration 48 to 72 h after PPD skin test administration.
Statistical methods.
All participants who received at least one vaccine dose were included in the demographic, reactogenicity, and safety analyses. The occurrences of solicited and unsolicited AEs are presented as point estimates with 95% CIs.
Immunogenicity analysis was performed on the according-to-protocol (ATP) data set, which included data for all participants who did not meet any elimination criteria during the study and for whom immunogenicity data were available.
Significant differences in the magnitude of Mtb72F-specific CD4+ T cells expressing at least 2 immune markers were calculated using the Wilcoxon rank-sum test.
RESULTS
Study population.
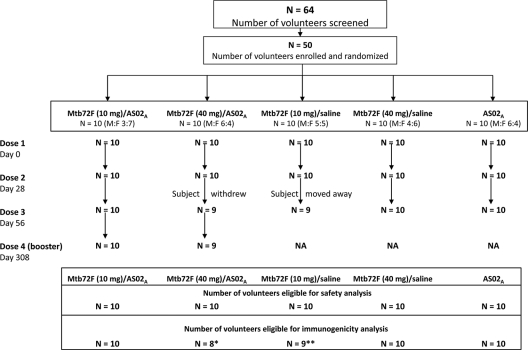
Figure 1 presents the participant flow. All participants were included in the safety analysis. Nineteen subjects returned for the booster dose. Two individuals in the Mtb72F/AS02A (40-μg) group were eliminated from the ATP analysis for immunogenicity: one withdrew from vaccination after dose 2 and the other was eliminated for noncompliance with the blood sampling schedule after dose 3. One participant from the 10-μg Mtb72F/saline group was not included in the ATP analysis for immunogenicity because he moved away from the study area after dose 2. The demographics were comparable between all groups. The mean age was 28.7 years (standard deviation = 5.9 years), and all participants were white (Caucasian). The overall female-to-male ratio was 1.08 (26 females/24 males).
FIG. 1.
Participant flow. M:F, ratio of males to females; NA, not applicable. *, two subjects from the Mtb72F (40 μg)/AS02A group were eliminated from the ATP immunogenicity analysis: one subject withdrew from vaccination at dose 3, and the other subject was out of interval for blood samples; **, one subject from the Mtb72F (40 μg)/saline group was not included in the ATP immunogenicity analysis because he moved away from the study area after dose 2.
Safety and reactogenicity.
The Mtb72F/AS02A vaccine formulations were considered to be clinically well tolerated. Most AEs reported were transient and resolved within 1 to 4 days, grade 3 AEs resolved or decreased in intensity within 48 h postvaccination, and the incidence of AEs did not increase with subsequent immunizations. All AEs resolved without sequelae, and in addition, no SAEs were judged by the investigator to be related to vaccination.
Injection-site pain was the most frequently reported AE in all groups (Table 1) after primary vaccination, with the incidence being comparable between the Mtb72F/AS02A and AS02A groups (89.7 to 100%, with overlapping CIs). There was a tendency for a lower frequency of reporting of local AEs in the Mtb72F/saline groups. The most frequently reported local grade 3 AE was injection-site swelling, reported only in the Mtb72F/AS02A groups (26.7% and 10.3% for the 10-μg and 40-μg groups, respectively) and the AS02A alone group (46.7%). There was a trend toward a lower frequency of reporting of local grade 3 AEs with the Mtb72F/AS02A (40-μg) vaccine than with the Mtb72F/AS02A (10-μg) vaccine and AS02A. No grade 3 local AEs were reported in the Mtb72F/saline groups. All solicited local AEs resolved within 5 days of vaccination.
TABLE 1.
Incidence of solicited adverse events reported during the 7-day follow-up period after all vaccinations of the primary vaccination phase
| AE | % AEs reported (95% CI) for each vaccine group |
||||
|---|---|---|---|---|---|
| Mtb72F (10 μg)/AS02A (na = 30) | Mtb72F (40 μg)/AS02A (n = 29) | Mtb72F (10 μg)/saline (n = 29) | Mtb72F (40 μg)/saline (n = 30) | AS02A (n = 30) | |
| Local AEs | |||||
| Pain | |||||
| All | 90.0 (73.5-97.9) | 89.7 (72.6-97.8) | 17.2 (5.8-35.8) | 30.0 (14.7-49.4) | 100.0 (88.4-100.0) |
| Grade 3 | 10.0 (2.1-26.5) | 3.4 (0.1-17.8) | 0.0 (0.0-11.9) | 0.0 (0.0-11.6) | 20.0 (7.7-38.6) |
