Abstract
Bradyzoite-to-tachyzoite conversion plays a role in the pathogenesis of recrudescence of ocular toxoplasmosis and disease in immunocompromised persons. The currently available medicines are ineffective on cysts and fail to prevent reactivation of latent toxoplasmosis. A previous study showed that the histone deacetylase inhibitor FR235222 has a dramatic effect on tachyzoite growth and induces tachyzoite-to-bradyzoite conversion in vitro. The present study shows that FR235222 can target in vitro-converted cysts and bradyzoites. Moreover, the compound is active on ex vivo T. gondii cysts. Free bradyzoites isolated after lysis of the cell wall did not proliferate in vitro when the cyst was treated with FR235222. The results imply that this compound is able to cross the T. gondii cystic cell wall. Fluorescent labeling shows that the compound impairs the capacity of the bradyzoites to convert without damaging the cyst wall integrity. In vivo inoculation of formerly treated cysts fails to infect mice when these cysts were treated with FR235222. We used our structural knowledge of FR235222 and its target, T. gondii HDAC3, to synthesize new FR235222 derivative compounds. We identified two new molecules that are highly active against tachyzoites. They harbor a better selectivity index that is more suitable for a future in vivo approach. These results identify FR235222 and its derivatives as new lead compounds in the range of therapeutics available for acute and chronic toxoplasmosis.
Toxoplasma gondii is the causative agent of toxoplasmosis and is considered one of the most common parasitic diseases, given its worldwide distribution and its broad range of intermediate hosts (18). T. gondii is an intracellular parasite that belongs to the Apicomplexa family, like Plasmodium species, with which it shares major biologic and genetic similarities (4, 35). Its life cycle is complex and is characterized by the interconversion phenomenon, which is the ability of the parasite to differentiate from a tachyzoite form to a cystic structure (containing the bradyzoite form) and vice versa. Cysts, which are responsible for the chronic form of toxoplasmosis, are believed to not cause symptoms in healthy people, but if they are reactivated, they can be potentially life-threatening in immunocompromised patients. Treatments of acute toxoplasmosis are currently based on the combination of pyrimethamine (PYR) and sulfadiazine, which can be associated with cytopenia and allergic skin reactions, respectively, as possible side effects. The antibiotics cotrimoxazole and clindamycin have been used as second-line treatments (14). In addition, medicines used so far in the clinical setting target the tachyzoite form, while the tissue cysts remain unaffected. Despite the highly active research on this parasite and the description of dozens of potential new molecular targets, no candidates presenting an anticystic activity have been identified, therefore providing no grounds for a treatment able to eliminate T. gondii from infected patients. Discovering new therapeutic agents that are effective on cysts is considered one of the important challenges in toxoplasmosis in the near future (5).
T. gondii completes its life cycle by successive processes of parasite differentiation that rely on tight control of gene expression to ensure appropriate protein profiles over time. How these changes are regulated at the molecular level remains to a large extent unknown. An unexpected feature is the apparent lack in apicomplexan parasites of large families of recognizable specific transcription factors (TFs) operating in other eukaryotes, with one exception being the plant-like AP2 DNA binding family (20). This observation is intriguing in light of the complex life cycle of the parasite, which inevitably requires tight gene regulation. Despite the paucity of recognizable TFs, apicomplexa are endowed with a rich repertoire of enzymes associated with epigenetics and chromatin remodeling, and this observation has fueled the idea that epigenetics could play an important role in the control of gene expression (7, 17, 34). Therefore, chromatin regulators make suitable targets for the development of therapeutics.
Increasing amounts of evidence indicate that acetylation of histones plays a substantial role in the control of gene expression during parasite interconversion (6, 32). Histone acetylation levels are controlled by histone acetylase (HAT) and histone deacetylase (HDAC) enzymes. HDACs counteract acetylase activity by catalyzing the removal of acetyl moieties from lysine residues in histone tails, thereby inducing chromatin condensation and transcriptional repression. Recently, it has been shown that inhibition of histone deacetylase activity with histone deacetylase inhibitors (HDACis) interferes with parasite differentiation (6). We previously showed that specific inhibition of T. gondii HDAC3 (TgHDAC3) by the cyclopeptide FR235222 disrupts the steady-state level of histone 4 (H4) acetylation across the genome, inducing derepression of stage-specific genes (6). In the same study, we isolated parasite lines resistant to the molecule; single-point mutations found in mutagenized parasites target amino acid T99 in TgHDAC3. Similar to drug inhibition of TgHDAC3, the TgHDAC3T99A and/or TgHDAC3T99I mutation led to bradyzoite marker expression in parasites carrying those mutations (6). We think that TgHDAC3 prevented the formation of tachyzoite daughter cells, while low doses of FR235222 induced conversion in bradyzoites. Comparison of different HDACis on T. gondii growth showed that inhibitors that belong to the class of cyclic tetrapeptides (such as apicidin [API], HC-toxin, and FR235222) are more effective in inhibiting Toxoplasma growth than other HDACis, such as trichostatin A (TSA) and the clinically relevant compound pyrimethamine (6, 12).
The study described here sought to determine the biological effects of the cyclic tetrapeptide HDACis on the cystic form of T. gondii. In light of the structural features of FR235222, we generated novel FR235222 derivative compounds by hemisynthesis in order to improve selectivity against the parasite. Five FR235222 derivatives were generated, and two of them showed significantly increased selectivity.
MATERIALS AND METHODS
Mice.
Outbred female Swiss mice weighing approximately 20 g each were used for all our in vivo experiments. Animal euthanasia was completed in an approved CO2 chamber.
Drugs.
The novel cyclic tetrapeptide FR235222 was kindly provided by Astellas Pharma Inc. (Osaka, Japan). The five FR235222 derivative compounds, W363, W371, W399, W406, and W425, were obtained by converting FR235222 in one or two synthetic steps. A stock solution of the HDACi cyclic tetrapeptide API (Sigma-Aldrich) was prepared in 100% dimethyl sulfoxide (DMSO), stored at −20°C, and diluted in tissue culture medium for in vitro experiments, with the final DMSO concentration in the culture medium being below 0.1%. PYR was used as a reference for anti-Toxoplasma activity and was purchased from Sigma-Aldrich; a 10 mM stock solution was prepared in ethanol. For proliferation assays, 2-fold serial dilutions of the drug were achieved in culture medium: from 90 nM to 2.8 nM for parasite strains and from 1 μM to 15 nM for human foreskin fibroblasts (HFFs). For the evaluation of the effects on cysts, FR235222 and API were used at a final concentration of 200 nM and PYR was used at a final concentration of 1 μM.