| Redness | |||||
| All | 36.7 (19.9-56.1) | 6.9 (0.8-22.8) | 0.0 (0.1-17.2) | 3.3 (0.0-11.9) | 23.3 (9.9-42.3) |
| Grade 3 | 16.7 (5.6-34.7) | 0.0 (0.0-11.9) | 0.0 (0.0-11.9) | 0.0 (0.0-11.6) | 16.7 (5.6-34.7) |
| Swelling | |||||
| All | 53.3 (34.3-71.7) | 31.0 (15.3-50.8) | 3.4 (0.8-22.1) | 6.7 (0.1-17.8) | 60.0 (40.6-77.3) |
| Grade 3 | 26.7 (12.3-45.9) | 10.3 (2.2-27.4) | 0.0 (0.0-11.9) | 0.0 (0.0-11.6) | 46.7 (28.3-65.7) |
| General AEs | |||||
| Fatigue | |||||
| All | 50.0 (31.3-68.7) | 51.7 (32.5-70.6) | 37.9 (20.7-57.7) | 26.7 (12.3-45.9) | 20.0 (7.7-38.6) |
| Grade 3 | 10.0 (2.1-26.5) | 6.9 (0.8-22.8) | 0.0 (0.0-11.9) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) |
| Feverb | |||||
| All | 13.3 (3.8-30.7) | 10.3 (2.2-27.4) | 3.4 (0.1-17.8) | 0.0 (0.0-11.6) | 3.3 (0.1-17.2) |
| Grade 3 | 0.0 (0.0-11.6) | 0.0 (0.0-11.9) | 0.0 (0.0-11.9) | 0.0 (0.0-11.6) | 0.0 (0.0-11.6) |
| Gastrointestinalc | |||||
| All | 16.7 (5.6-34.7) | 17.2 (5.8-35.8) | 20.7 (8.0-39.7) | 16.7 (5.6-34.7) | 20.0 (7.7-38.6) |
| Grade 3 | 0.0 (0.0-11.6) | 6.9 (0.8-22.8) | 3.4 (0.1-17.8) | 0.0 (0.0-11.6) | 3.3 (0.1-17.2) |
| Headache | |||||
| All | 33.3 (17.3-52.8) | 34.5 (17.9-54.3) | 20.7 (8.0-39.7) | 26.7 (12.3-45.9) | 36.7 (19.9-56.1) |
| Grade 3 | 0.0 (0.0-11.6) | 0.0 (0.0-11.9) | 0.0 (0.0-11.9) | 3.3 (0.1-17.2) | 0.0 (0.0-11.6) |
n, total number of doses administered.
Fever was defined as an axillary temperature of ≥37.5°C (99.5°F).
Gastrointestinal AEs were nausea, vomiting, diarrhea, and/or abdominal pain.
The most commonly reported solicited general AEs after primary vaccination were fatigue and headache. The incidence of fatigue was similar in the Mtb72F/AS02A groups (50.0 to 51.7%) and lower in the control/comparator groups (<38%). The incidence of headache was comparable in all groups and ranged from 20.7 to 36.7%. Solicited general grade 3 AEs were infrequent; no cases of grade 3 fever were reported.
The most frequently reported causally related unsolicited AEs after primary vaccination with Mtb72F/AS02A or AS02A were transient mild to moderate influenza-like illness (10.0 to 23.3%) and injection-site warmth (23.3 to 30.0%). Causally related unsolicited AEs were not frequently reported in the Mtb72F/saline groups (≤10.3%).
Following booster vaccination with the Mtb72F/AS02A vaccines, comparable frequencies of unsolicited AEs were observed, with transient mild to moderate influenza-like illness (15.0 to 20.0%) and injection-site warmth (26.0 to 30.0%) being the most frequently reported AEs. No increase in the overall frequency of AEs was observed, and the frequencies were comparable in both Mtb72F/AS02A vaccine groups.
No clinically relevant changes in the biochemistry or hematology parameters were noted throughout the study (results not shown).
Two SAEs were reported during the study period. One individual in the Mtb72F/AS02A (40 μg) group reported appendicitis, and one in the Mtb72F/AS02A (10 μg) group suffered a broken ankle. Both SAEs were judged to be not related to vaccination by the investigator.
Immunogenicity. (i) CD4+ and CD8+ T-cell responses by ICS and flow cytometry.
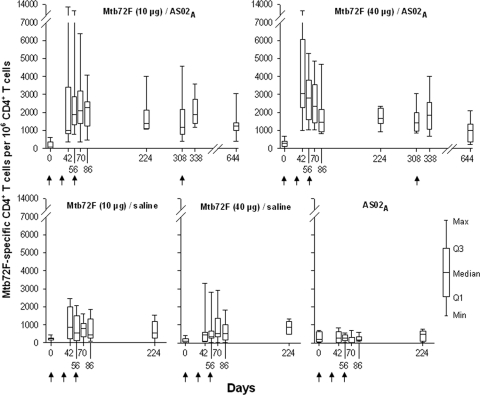
In order to evaluate the magnitude of the cell-mediated immune response elicited by the Mtb72F/AS02A vaccines, PBMCs were restimulated in vitro and analyzed by ICS and flow cytometry. A high frequency of Mtb72F-specific CD4+ T cells expressing two or more immune markers among the markers CD40L, IL-2, TNF-α, and IFN-γ was measured after 2 and 3 immunizations for 10 μg Mtb72F/AS02A (median frequencies, 0.19% and 0.23%, respectively) and 40 μg Mtb72F/AS02A (median frequencies, 0.28% and 0.15%, respectively) (Fig. 2). These responses were significantly higher than those for their respective saline comparators (Wilcoxon rank-sum test, P < 0.05 at 1 month after doses 2 and 3 for both the 40-μg and 10-μg vaccines).
FIG. 2.
Frequency of Mtb72F-specific CD4+ T cells expressing at least two immune markers among IFN-γ, IL-2, TNF-α, and CD40L. PBMCs from blood samples from all study groups were obtained at prevaccination (day 0), 2 weeks after dose 2 (day 42), 1 month after dose 2 (day 56), 2 weeks after dose 3 (day 70), 1 month after dose 3 (day 86), and 6 months after dose 3 (day 224). The Mtb72F/AS02A-vaccinated groups were followed up prebooster (day 308), 1 month postbooster (day 338), and 1 year postbooster (day 644). PBMCs were evaluated by ICS and flow cytometry after short-term in vitro stimulation with overlapping 15-mer peptide pools spanning the Mtb32A and Mtb39A proteins. Data are represented in box-and-whiskers plots as the number of Mtb72F-specific CD4+ T cells expressing at least two immune markers per 106 CD4+ T cells. Arrows indicate the time of vaccination.
The magnitude of the CD4+ T-cell response at 1 month after dose 2 (day 56) was not significantly different from the response at 1 month after dose 3 (day 86) for either the 10- or 40-μg Mtb72F/AS02A vaccines (Wilcoxon rank-sum test, P = 0.93 and P = 0.13, respectively).
Boosting by a fourth vaccine dose did not increase the magnitude of the CD4+ T-cell response (at 1 month postbooster, day 338) in either of the Mtb72F/AS02A vaccine groups beyond that observed after the second and third immunizations. However, these cellular responses persisted at 1 year postbooster (day 644).
The magnitude of the CD4+ T-cell responses induced by the 10-μg and 40-μg Mtb72F/AS02A vaccines were not significantly different from each other at 1 month after dose 2 (day 56; Wilcoxon rank-sum test, P = 0.47), 1 month after dose 3 (day 86; Wilcoxon rank-sum test, P = 0.74), 9 months after dose 3 (day 308; Wilcoxon rank-sum test, P = 0.64), and 1 year postbooster (day 644; Wilcoxon rank-sum test, P = 0.36).
No Mtb72F-specific CD4+ T-cell response was observed in the AS02A group.
No vaccine-induced Mtb72F-specific CD8+ T cells were detected (data not shown).
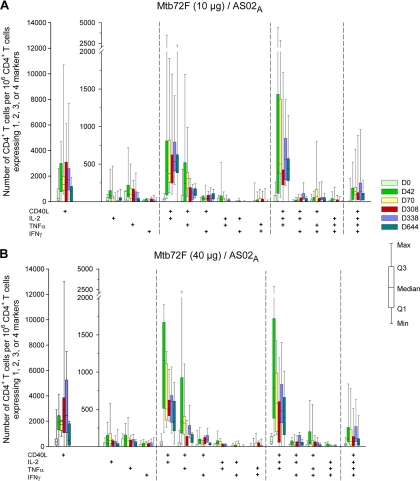
Functional characterization of the Mtb72F-specific CD4+ T cells showed that both Mtb72F/AS02A vaccines induced polyfunctional CD4+ T cells after vaccination, which persisted (Fig. 3A and B). The predominant phenotypes of the vaccine-induced Mtb72F-specific CD4+ T cells were CD40L+ IL-2+, CD40L+ TNF-α+, polyfunctional CD40L+ IL-2+ TNF-α+, and to a lesser extent, polyfunctional CD40L+ IL-2+ IFN-γ+ TNF-α+. Low frequencies of monofunctional CD4+ T cells expressing IL-2, TNF-α, or IFN-γ were also induced. High frequencies of antigen-specific CD4+ T cells expressing only the activation marker CD40L were elicited by Mtb72F/AS02A.
FIG. 3.
Polyfunctional profiles of Mtb72F-specific CD4+ T cells expressing any combination of immune markers among IFN-γ, IL-2, TNF-α, and CD40L. PBMCs from subjects vaccinated with 10 μg Mtb72F/AS02A (A) and 40 μg Mtb72F/AS02A (B) were obtained at prevaccination (day 0), 2 weeks after dose 2 (day 42), 2 weeks after dose 3 (day 70), prebooster (day 308), 1 month postbooster (day 338), and 1 year postbooster (day 644). PBMCs were evaluated using ICS and flow cytometry after short-term in vitro stimulation with 15-mer peptide pools spanning the Mtb32A and Mtb39A antigens. Data are represented in box-and-whiskers plots as the number of Mtb72F-specific CD4+ T cells expressing single markers and any combination of IFN-γ, IL-2, TNF-α, and/or CD40L per 106 CD4+ T cells.
(ii) Serum IFN-γ and TNF-α responses by ELISA induced by Mtb72F/AS02A vaccines.