Proliferation assay with T. gondii strains and human cells.
Type I T. gondii reference laboratory strain RH was used for 50% effective concentration (EC50) determination, and the cystogenic type II PRU strain was used for in vitro and in vivo cyst experiments. FR235222-resistant mutant R20D9 carrying the TgHDAC3T99A mutation was used in a second step. All strains were maintained by serial passage in an HFF monolayer under tachyzoite growth conditions in Dulbecco's modified Eagle medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 4 mM glutamine, 500 U/ml penicillin, and 250 μg/ml streptomycin at 37°C in 5% CO2.
In vitro inhibitory concentrations for FR235222 and derivative compounds against the RH strain were determined by measuring the incorporation of [3H]uracil by intracellular tachyzoites, as described previously (6). Twenty-four-well plates of confluent HFFs were infected with 3 × 102 tachyzoites/well for the RH strain or 10e4 tachyzoites/well for the R20D9 strain. After 72 h, 5 μCi of [3H]uracil was added for a 4-h pulse. [3H]uracil was incorporated only by living parasites (30). After 4 h, the monolayers were washed with phosphate-buffered saline (PBS) to remove residual [3H]uracil, and the remaining material was solubilized in 500 μl of lysis scintillation solution (Optiphase super mix; Perkin Elmer). The radioactivity was quantified using a liquid scintillation counter (MicroBeta TriLux; Perkin Elmer).
In vitro inhibitory concentrations for FR235222 and derivative compounds for HFF were determined using the Cell Titer Aqueous One Solution cell proliferation assay (Promega), in accordance with the manufacturer's procedure. A total of 2 × 103 cells per well were inoculated for initial proliferation, and the tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt, was added directly to culture wells after a 72-h proliferation phase. The bioreduced colored formazan produced by viable cells was quantified with a 96-well plate reader (absorbance, 490 nm). The EC50 for each drug used in the in vitro tests was determined by nonlinear regression analysis using the HN-NonLin (version 1.1) computer program developed for the analysis of in vitro drug sensitivity data (http://www.who.int/malaria/rbm/Attachment/20041108/NonLin_V1.1.xls). The selectivity index (SI) of each drug was defined as the ratio of the HFF cell EC50 to the RH strain EC50. According to definitions and activity criteria for hits and leads in drug development, an SI corresponds to the 50% inhibitory concentration (IC50) for the mammalian cell line/IC50 for the parasite. Hit activity criteria for protozoa are considered when the EC50 is <0.2 μg/ml and the SI is >100 (27). In our experiments, a high SI indicates that the compound specifically targets Toxoplasma proliferation and has negligible effect on HFF cells.
Western blot analysis.
Whole-cell extracts were prepared as follows: approximately 107 extracellular parasites (needle passed and filtered using a 3-μm-pore-size Nuclepore membrane) were lysed in 50 μl of lysis buffer (10 mM Tris-HCl [pH 6.8], 0.5% [vol/vol] SDS, 10% [vol/vol] glycerol, 1 mM EDTA) and sonicated. The protein concentration was determined using a Bradford protein assay kit (Bio-Rad Laboratories) with bovine serum albumin as the standard. A total of 10 μg of protein per sample was analyzed using NuPAGE 4% to 12% bis-Tris polyacrylamide gels with morpholineethanesulfonic acid-SDS running buffer (Invitrogen); transferred onto a nitrocellulose membrane (Hybond-ECL; GE Healthcare); and probed with a 1:1,000 dilution of an anti-acetyl-H4 (anti-AcH4) antiserum (AcH4K5K8K12K16; Millipore), a 1:1,000 dilution of an anti-H3 antiserum (H3; Abcam), and a 1:2,000 dilution of a monoclonal anti-α-tubulin antibody (Sigma-Aldrich). The blots were developed with a SuperSignal West Pico chemiluminescent substrate kit (Thermo Fisher Scientific).
Immunofluorescence microscopy.
For immunofluorescence labeling, HFFs grown on coverslips infected with the PRU or RH strain were fixed for 20 min with PBS containing 5% formaldehyde and permeabilized for 20 min with 0.2% Triton X-100. Blocking was performed with PBS containing 5% FBS and 5% goat serum for 1 h. All antibodies were diluted in 1% FBS. Coverslips were incubated for 1 h with the primary antibodies anti-P36 or anti-IMC-1 monoclonal mouse antibodies and anti-small ubiquitin-like modifier (anti-SUMO) (8) or anti-acetyl-H4 polyclonal rabbit antibodies (Millipore), followed by the secondary antibodies goat anti-mouse IgG or goat anti-rabbit IgG coupled with either Alexa Fluor 568 or Alexa Fluor 488 dye (Invitrogen) at a 1:1,000 dilution. Anti-P36 reveals the SRS9 and BSR4 proteins, two specific markers of the bradyzoite stage (36). Anti-T. gondii SUMO (TgSUMO) labels not only the surface of in vitro-induced cysts but also the parasite nucleus (8). The nuclei of host cells and parasites were stained for 10 min with Hoechst 33258 dye at 2 μg/ml in PBS. After 3 washes, coverslips were mounted on a glass slide with Mowiol mounting medium, and images were acquired with a fluorescence microscope (Axioplan 2; Carl Zeiss, Inc.). G. E. Ward (University of Vermont, Burlington, VT), U. Gross (Institute of Medical Microbiology, University of Göttingen), and J. F. Dubremetz (Centre National de la Recherche Scientifique, Montpellier, France) provided antibodies against IMC-1, CC2, and P36 (SRS9-BSR4), respectively.
In vitro FR235222 activity on tachyzoites and bradyzoites.
HFFs were grown in 24-well plates covered with glass coverslips. For the RH strain, 5e4 extracellular tachyzoites were allowed to invade HFF cells; the medium was replaced by medium containing either FR235222 60 nM (the IC100) or 0.1% DMSO as the control. After 24 h, the slides were fixed and used for IMC-1 and AcH4 labeling, as described above.