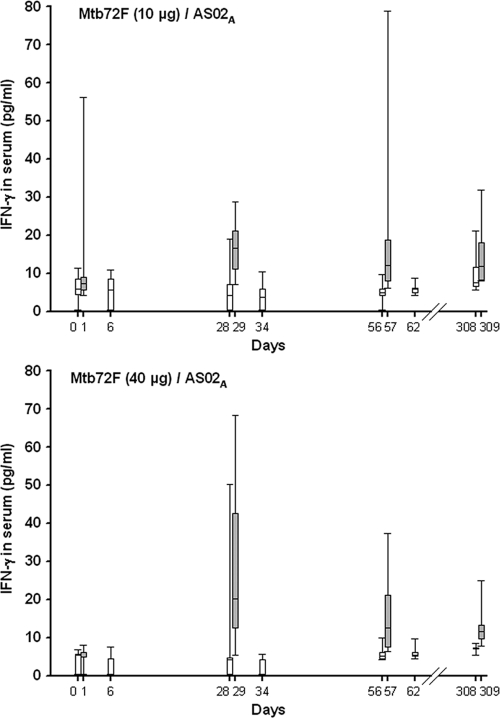
To evaluate whether the Mtb72F/AS02A vaccine upregulated the expression of Th1 cytokines in the blood, IFN-γ and TNF-α levels were evaluated shortly after vaccine administration. No vaccine-induced serum IFN-γ was detected after dose 1 in the majority of the individuals (Fig. 4). IFN-γ was detected in the serum at 1 day after doses 2 and 3 (days 29 and 57, respectively) but had decreased to baseline levels at 6 days after doses 2 and 3 (days 34 and 62, respectively). At 1 day postbooster (day 309), median serum levels of IFN-γ were comparable to those detected at 1 day after dose 3 (day 57) in the Mtb72F/AS02A groups. Only baseline responses were observed in the Mtb72F/saline and AS02A groups (data not shown). To investigate whether these cytokines were induced early after vaccination, blood samples from additional postbooster time points were evaluated. However, no IFN-γ was detected at 2, 4, and 6 h postbooster. No TNF-α was detected in any of the groups at any time points tested (data not shown).
FIG. 4.
Serum IFN-γ levels after Mtb72F/AS02A vaccine administration. Serum from subjects vaccinated with 10 μg Mtb72F/AS02A and 40 μg Mtb72F/AS02A were obtained on the day of vaccination (days 0, 28, 56, and 308), 1 day later (days 1, 29, 57, and 309), and 6 days later (days 6, 34, and 62) and were evaluated using ELISA to measure serum levels of IFN-γ. Data are represented in box-and-whiskers plots with a line at the median response and lower and higher quartiles. Whiskers indicate minimum and maximum responses.
(iii) Mtb72F-specific antibody responses.
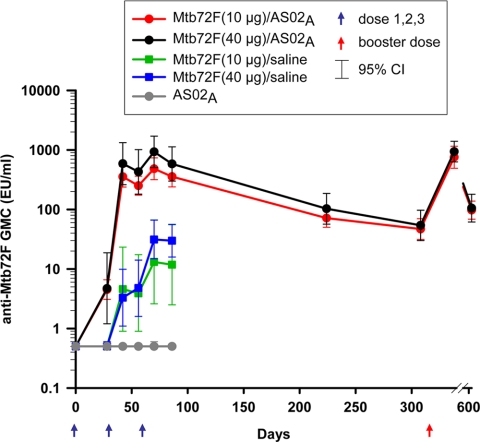
Two weeks after the second vaccine dose (day 42), all participants who had received Mtb72F/AS02A were seropositive for anti-Mtb72F IgG antibodies (Fig. 5). During the primary vaccination course, peak levels of anti-Mtb72F antibodies were measured at 2 weeks after dose 3 (day 70). There was a trend toward increased humoral responses with 40 μg Mtb72F/AS02A compared to those with 10 μg Mtb72F/AS02A. All but one vaccinee in the Mtb72F/saline groups became seropositive at 2 weeks after dose 3 (day 70), but the GMCs were significantly lower with no overlapping CIs compared with the values for the Mtb72F/AS02A groups (13.1 and 31.3 EU/ml for the 10- and 40-μg Mtb72F/saline groups, respectively).
FIG. 5.
Anti-Mtb72F IgG antibody responses. Serum samples from all groups were obtained prevaccination (day 0), 2 weeks after dose 2 (day 42), 1 month after dose 2 (day 56), 2 weeks after dose 3 (day 70), 1 month after dose 3 (day 86), and 6 months after dose 3 (day 224). The Mtb72F/AS02A-vaccinated groups were followed up prebooster (day 308), 1 month postbooster (day 338), and 1 year postbooster (day 644) and were evaluated by ELISA. The Mtb72F-specific IgG antibody responses are represented using geometric mean concentrations with 95% CIs. Arrows indicate the time of vaccination.
Antibody responses after primary vaccination with Mtb72F/AS02A persisted over 9 months. A fourth administration of the Mtb72F/AS02A vaccines boosted the antibody response, and 1 month later (day 338), they were approximately twice those observed after dose 3 (753.7 and 354.9 EU/ml after doses 4 and 3, respectively, for the 10-μg antigen dose and 938.3 and 581.4 EU/ml after doses 4 and 3, respectively, for the 40-μg antigen dose). All subjects receiving Mtb72F/AS02A remained seropositive at 1 year after the booster dose. No anti-Mtb72F IgG response was observed in the AS02A group.
(iv) PPD skin test reactivity.
To assess whether vaccination with Mtb72F/AS02A had an effect on PPD status, participants were evaluated 1 month after the primary vaccination course (i.e., ∼day 86 after the first vaccine dose) by intradermal PPD skin test. None of the participants converted from PPD-negative to PPD-positive status during the study.
DISCUSSION
The safety, reactogenicity, and immunogenicity of Mtb72F/AS02A have recently been evaluated in PPD-negative U.S. adults (42). This randomized, controlled study allowed a better evaluation of safety and was also designed to provide a justification for use of the adjuvant. Therefore, the reactogenicity and immunogenicity induced by the adjuvanted vaccine (Mtb72F/AS02A) were compared to those of the antigen in saline (Mtb72F/Saline) and the adjuvant alone (AS02A). A head-to-head comparison was also needed to examine whether a higher concentration of antigen (40 μg) was well tolerated and was able to elicit better immune responses compared to those elicited with the 10-μg concentration used previously. Here we also assessed if a fourth vaccine administration could boost the persistent immune responses. Finally, the quality of the T-cell response was extensively analyzed in order to achieve a full characterization of the vaccine-elicited mono- and polyfunctional T-cell phenotypes. This study demonstrates that up to four immunizations with the Mtb72F/AS02A vaccine (10 and 40 μg) are clinically well tolerated in PPD-negative healthy adults. No vaccine-related SAEs were reported. The local reactogenicity profile of Mtb72F/AS02A was comparable to that seen with AS02A alone. Fewer AEs were observed in the Mtb72F/saline groups, suggesting that AS02A rather than the Mtb72F antigen contributes to the observed injection-site reactions. The solicited general AEs fatigue and fever were reported more frequently in the Mtb72F/AS02A groups than in the Mtb72F/saline or AS02A groups, suggesting that this may be the expected consequence of the vigorous antigen-specific immune response observed after Mtb72F/AS02A administration. All AEs reported were transient, and most resolved within 1 to 4 days. The pattern of AEs observed has previously been reported with other vaccines containing AS02A (14, 16, 37, 40-42). No clinically significant changes in laboratory test (biochemistry and hematology) values related to vaccination were observed. In a study described in a previous publication (42), eosinophilia and decreased hemoglobin levels were frequently reported when the vaccine was given to healthy PPD-negative adults, although these were reported to be mainly mild and not clinically significant. In this study, which was controlled, in contrast to the previous study, which had no comparators, there were infrequent and comparable reports of mild eosinophilia and a decrease in hemoglobin levels in all vaccine and control groups (maximum of 1 to 3 individuals per group). Most of these mild fluctuations occurred either at prevaccination or screening, and no vaccination-related pattern emerged. These reports were also not considered to be clinically significant, in line with what was previously reported.
It is interesting to note that there appeared to be a trend toward a lower frequency of reporting of AEs in the 40-μg Mtb72F/AS02A group than in the 10-μg Mtb72F/AS02A and AS02A groups. However, given the small sample size and overlapping CIs, this observation appears not to be clinically significant. The Mtb72F/AS02A vaccines induced a strong Mtb72F-specific CD4+ T-cell response compared to that induced by Mtb72F/saline, showing that the AS02A adjuvant system enhanced the immunogenicity of the Mtb72F antigen. No Mtb72F-specific immune responses were observed in the group receiving AS02A alone.
A robust CD4+ T-cell response was induced after two doses of Mtb72F/AS02A, with no increase in the magnitude of the response occurring after a third and a fourth vaccine dose. The Mtb72F-specific CD4+ T-cell response was persistent in the Mtb72F/AS02A vaccine groups at 9 months after the primary vaccination course. A booster dose increased Mtb72F-specific CD4+ T-cell responses to magnitudes comparable to those observed after primary vaccination and persisted at 1 year postbooster, suggesting that memory CD4+ T cells may have been induced. There was no significant difference between the magnitude of the CD4+ T-cell response induced by the 10-μg and 40-μg Mtb72F/AS02A vaccines. The magnitude of the response after 1 month after dose 3 was comparable to that seen previously with the 10-μg Mtb72F/AS02A vaccine when it was tested in PPD-negative U.S. adults (10). Functional characterization showed that Mtb72F-specific polyfunctional CD4+ T cells were induced by both Mtb72F/AS02A vaccines and persisted 1 year after boosting. The predominant CD4+ T-cell phenotypes were CD40L+, CD40L+ IL-2+, CD40L+ TNF-α+, and CD40L+ IL-2+ TNF-α+. Mtb72F/AS02A also induced Mtb72F-specific CD40L+ IL-2+ TNF-α+ IFN-γ+ CD4+ T cells, albeit at a lower frequency. Remarkably, a higher frequency of single positive CD40L+ CD4+ T cells was also observed, indicating that a distinct population of antigen-specific activated CD4+ T cells was elicited by the Mtb72F/AS02A vaccine. This is the first study which demonstrates the polyfunctionality of vaccine-induced CD4+ T cells in PPD-negative subjects. Other studies of TB vaccine candidates have demonstrated polyfunctional CD4+ T-cell responses in PPD-positive adults (2, 5, 18, 31). Earlier work showed that the MVA85a TB vaccine candidate was immunogenic in PPD-negative British adults and that the magnitude of the IFN-γ enzyme-linked immunospot assay response was positively influenced by BCG priming (26).