For the type II cystogenic PRU strain, 1.5 × 104 extracellular tachyzoites were allowed to invade HFF cells. The strain had recently been isolated from mouse brain to ensure that tachyzoites could interconvert naturally in vitro. Natural cystogenesis of our PRU strain was obtained on day 7. Bradyzoite induction was assessed for P36 expression by immunofluorescence assay on one of the coverslips. The medium was removed and replaced by medium containing FR235222 at 30 nM and 50 nM or DMSO at 0.1% under the control condition. After 24, 48, and 72 h, the medium was removed and the effect on bradyzoite and cyst stage-specific P36 and SUMO expression by in vitro cysts was assessed by immunofluorescence assay (IFA) (8). The same experiment was done after tachyzoites were switched into bradyzoites using alkaline medium (pH 8.2) on day 3; induced cysts were obtained and treated as described before for 3 more days.
HDACi activity on ex vivo T. gondii cysts and bradyzoites.
Cysts were isolated from brains of mice chronically infected with the PRU strain, using the Percoll gradient method, as described previously (11). In order not to deteriorate the cyst cell wall, neither saponin nor trypsin was added at the end of the experiment. Cysts were resuspended in 1 ml culture medium containing FR235222 (200 nM), apicidin (200 nM), or pyrimethamine (1 μM) in six-well plates at 37°C in 5% CO2. Cysts treated with 0.1% DMSO, 0.1% ethanol, or medium only were used as controls. Not knowing the EC50 for bradyzoites (which was impossible to obtain because bradyzoites do not proliferate) and working on the cyst form, which is known to be highly resistant, we arbitrarily chose a 200 nM concentration, which is quite high compared to the IC100 for tachyzoites (about three times the IC100 for free tachyzoites) but which is still far less than the concentrations of drugs currently used in toxoplasmosis (24). The activity against tissue cysts was evaluated using three different approaches. Each experiment was conducted at least in triplicate.
On day 7, in a first series of experiments, the cysts were counted, resuspended, pelleted, washed twice in PBS buffer, and stained with a solution of acridine orange and ethidium bromide (AO-EB), as described previously (1). This technique assesses the viability of treated cysts and is easy to use as a screening method. Viable cysts stained green, whereas cysts affected by treatment presented a color from yellow-green to orange-red. All samples were identified by numbers so the reader was not influenced by the cyst treatment. In a second series of experiments, cysts treated with FR232222 or DMSO for 7 days were stained with Dolichos biflorus lectin conjugated to fluorescein in order to evaluate the integrity of the cell wall. Briefly, the cysts were washed with PBS, fixed in 5% paraformaldehyde for 20 min, washed twice with PBS, and then permeabilized and blocked for 20 min in a solution containing 5% FBS and 0.2% Triton X-100 (final concentrations). The washed cysts were stained with 1:100-diluted Dolichos lectin for 30 min. The stained cysts were examined with a fluorescence microscope (Axioplan 2; Carl Zeiss, Inc).
In a second, independent experiment, in order to evaluate the interconversion capacities of bradyzoites within the treated cyst, cysts treated for 7 days were washed three times and inoculated on an HFF monolayer in fresh medium for 7 additional days. To ensure that the observed effect was independent of the cystic cell wall (hypothetically, remaining drug is trapped in the cystic cell wall), washed cysts were lysed so that only free-living bradyzoites were inoculated on the HFF monolayer. Briefly, after 7 days of treatment, the remaining red cells were lysed using 0.1% saponin for 5 min and then washed twice in PBS, and the cystic cell wall was lysed with 0.25% trypsin solution (Gibco) for 1 min at 37°. Washed bradyzoites were inoculated in fresh medium for 7 more days in a six-well plate formerly coated with an HFF monolayer. The medium was changed once on day 3 for each type of experiment. Viable bradyzoites are able to convert to the tachyzoite replicative form. The parasite load in each well was quantified on day 7, after DNA extraction of the pellet (QIAmp DNA minikit; Qiagen) using the reverse transcription-PCR (RT-PCR) targeting the B1 gene, as described previously (9).
A third series of experiments was conducted to assess the infective power of the FR232222-treated cysts and the control cysts. The cysts were treated in vitro with FR235222 (200 nM), PYR (1 μM), or DMSO as a control. After 7 days, the presence of cysts was confirmed with an inverted microscope and the treated cyst suspensions were washed, pelleted, and inoculated intraperitoneally into four mice for each condition (from 25 to 50 cysts were injected per mouse, depending on the experiment). Six weeks after inoculation, the mice were euthanatized, the brains were isolated and homogenized in 4 ml PBS with an adapted potter, and 300 μl of crushed brain was evaluated for the presence of cysts using the Dolichos lectin staining method, as described above. The serum of each mouse was checked for Toxoplasma antibodies using a direct agglutination method (Toxo-Screen) and Western blot analysis of the IgG immune response using the WB LD-bio diagnostic test (LB-Bio Diagnostic, Lyon, France) with an anti-mouse IgG-alkaline phosphatase conjugate.
RESULTS
FR235222 effects on tachyzoites and bradyzoites induced in vitro.
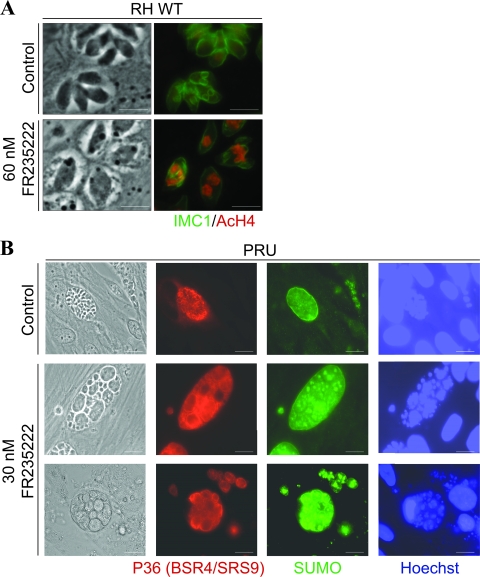
FR235222 induced multiple defects on multiplying tachyzoites, such as polynucleated parasites, the disappearance of IMC-1-delineated daughter cells, and increased histone H4 acetylation levels (Fig. 1 A). To determine whether FR235222 had an effect on bradyzoite differentiated parasites, in vitro cysts were cultured in the presence of FR235222 and observed by IFA. Figure 1B shows that the morphology of the treated parasites was dramatically altered: giant bradyzoites with multiple nuclei were observed, as assessed by anti-P36 and anti-SUMO labeling. These phenotypic aspects were observed for every treated cyst (100% altered cysts 24 h after treatment with the lowest concentration [30 nM] of FR235222), contrary to those of control cysts, which showed a classic structure (normally sized bradyzoites with one nucleus). All the in vitro-induced T. gondii cysts also stained for the specific cyst cell wall monoclonal antibody (MAb) anti-CC2 (16), confirming that they were not parasitophorous vacuole (see data set A in the supplemental material). These data indicated that FR235222 was able to affect bradyzoites differentiated in vitro.