Our results are promising, since studies in mouse models showed that induction of polyfunctional T cells correlated with protection against tuberculosis (1, 19) and that assessment in humans of T-cell polyfunctionality might allow more accurate identification of a protective T-cell population (9). Moreover, it is generally accepted that the induction of cellular Th1 immune responses is associated with protection against TB (36). CD4+ T cells are believed to contribute to protection against M. tuberculosis by the production of Th1 cytokines. Among these cytokines, IFN-γ (3, 4, 22, 36) and TNF-α (11, 28) seem to be the central effector molecules, leading to autophagy, mycobacterial killing, and macrophage death (12, 17, 21, 29, 33). IL-2 is a potent inducer of T-cell proliferation and differentiation and is key to the optimal survival and function of memory cells (20). CD40 ligand has been identified as a marker for activated antigen-specific T cells; it is a costimulatory ligand needed to provide T-cell help (7, 13, 38). A role for CD8+ T cells in the protection against TB has been demonstrated (15, 23, 27, 32). Similar to results from an earlier study with Mtb72F/AS02A (42) and studies with the MVA85a TB vaccine candidate (5, 18, 31), no vaccine-induced antigen-specific CD8+ T cells could be detected by ICS using a short-term in vitro restimulation.
We demonstrated that vaccination with Mtb72F/AS02A induced IFN-γ secretion in the serum at 1 day after vaccination. No TNF-α was detected. The transient presence of IFN-γ in the serum was clearly linked to Mtb72F/AS02A vaccination, as no such response was observed in the other study groups.
Robust and persistent antibody responses were induced by the Mtb72F/AS02A vaccines, with 100% seropositivity occurring after the second dose. Each vaccine administration increased the humoral immune response during the primary vaccination course, and the antibody responses induced by the Mtb72F/AS02A vaccines were significantly higher than those induced by their Mtb72F/saline counterparts. Anti-Mtb72F titers tended to be higher in the Mtb72F/AS02A (40-μg) group than in the Mtb72F/AS02A (10-μg) group in the primary vaccination course. The antibody response could be boosted to the same level for both Mtb72F/AS02A vaccines by a fourth dose 9 months later. All subjects remained seropositive 1 year postbooster.
The contribution of humoral responses in protection against TB is somewhat controversial, but it has been suggested that antibody-mediated immunity can alter the course of M. tuberculosis infection in certain situations (15).
In conclusion, the Mtb72F/AS02A candidate vaccine is clinically well tolerated and highly immunogenic in PPD-negative adults. A favorable safety profile was observed with 4 injections of the two Mtb72F/AS02A vaccines (10 μg and 40 μg) tested in this study. The vaccine induced a robust Mtb72F-specific humoral and polyfunctional CD4+ T-cell response.
The next steps in the clinical development of this vaccine will be to evaluate Mtb72F/AS02A in adults who are PPD positive, either from natural exposure to M. tuberculosis or from prior vaccination with BCG, and to initiate its evaluation in individuals living in regions where TB is endemic.
Acknowledgments
We are indebted to all trial participants and acknowledge the contributions of the clinicians, nurses, and laboratory technicians. We thank Anne Bollaerts, Pascale Van Belle, and Marc Fourneau for their statistical input; Michel Janssens, Yannick Vanloubbeeck, and Marie-Noëlle Donner for performing CMI characterization; Pascal Mettens, Patricia Bourguignon, and Erik Jongert for their thorough review of the manuscript; Priya Pavithran for preparing the clinical study documents; and Ilse Vastiau, Ulrike Krause, and Julia Donnelly for editorial assistance in preparing the manuscript.
Opokua Ofori-Anyinam, Philippe Moris, Marguerite Koutsoukos, Marie-Claude Dubois, Marie-Ange Demoitié, Joe Cohen, and W. Ripley Ballou are present employees of GlaxoSmithKline Biologicals. Els De Kock was employed by GSK Biologicals at the time of the study. Geert Leroux-Roels has been principal investigator of vaccine trials for several manufacturers, including GlaxoSmithKline Biologicals, for which his institution obtained research grants. Geert Leroux-Roels has received honoraria for participation on scientific advisory boards. Isabel Leroux-Roels and Geert Leroux-Roels received speaker fees and travel grants from several manufacturers, including GlaxoSmithKline Biologicals. Frédéric Clement has no declared conflict of interest.
GSK Biologicals was the funding source of this clinical trial.