FIG. 1.
Effect of FR235222 on RH strain tachyzoites and PRU strain bradyzoites and cysts induced in vitro. (A) An effect on RH strain tachyzoites was observed after 24 h with 60 nM FR235222. The morphology was highly altered, with giant polynucleated parasites being detected, and an enhanced level of acetyl-H4 labeling was found. Bars, 5 μm. (B) PRU strain cysts obtained in vitro on day 7 were treated for 24 h with 30 nM FR235222: all treated cysts were oversized, with giant polynucleated bradyzoites being detected, as assessed by P36, TgSUMO, and Hoechst fluorescent labeling. Bars, 10 μM.
FR235222 effect on ex vivo cysts. (i) In vitro experiments.
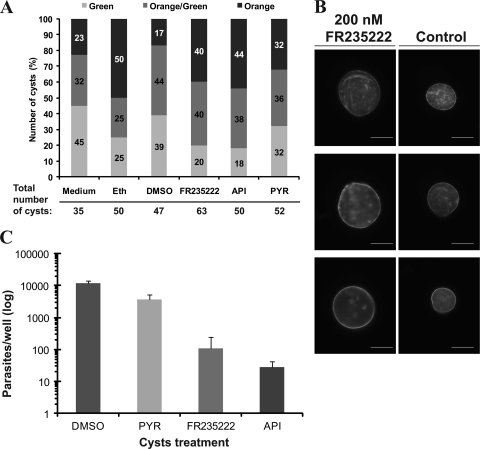
To specify the potential activity of FR235222 on Toxoplasma tissue cysts, chronically infected mice were euthanatized and the cysts were isolated and treated with different compounds ex vivo for 7 days. An objective count of AO-EB-stained cysts after a 7-day treatment revealed that there were no major differences between any of the different treatments compared to the results for the DMSO or ethanol controls (Fig. 2 A). Contrary to the findings of Araujo et al. (2), who obtained a majority of dead cysts when the cysts were treated with atovaquone, a maximum 44% of our cysts treated with an HDACi (API or FR235222) were orange and considered not viable. Under all conditions, there was a high rate of noninformative cysts within the group of orange-green cysts (Fig. 2A). We concluded that this method was not suitable to test cyst viability under the conditions tested. The total cyst count in the presence of drugs did not significantly differ from day 1 to day 7, suggesting that the cysts were not disrupted by the drugs during the incubation time. Dolichos lectin staining of the cyst wall after a 7-day treatment with FR235222 or DMSO as a control confirmed that the cyst wall remained unaffected under both conditions (Fig. 2B). In an independent experiment, after the 7-day treatment, bradyzoites were released from treated and untreated cysts and labeled with the SRS9-BSR4 MAb. All parasites were positive for SRS9-BSR4 MAb labeling, confirming (i) the presence of parasites after treatment and (ii) the bradyzoite stage of the inoculated parasite (see data set SB in the supplemental material).
FIG. 2.
Effect of FR235222 on ex vivo cysts. In vitro conditions. (A) Acridine orange EB staining of ex vivo-treated cysts. Counts of ex vivo cysts after acridine orange EB staining after a 7-day treatment with HDACi compounds (API, FR235222) and control agents (PYR, DMSO, ethanol [Eth]). No major differences in distribution between green, orange-green, and orange cysts were observed when the different conditions and treatments were considered. (B) Dolichos lectin staining of the cyst cell wall. Ex vivo cysts were treated for 7 days with compound FR235222 or control (DMSO). After 7 days, the cyst cell walls were labeled with Dolichos lectin. No morphological disruption of the cyst cell wall was observed in cysts treated with FR235222. Magnification, ×400; bars, 40 μm. (C) Interconversion assay with free bradyzoites isolated from treated cysts: effect of FR235222 on bradyzoite interconversion (average of two independent experiments; logarithmic scale). Bradyzoites were able to interconvert and proliferate when cysts were treated with DMSO or PYR, and fewer numbers were obtained from cysts treated with FR235222 or API, suggesting the marked reduction in interconversion and proliferation.
Considering phenotypic aspects of treated cysts observed in vitro, the ex vivo-treated cysts at first seemed to be poorly affected by FR235222. To further explore cyst viability after FR235222 treatment, a proliferation assay was conducted. After 7 days of incubation of the cysts in the presence of FR235222 in the culture medium, the cysts were washed and allowed to differentiate into tachyzoites over a confluent HFF monolayer. Tachyzoite-induced lysis plaques were observed in the cultures with DMSO and pyrimethamine, whereas no lysis plaques were observed in the cultures with FR235222 or apicidin, another cyclopeptide inhibitor. We confirmed that this absence of proliferation was due to an effect of the drug on bradyzoites themselves, because the same effects were observed when the cystic cell wall was lysed with trypsin and when only bradyzoites were incubated in the medium. Thus, these data suggest that HDACis prevent bradyzoite-to-tachyzoite conversion in vitro, which was confirmed by quantitative analysis of the B1 gene (Fig. 2C). Bradyzoites were able to interconvert and proliferate when cysts were treated with DMSO or pyrimethamine, as assessed by the amount of DNA in each well. On the contrary, B1 DNA was detected in only small amounts when bradyzoite-containing cysts were treated with either FR235222 or apicidin (420-fold less compared to control) (Fig. 2C), reflecting the near absence of parasite growth. The very small amount of T. gondii DNA could be explained by the presence of residual parasites.
(ii) In vivo experiments.
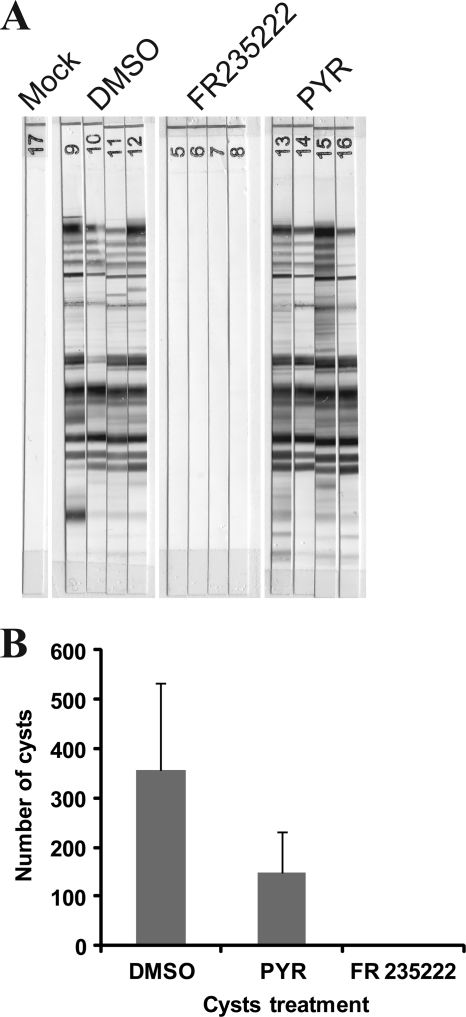
Intraperitoneally injected living cysts usually induce parasite dissemination, and mice become seropositive for anti-Toxoplasma IgG and chronically infected with Toxoplasma cysts in their brain tissue. The reinoculation of treated cysts is a good way to test the efficacy of a drug against these cysts (2). Six weeks after inoculation of drug- or DMSO-treated cysts, the mice were euthanatized. The presence or absence of cysts in their brain tissue was assessed by Dolichos lectin staining, which stains the cyst cell wall fluorescent green. While a significant number of cysts were detected in mice that received DMSO control- or PYR-treated cysts, no cysts were detected in mice inoculated with FR235222-treated cysts, suggesting the absence of toxoplasmosis in these mice (Fig. 3 C). Serological screening for T. gondii antibodies for each mouse confirmed the absence of any detectable serological reaction against the parasite using both the agglutination test and the Western blot methods (Fig. 3A).
FIG. 3.
Effect of FR235222 on ex vivo cysts under in vivo conditions by mouse infection assay. (A) Serological status by Western blot analysis 6 weeks after intraperitoneal inoculation of cysts formerly treated with DMSO, FR235222, or PYR, confirming the absence of any serological response against injected cysts formerly treated with FR235222. Cysts were treated with FR235222, PYR, or DMSO and were mock treated (the mice were not infected). (B) Average brain cyst count of mice infected with ex vivo-treated cysts. Pretreatments were with PYR, DMSO, and FR235222 (four mice for each pretreatment).
Proliferation assay with FR235222 derivatives.
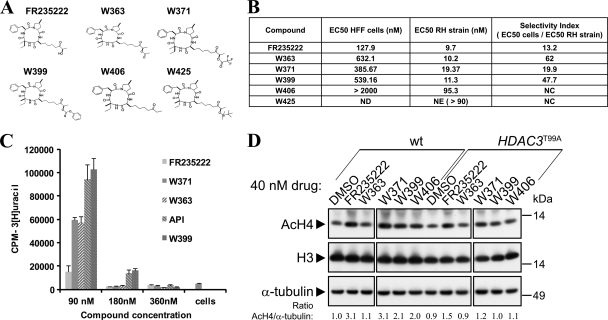
In vivo experiments require the use of nontoxic compounds to ensure a specific action on the target disease. FR235222 is a cyclic tetrapeptide that displays remarkable chemical and biophysical properties. Its typical drug profile is characterized by good membrane permeation and a high affinity with the targeted enzyme. It turns out that the effectiveness of FR235222 relies on the presence of certain residues present only on parasitic HDAC3 and not on human homologs, which makes it a good candidate for specific antiparasitic action (6). This compound is also an attractive lead for the generation of new and more effective derivatives. The conversion of FR235222 by hemisynthesis was easily achieved in one or two steps, and five derivatives have been made so far (Fig. 4 A). All EC50 results and the corresponding selectivity indexes of the tested drugs are shown in Fig. 4B. On RH strain tachyzoites, derived compounds W363 and W399 showed EC50s equivalent to the FR235222 EC50 (10.2 nM, 11.3 nM, and 9.7 nM, respectively). Other compounds were either less effective on the parasite (W371 EC50 = 19.3 nM; W406 EC50 = 95.3 nM) or failed to exhibit any potent activity (W425). The W363, W371, and W399 EC50s on dividing HFF host cells (632 nM, 385 nM, and 539 nM respectively) were above the FR235222 EC50 (127.9 nM). Consequently, W363 and W399 had improved selectivity against T. gondii, with the SIs (ratios of EC50 for cells/EC50 for the RH strain) being 62 and 47.7, respectively (Fig. 4B). It should be noted that FR235222 showed the lowest SI (13.2) among the compounds tested.
FIG. 4.
Study of FR235222 derivative drugs. (A) Structures of FR235222 and derivatives. (B) Selectivity indexes of the new molecular compounds, derivatives of FR235222. The reference strain for T. gondii was the RH strain, and toxicity on proliferating HFFs, commonly used as host cells for T. gondii, was evaluated. ND, not done; NE, not effective; NC, not calculated. (C) Proliferation assay of the mutant strain R20D9 TgHDAC3T99A with FR235222 and derivative compounds. The FR235222-resistant mutant showed a high rate of proliferation in the presence of the FR235222 derivative compounds, as was also the case for API, suggesting the central role of the T99A amino acid mutation in the drug susceptibility of normal T. gondii strains and the more specific targeting of these new compounds to the HDAC3 T99 residue. (D) Western blot analysis of the AcH4 signals before and after treatment with the different HDACi derivatives in the wild type (wt) and the TgHDAC3T99A resistant mutant. The experiment was done on the same blot, and the blot was subsequently cut for presentation in the figure.