Footnotes
Published ahead of print on 22 September 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Aagaard, C., T. T. Hoang, A. Izzo, R. Billeskov, J. Troudt, K. Arnett, A. Keyser, T. Elvang, P. Andersen, and J. Dietrich. 2009. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One 4:e5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel, B., M. Tameris, N. Mansoor, S. Gelderbloem, J. Hughes, D. Abrahams, L. Makhethe, M. Erasmus, M. de Kock, L. van der Merwe, A. Hawkridge, A. Veldsman, M. Hatherill, G. Schirru, M. G. Pau, J. Hendriks, G. J. Weverling, J. Goudsmit, D. Sizemore, J. B. McClain, M. Goetz, J. Gearhart, H. Mahomed, G. D. Hussey, J. C. Sadoff, and W. A. Hanekom. 2010. The novel TB vaccine, AERAS-402, induces robust and polyfunctional CD4 and CD8 T cells in adults. Am. J. Respir. Crit. Care Med. 181:1407-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agger, E. M., and P. Andersen. 2001. Tuberculosis subunit vaccine development: on the role of interferon-gamma. Vaccine 19:2298-2302. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, P., and T. M. Doherty. 2005. TB subunit vaccines—putting the pieces together. Microbes Infect. 7:911-921. [DOI] [PubMed] [Google Scholar]
- 5.Beveridge, N. E., D. A. Price, J. P. Casazza, A. A. Pathan, C. R. Sander, T. E. Asher, D. R. Ambrozak, M. L. Precopio, P. Scheinberg, N. C. Alder, M. Roederer, R. A. Koup, D. C. Douek, A. V. Hill, and H. McShane. 2007. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur. J. Immunol. 37:3089-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt, L., Y. A. Skeiky, M. R. Alderson, Y. Lobet, W. Dalemans, O. C. Turner, R. J. Basaraba, A. A. Izzo, T. M. Lasco, P. L. Chapman, S. G. Reed, and I. M. Orme. 2004. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect. Immun. 72:6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chattopadhyay, P. K., J. Yu, and M. Roederer. 2005. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat. Med. 10:1113-1117. [DOI] [PubMed] [Google Scholar]
- 8.Colditz, G. A., C. S. Berkey, F. Mosteller, T. F. Brewer, M. E. Wilson, E. Burdick, and H. V. Fineberg. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29-35. [PubMed] [Google Scholar]
- 9.De Rosa, S. C., F. X. Lu, J. Yu, S. P. Perfetto, J. Falloon, S. Moser, T. G. Evans, R. Koup, C. J. Miller, and M. Roederer. 2004. Vaccination in humans generates broad T cell cytokine responses. J. Immunol. 173:5372-5380. [DOI] [PubMed] [Google Scholar]
- 10.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 12.Fremond, C., N. Allie, I. Dambuza, S. I. Grivennikov, V. Yeremeev, V. F. Quesniaux, M. Jacobs, and B. Ryffel. 2005. Membrane TNF confers protection to acute mycobacterial infection. Respir. Res. 6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frentsch, M., O. Arbach, D. Kirchhoff, B. Moewes, M. Worm, M. Rothe, A. Scheffold, and A. Thiel. 2005. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat. Med. 11:1118-1124. [DOI] [PubMed] [Google Scholar]
- 14.Garçon, N., P. Chomez, and M. Van Mechelen. 2007. GlaxoSmithKline adjuvant systems in vaccines: concepts; achievements and perspectives. Expert Rev. Vaccines 6:723-739. [DOI] [PubMed] [Google Scholar]
- 15.Glatman-Freedman, A. 2006. The role of antibody-mediated immunity in defense against Mycobacterium tuberculosis: advances toward a novel vaccine strategy. Tuberculosis (Edinb.) 86:191-197. [DOI] [PubMed] [Google Scholar]
- 16.Goepfert, P. A., G. D. Tomaras, H. Horton, D. Montefiori, G. Ferrari, M. Deers, G. Voss, M. Koutsoukos, L. Pedneault, P. Vandepapeliere, M. J. McElrath, P. Spearman, J. D. Fuchs, B. A. Koblin, W. A. Blattner, S. Frey, L. R. Baden, C. Harro, T. Evans, and NIAID HIV Vaccine Trials Network. 2007. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine 25:510-518. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez, M. G., S. S. Master, S. B. Singh, G. A. Taylor, M. I. Colombo, and V. Deretic. 2004. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119:753-766. [DOI] [PubMed] [Google Scholar]
- 18.Hawkridge, T., T. J. Scriba, S. Gelderbloem, E. Smit, M. Tameris, S. Moyo, T. Lang, A. Veldsman, M. Hatherill, L. Merwe, H. A. Fletcher, H. Mahomed, A. V. Hill, W. A. Hanekom, G. D. Hussey, and H. McShane. 2008. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J. Infect. Dis. 198:544-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoang, T. T., A. Nansen, S. Roy, R. Billeskov, C. Aagaard, T. Elvang, J. Dietrich, and P. Andersen. 2009. Distinct differences in the expansion and phenotype of TB10.4 specific CD8 and CD4 T cells after infection with Mycobacterium tuberculosis. PLoS One 4:e5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoyer, K. K., H. Dooms, L. Barron, and A. K. Abbas. 2008. Interleukin-2 in the development and control of inflammatory disease. Immunol. Rev. 226:19-28. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs, M., D. Togbe, C. Fremond, A. Samarina, N. Allie, T. Botha, D. Carlos, S. K. Parida, S. Grivennikov, S. Nedospasov, A. Monteiro, M. Le Bert, V. Quesniaux., and B. Ryffel. 2007. Tumor necrosis factor is critical to control tuberculosis infection. Microbes Infect. 9:623-628. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann, S. H. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 1:20-30. [DOI] [PubMed] [Google Scholar]