To ascertain that the new derivatives are still acting directly on TgHDAC3, wild-type and mutant (R20D9) tachyzoites were incubated with the compounds and growth was monitored. All the compounds that remained active on the wild-type strain (W363, W399, W371, and W406) were no longer active on the FR235222-resistant mutant. As shown in Fig. 4C, the FR235222-resistant mutant (R20D9) was able to proliferate at an elevated rate in the presence of 90 nM the three active derivative compounds (W363, W399, W371) as well as apicidin. Residual parasite growth was noted even at 180 nM for W399 and apicidin. Therefore, these data indicate that the new compounds were still acting as HDACis and that TgHDAC3 remained the targeted enzyme. To determine whether growth inhibition by the compounds was associated with hyperacetylation of histone H4, AcH4 levels were analyzed by immunoblotting (Fig. 4D). As expected, parasites treated with most of the FR235222 derivatives (W371, W399, and W406) induced histone H4 hyperacetylation. The relative level of histone H4 acetylation was also reduced when these compounds were used on resistant TgHDAC3T99A mutant R20D9. Unexpectedly, the W363 compound failed to alter the levels of H4 acetylation (Fig. 4D), suggesting that its ability to inhibit tachyzoite growth is not mediated by or linked to the overall level of histone acetylation. This discrepancy is interesting and calls for further investigation (see Discussion).
DISCUSSION
New therapeutic targets are needed because of the poor therapeutic armamentarium for Apicomplexa-related disease and the rapid emergence of drug resistance (28).
One-third of the human population is currently and chronically infected with T. gondii cysts (25). Cysts containing bradyzoites are usually considered harmless in healthy individuals but may be a time bomb in the immunocompromised patient, because of their ability to liberate freshly converted invading tachyzoites. Once infection has spread, the rate of mortality among highly fragile patients, such as patients with hematological malignancies, is up to 90% (13). For these patients, a therapeutic goal would be to prevent bradyzoite conversion into spreading tachyzoites. So far, no drugs commonly used for toxoplasmosis have shown efficacy against tissue cysts. A first report in 1992 suggested that hydroxynaphthoquinone (atovaquone) had partial activity on cyst tissue in a mouse model (1). Unfortunately, the parasites have a strong ability to spontaneously develop drug resistance by mutation of the atovaquone-binding site on cytochrome b, and clinical cases of resistance have been described (3, 23). Atovaquone has never become a first-line treatment for toxoplasmosis, and its partial efficacy on parasite cysts has never been further explored (10).
Knowing the effect of FR235222 on the tachyzoite form of T. gondii, we logically sought to check its potential activity on the bradyzoite. FR235222 has a spectacular effect on bradyzoites obtained in vitro. This first observation leads us to believe that the HDACi FR235222 is able to cross the cell wall, alter the in vitro-induced cysts, and reach the contained bradyzoites. This hypothesis was tested on ex vivo Toxoplasma cysts. We first decided to use a rapid screening method using AO-BET staining, as described before with atovaquone (19). Using the first method, no drastic effect on cysts was evidenced with FR235222. Even more important, the external structure of the cyst was proved to remain intact under FR235222 conditions using Dolichos lectin staining. Considering that FR235222 pushed tachyzoites to differentiate by triggering the transcription of certain bradyzoite-sporozoite-specific genes (6), the question was raised as to whether this impact on the natural course of the T. gondii life cycle occurred on bradyzoites as well. In agreement with FR235222 inducing tachyzoite-to-bradyzoite conversion, in vitro and in vivo experiments proved that FR235222-treated cysts and bradyzoites were not able to convert into multiplying tachyzoites. Furthermore, even with 50 treated and injected cysts, no signs of any serological reaction could be detected in mice.
Altogether, FR235222 appeared to be a bradyzoite-to-tachyzoite conversion inhibitor. However, the molecular mechanism remains to be defined. Regarding FR235222 activity (i.e., as an HDACi), one can assume that epigenetic factors involved in bradyzoite stage conversion are probably profoundly and definitively disturbed. So far, stage conversion studies have focused on the tachyzoite-to-bradyzoite processes (21, 22, 32). This is easily explained by two main technical issues: bradyzoites have to be freshly isolated from tissue cysts, and once they are in culture, they inexorably convert into tachyzoites, preventing any further analysis. Nevertheless, bradyzoite-to-tachyzoite conversion is a major clinical event. Preventing the parasite differentiation process could be an effective way to prevent the parasite from spreading.
The HDACi cyclic tetrapeptide FR235222 has proved to inhibit T. gondii and Plasmodium species proliferation at the nanomolar level. Using genetic approaches, parasite lines resistant to this molecule were isolated; single-point mutations within TgHDAC3 (T99A or T99I) were sufficient to decrease the sensitivity of T. gondii parasites to FR235222 or apicidin, thus providing genetic evidence that TgHDAC3 is the drug-targeted enzyme. Interestingly, residue T99 along with amino acid A98 create an insertion within the catalytic site of the enzyme that is exclusively conserved in the Apicomplexa HDAC3 family of proteins and absent in any other eukaryotic HDACs characterized thus far (6). We observed that FR235222 inhibits parasite proliferation without affecting the human host cells monolayer and that parasites are 13 times more sensitive to FR235222 than HFF cells under proliferative conditions, thus revealing a certain specificity of action of FR235222 toward parasites. Though FR235222 is relatively nontoxic to primary host human cells (26), an increase in AcH4 levels in human cells was observed at high concentrations (1 μM) of FR235222, indicating that this compound is somehow active on one or multiple mammalian HDACs (15). Moreover, FR235222 has been proved to have some efficacy on a human leukemia cell line, with induction of apoptosis occurring at a high concentration (0.5 μM) (29). In the present study, we tailored the synthesis of new FR235222 derivative compounds to optimize this specific antiparasitic effect and to minimize potential effects on human cells. The new molecules W363 and W399 appeared to be effective on tachyzoites, with their EC50s being comparable to the FR235222 EC50. Interestingly, they are less effective than the parental drug, FR235222, on human cell proliferation, increasing the selectivity index. This correlates quite well with the increase in the levels of acetylated H4 upon treatment with these compounds and remained unchanged in the mutant background involving TgHDAC3 as a major target for these new molecules. Surprisingly, the W363 molecule is unable to enhance the level of acetylation of histone H4, despite its ability to inhibit growth efficiently. Currently, we do not have an explanation. A possible hypothesis could be that W363 affects TgHDAC3 activity in a substrate-specific manner; given that HDACs work in multisubunit complexes to deacetylate different substrates (33), it is therefore possible that W363 targets a particular HDAC complex in charge of regulating the acetylation status of only a subset of histones at certain loci or a specific nonhistone protein directly responsible for parasite growth arrest. Overall, the antiparasitic activity of this drug family contributes an attractive perspective to developing new compounds effective against toxoplasmosis, malaria, and other apicomplexan diseases.
The findings of the present study extend our knowledge of how FR235222 and derivatives act on the tachyzoite and bradyzoite forms. Immunofluorescence microscopy analysis indicated that FR235222 induced a polyploidy of in vitro-induced bradyzoites in a fashion similar to that for tachyzoites, and in vivo experiments indicated that FR235222 is able to access the bradyzoites within the cyst: we observed that brain cysts treated with FR235222 were no longer capable of inducing toxoplasmosis in the mouse model. The ability of FR235222 to permeate the membrane wall is a major advantage for crossing the blood-brain barrier and central nervous system tissue where Toxoplasma cysts are located (31). This opens a promising way to develop drugs that are selective against Toxoplasma and that have sterilizing activity, especially in patients with cysts, who are at risk for reactivating acute toxoplasmosis (patients with HIV infection, hematological malignancies, or transplantation). Still, its effectiveness in vivo against chronically infected mice remains to be directly demonstrated.
Supplementary Material
Acknowledgments
This study was supported by grants from CNRS (ATIP+), ANR (Agence National de la Recherche, MIME Program), Lyon-Biopôle, and INSERM (Contrat d'Interface).
We thank Astellas Pharma Inc. for the kind gift of the FR235222 compound. We are grateful to C. Barois, C. Mur, and A. Meunier for technical support with RT-PCR. We thank L. Northrup for editing the English manuscript.
Footnotes
Published ahead of print on 16 August 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Araujo, F. G., J. Huskinson-Mark, W. E. Gutteridge, and J. S. Remington. 1992. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob. Agents Chemother. 36:326-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo, F. G., J. Huskinson, and J. S. Remington. 1991. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob. Agents Chemother. 35:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baatz, H., A. Mirshahi, J. Puchta, H. Gumbel, and L. O. Hattenbach. 2006. Reactivation of Toxoplasma retinochoroiditis under atovaquone therapy in an immunocompetent patient. Ocul. Immunol. Inflamm. 14:185-187. [DOI] [PubMed] [Google Scholar]
- 4.Beck, H. P., D. Blake, M. L. Darde, I. Felger, S. Pedraza-Diaz, J. Regidor-Cerrillo, M. Gomez-Bautista, L. M. Ortega-Mora, L. Putignani, B. Shiels, A. Tait, and W. Weir. 2009. Molecular approaches to diversity of populations of apicomplexan parasites. Int. J. Parasitol. 39:175-189. [DOI] [PubMed] [Google Scholar]
- 5.Boothroyd, J. C. 2009. Toxoplasma gondii: 25 years and 25 major advances for the field. Int. J. Parasitol. 39:935-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bougdour, A., D. Maubon, P. Baldacci, P. Ortet, O. Bastien, A. Bouillon, J. C. Barale, H. Pelloux, R. Menard, and M. A. Hakimi. 2009. Drug inhibition of HDAC3 and epigenetic control of differentiation in Apicomplexa parasites. J. Exp. Med. 206:953-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bougdour, A., C. F. Sautel, D. Cannella, L. Braun, and M. A. Hakimi. 2008. Toxoplasma gondii gene expression is under the control of regulatory pathways acting through chromatin structure. Parasite 15:206-210. [DOI] [PubMed] [Google Scholar]
- 8.Braun, L., D. Cannella, A. M. Pinheiro, S. Kieffer, H. Belrhali, J. Garin, and M. A. Hakimi. 2009. The small ubiquitin-like modifier (SUMO)-conjugating system of Toxoplasma gondii. Int. J. Parasitol. 39:81-90. [DOI] [PubMed] [Google Scholar]
- 9.Brenier-Pinchart, M. P., V. Morand-Bui, H. Fricker-Hidalgo, V. Equy, R. Marlu, and H. Pelloux. 2007. Adapting a conventional PCR assay for Toxoplasma gondii detection to real-time quantitative PCR including a competitive internal control. Parasite 14:149-154. [DOI] [PubMed] [Google Scholar]
- 10.Chirgwin, K., R. Hafner, C. Leport, J. Remington, J. Andersen, E. M. Bosler, C. Roque, N. Rajicic, V. McAuliffe, P. Morlat, D. T. Jayaweera, J. L. Vilde, and B. J. Luft. 2002. Randomized phase II trial of atovaquone with pyrimethamine or sulfadiazine for treatment of toxoplasmic encephalitis in patients with acquired immunodeficiency syndrome: ACTG 237/ANRS 039 Study. AIDS Clinical Trials Group 237/Agence Nationale de Recherche sur le SIDA, Essai 039. Clin. Infect. Dis. 34:1243-1250. [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen, A. W., J. P. Overdulve, and J. M. Hoenderboom. 1981. Separation of Isospora (Toxoplasma) gondii cysts and cystozoites from mouse brain tissue by continuous density-gradient centrifugation. Parasitology 83:103-108. [DOI] [PubMed] [Google Scholar]
- 12.Darkin-Rattray, S. J., A. M. Gurnett, R. W. Myers, P. M. Dulski, T. M. Crumley, J. J. Allocco, C. Cannova, P. T. Meinke, S. L. Colletti, M. A. Bednarek, S. B. Singh, M. A. Goetz, A. W. Dombrowski, J. D. Polishook, and D. M. Schmatz. 1996. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. U. S. A. 93:13143-13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fricker-Hidalgo, H., C. E. Bulabois, M. P. Brenier-Pinchart, R. Hamidfar, F. Garban, J. P. Brion, J. F. Timsit, J. Y. Cahn, and H. Pelloux. 2009. Diagnosis of toxoplasmosis after allogeneic stem cell transplantation: results of DNA detection and serological techniques. Clin. Infect. Dis. 48:e9-e15. [DOI] [PubMed] [Google Scholar]