- 23.Lazarevic, V., and J. Flynn. 2002. CD8+ T cells in tuberculosis. Am. J. Respir. Crit. Care Med. 166:1116-1121. [DOI] [PubMed] [Google Scholar]
- 24.Maecker, H. T., H. S. Dunn, M. A. Suni, E. Khatamzas, C. J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T. M. Fu, E. Sinclair, B. M. Bredt, J. M. McCune, V. C. Maino, F. Kern, and L. J. Picker. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 255:27-40. [DOI] [PubMed] [Google Scholar]
- 25.Maecker, H. T., V. C. Maino, and L. J. Picker. 2000. Immunofluorescence analysis of T-cell responses in health and disease. J. Clin. Immunol. 20:391-399. [DOI] [PubMed] [Google Scholar]
- 26.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 27.Pathan, A. A., K. A. Wilkinson, R. J. Wilkinson, M. Latif, H. McShane, G. Pasvol, A. V. Hill, and A. Lalvani. 2000. High frequencies of circulating IFN-gamma-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur. J. Immunol. 30:2713-2721. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer, K. 2003. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 14:185-191. [DOI] [PubMed] [Google Scholar]
- 29.Randhawa, A. K., H. J. Ziltener, and R. W. Stokes. 2008. CD43 controls the intracellular growth of Mycobacterium tuberculosis through the induction of TNF-alpha-mediated apoptosis. Cell. Microbiol. 10:2105-2117. [DOI] [PubMed] [Google Scholar]
- 30.Reed, S. G., R. N. Coler, W. Dalemans, E. V. Tan, E. C. DeLa Cruz, R. J. Basaraba, I. M. Orme, Y. A. Skeiky, M. R. Alderson, K. D. Cowgill, J. P. Prieels, R. M. Abalos, M. C. Dubois, J. Cohen, P. Mettens, and Y. Lobet. 2009. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in Cynomolgus monkeys. Proc. Natl. Acad. Sci. U. S. A. 106:2301-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sander, C. R., A. A. Pathan, N. E. Beveridge, I. Poulton, A. Minassian, N. Alder, J. Van Wijgerden, A. V. Hill, F. V. Gleeson, R. J. Davies, G. Pasvol, and H. McShane. 2009. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am. J. Respir. Crit. Care Med. 179:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serbina, N. V., and J. L. Flynn. 2001. CD8(+) T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect. Immun. 69:4320-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh, S. B., A. S. Davis, G. A. Taylor, and V. Deretic. 2006. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 313:1438-1441. [DOI] [PubMed] [Google Scholar]
- 34.Skeiky, Y. A., M. R. Alderson, P. J. Ovendale, J. A. Guderian, L. Brandt, D. C. Dillon, A. Campos-Neto, Y. Lobet, W. Dalemans, I. M. Orme, and S. G. Reed. 2004. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 172:7618-7628. [DOI] [PubMed] [Google Scholar]
- 35.Skeiky, Y. A., M. J. Lodes, J. A. Guderian, R. Mohamath, T. Bement, M. R. Alderson, and S. G. Reed. 1999. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect. Immun. 67:3998-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stenger, S., and R. L. Modlin. 1999. T cell mediated immunity to Mycobacterium tuberculosis. Curr. Opin. Microbiol. 2:89-93. [DOI] [PubMed] [Google Scholar]
- 37.Stoute, J. A., D. G. Heppner, Jr., C. J. Mason, J. Siangla, M. O. Opollo, K. E. Kester, L. Vigneron, G. Voss, M. J. Walter, N. Tornieporth, J. D. Cohen, and W. R. Ballou. 2006. Phase 1 safety and immunogenicity trial of malaria vaccine RTS, S/AS02A in adults in a hyperendemic region of western Kenya. Am. J. Trop. Med. Hyg. 75:166-170. [PubMed] [Google Scholar]
- 38.Stubbe, M., N. Vanderheyde, M. Goldman, and A. Marchant. 2006. Antigen-specific central memory CD4+ T lymphocytes produce multiple cytokines and proliferate in vivo in humans. J. Immunol. 177:8185-8190. [DOI] [PubMed] [Google Scholar]
- 39.Tsenova, L., R. Harbacheuski, A. L. Moreira, E. Ellison, W. Dalemans, M. R. Alderson, B. Mathema, S. G. Reed, Y. A. Skeiky, and G. Kaplan. 2006. Evaluation of the Mtb72F polyprotein vaccine in a rabbit model of tuberculous meningitis. Infect. Immun. 74:2392-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandepapeliere, P., R. Barrasso, C. J. Meijer, J. M. Walboomers, M. Wettendorff, L. R. Stanberry, and C. J. Lacey. 2005. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J. Infect. Dis. 192:2099-2107. [DOI] [PubMed] [Google Scholar]
- 41.Vandepapeliere, P., B. Rehermann, M. Koutsoukos, P. Moris, N. Garçon, M. Wettendorff, and G. Leroux-Roels. 2005. Potent enhancement of cellular and humoral immune responses against recombinant hepatitis B antigens using AS02A adjuvant in healthy adults. Vaccine 23:2591-2601. [DOI] [PubMed] [Google Scholar]
- 42.Von Eschen, K., R. Morrison, M. Braun, O. Ofori-Anyinam, E. De Kock, P. Pavithran, M. Koutsoukos, P. Moris, D. Cain, M. C. Dubois, J. Cohen, and W. R. Ballou. 2009. The candidate tuberculosis vaccine Mtb72F/AS02A: tolerability and immunogenicity in humans. Hum. Vaccin. 5:475-482. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. 2009. Global Tuberculosis Control: a short update of the 2009 report. World Health Organization, Geneva, Switzerland. http://whqlibdoc.who.int/publications/2009/9789241598866_eng.pdf. Accessed 27 July 2010.