- 14.Fung, H. B., and H. L. Kirschenbaum. 1996. Treatment regimens for patients with toxoplasmic encephalitis. Clin. Ther. 18:1037-1056. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Paloma, L., I. Bruno, E. Cini, S. Khochbin, M. Rodriquez, M. Taddei, S. Terracciano, and K. Sadoul. 2007. Design and synthesis of cyclopeptide analogues of the potent histone deacetylase inhibitor FR235222. ChemMedChem 2:1511-1519. [DOI] [PubMed] [Google Scholar]
- 16.Gross, U., H. Bormuth, C. Gaissmaier, C. Dittrich, V. Krenn, W. Bohne, and D. J. Ferguson. 1995. Monoclonal rat antibodies directed against Toxoplasma gondii suitable for studying tachyzoite-bradyzoite interconversion in vivo. Clin. Diagn. Lab. Immunol. 2:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakimi, M. A., and K. W. Deitsch. 2007. Epigenetics in Apicomplexa: control of gene expression during cell cycle progression, differentiation and antigenic variation. Curr. Opin. Microbiol. 10:357-362. [DOI] [PubMed] [Google Scholar]
- 18.Hill, D. E., S. Chirukandoth, and J. P. Dubey. 2005. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 6:41-61. [DOI] [PubMed] [Google Scholar]
- 19.Huskinson-Mark, J., F. G. Araujo, and J. S. Remington. 1991. Evaluation of the effect of drugs on the cyst form of Toxoplasma gondii. J. Infect. Dis. 164:170-171. [DOI] [PubMed] [Google Scholar]
- 20.Iyer, L. M., V. Anantharaman, M. Y. Wolf, and L. Aravind. 2008. Comparative genomics of transcription factors and chromatin proteins in parasitic protists and other eukaryotes. Int. J. Parasitol. 38:1-31. [DOI] [PubMed] [Google Scholar]
- 21.Kim, S. K., and J. C. Boothroyd. 2005. Stage-specific expression of surface antigens by Toxoplasma gondii as a mechanism to facilitate parasite persistence. J. Immunol. 174:8038-8048. [DOI] [PubMed] [Google Scholar]
- 22.Lyons, R. E., R. McLeod, and C. W. Roberts. 2002. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 18:198-201. [DOI] [PubMed] [Google Scholar]
- 23.Megged, O., I. Shalit, I. Yaniv, J. Stein, S. Fisher, and I. Levy. 2008. Breakthrough cerebral toxoplasmosis in a patient receiving atovaquone prophylaxis after a hematopoietic stem cell transplantation. Pediatr. Transplant. 12:902-905. [DOI] [PubMed] [Google Scholar]
- 24.Meneceur, P., M. A. Bouldouyre, D. Aubert, I. Villena, J. Menotti, V. Sauvage, J. F. Garin, and F. Derouin. 2008. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob. Agents Chemother. 52:1269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 26.Mori, H., Y. Urano, F. Abe, S. Furukawa, S. Furukawa, Y. Tsurumi, K. Sakamoto, M. Hashimoto, S. Takase, M. Hino, and T. Fujii. 2003. FR235222, a fungal metabolite, is a novel immunosuppressant that inhibits mammalian histone deacetylase (HDAC). I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. (Tokyo) 56:72-79. [PubMed] [Google Scholar]
- 27.Nwaka, S., and A. Hudson. 2006. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 5:941-955. [DOI] [PubMed] [Google Scholar]
- 28.Olliaro, P. 2005. Drug resistance hampers our capacity to roll back malaria. Clin. Infect. Dis. 41(Suppl. 4):S247-S257. [DOI] [PubMed] [Google Scholar]
- 29.Petrella, A., C. W. D'Acunto, M. Rodriquez, M. Festa, A. Tosco, I. Bruno, S. Terracciano, M. Taddei, L. G. Paloma, and L. Parente. 2008. Effects of FR235222, a novel HDAC inhibitor, in proliferation and apoptosis of human leukaemia cell lines: role of annexin A1. Eur. J. Cancer 44:740-749. [DOI] [PubMed] [Google Scholar]
- 30.Pfefferkorn, E. R., and L. C. Pfefferkorn. 1977. Specific labeling of intracellular Toxoplasma gondii with uracil. J. Protozool. 24:449-453. [DOI] [PubMed] [Google Scholar]
- 31.Rezai, T., J. E. Bock, M. V. Zhou, C. Kalyanaraman, R. S. Lokey, and M. P. Jacobson. 2006. Conformational flexibility, internal hydrogen bonding, and passive membrane permeability: successful in silico prediction of the relative permeabilities of cyclic peptides. J. Am. Chem. Soc. 128:14073-14080. [DOI] [PubMed] [Google Scholar]
- 32.Saksouk, N., M. M. Bhatti, S. Kieffer, A. T. Smith, K. Musset, J. Garin, W. J. Sullivan, Jr., M. F. Cesbron-Delauw, and M. A. Hakimi. 2005. Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol. Cell. Biol. 25:10301-10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahbazian, M. D., and M. Grunstein. 2007. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 76:75-100. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan, W. J., Jr., and M. A. Hakimi. 2006. Histone mediated gene activation in Toxoplasma gondii. Mol. Biochem. Parasitol. 148:109-116. [DOI] [PubMed] [Google Scholar]
- 35.Tardieux, I., and R. Menard. 2008. Migration of Apicomplexa across biological barriers: the Toxoplasma and Plasmodium rides. Traffic 9:627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van, T. T., S.-K. Kim, M. Camps, J. C. Boothroyd, and L. J. Knoll. 2007. The BSR4 protein is up-regulated in Toxoplasma gondii bradyzoites, however the dominant surface antigen recognised by the P36 monoclonal antibody is SRS9. Int. J. Parasitol. 37:877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.