Abstract
ACHN-490 is a neoglycoside, or “next-generation” aminoglycoside (AG), that has been identified as a potentially useful agent to combat drug-resistant bacteria emerging in hospitals and health care facilities around the world. A focused medicinal chemistry campaign produced a collection of over 400 sisomicin analogs from which ACHN-490 was selected. We tested ACHN-490 against two panels of Gram-negative and Gram-positive pathogens, many of which harbored AG resistance mechanisms. Unlike legacy AGs, ACHN-490 was active against strains expressing known AG-modifying enzymes, including the three most common such enzymes found in Enterobacteriaceae. ACHN-490 inhibited the growth of AG-resistant Enterobacteriaceae (MIC90, ≤4 μg/ml), with the exception of Proteus mirabilis and indole-positive Proteae (MIC90, 8 μg/ml and 16 μg/ml, respectively). ACHN-490 was more active alone in vitro against Pseudomonas aeruginosa and Acinetobacter baumannii isolates with AG-modifying enzymes than against those with altered permeability/efflux. The MIC90 of ACHN-490 against AG-resistant staphylococci was 2 μg/ml. Due to its promising in vitro and in vivo profiles, ACHN-490 has been advanced into clinical development as a new antibacterial agent.
Aminoglycosides (AGs) are highly potent, broad-spectrum antibiotics used for treating serious bacterial infections. They are a well-established class of antibacterials that act by binding to the A-site of the bacterial ribosome and interfering with normal protein synthesis. They are frequently used empirically for treating complicated urinary tract infections, nosocomial respiratory tract infections, complicated intra-abdominal infections, and septicemia (4). AGs and β-lactam antibiotics are sometimes used in combination for treating suspected or confirmed Pseudomonas aeruginosa infections, as well as empirically in endocarditis.
As seen with other antibiotic classes, resistance to AGs has emerged in the 50 years since their introduction. For example, gentamicin (GEN) resistance (MIC, >4 μg/ml) was seen in 31.4% of P. aeruginosa isolates from urinary tract infections collected in Europe and the Americas in 2000 (SENTRY Antimicrobial Surveillance Program) (12). Amikacin (AMK) resistance (MIC, >16 μg/ml) was seen in 16.7% of the same isolates. Enterobacteriaceae have also shown resistance to AMK (9). Among isolates collected from intra-abdominal infections worldwide in 2004 (Study for Monitoring Antibiotic Resistance Trends [SMART]), 9.0% of extended-spectrum β-lactamase-positive (ESBL+) Escherichia coli and 31.5% of ESBL+ Klebsiella pneumoniae were resistant to AMK (19). Other AGs were not examined in that study.
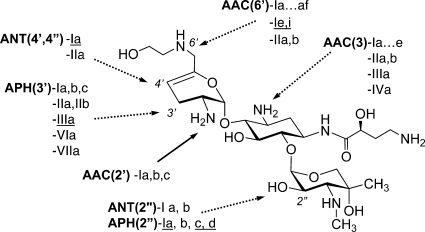
At present, the most significant contribution to clinical resistance is represented by diverse AG-modifying enzymes (AMEs) (Fig. 1) that inactivate AGs by N-acetylation (AG acetyltransferases [AAC]), O-adenylylation (AG nucleotidyltransferases [ANT]), or O-phosphorylation (AG phosphotransferases [APH]) (11). Numerous such enzymes have been identified, and they often occur in combinations that can impart broad AG resistance (15). Less common AG resistance mechanisms (AGRMs) among the Enterobacteriaceae involve the regulation of intracellular drug concentration by overexpression of efflux pumps and the expression of enzymes that modify the ribosomal target (10). As broad-spectrum antibiotics, including the existing AGs cephalosporins, carbapenems, and fluoroquinolones, are rendered ineffective by increasing resistance, new AGs are an attractive option for the treatment of serious infections, especially if developed with optimized dosing regimens to maximize safety and efficacy (7).
FIG. 1.
ACHN-490 structure and AMEs from Gram-negative and Gram-positive (underlined) organisms. AMEs shown with dotted arrows cannot modify ACHN-490.
In the present study, we modified sisomicin (SIS) in search of substituents that would improve the in vitro potency against AG-resistant (AG-R) bacterial strains. Among the SIS derivatives we synthesized, the novel compound ACHN-490 showed promise in a screening panel and thus was tested against a larger panel that included 461 recent clinical isolates of Gram-negative and Gram-positive organisms. The majority of the pathogens in these panels carried AGRMs, and many were also resistant to other classes of antibiotics. The broad-spectrum in vitro activity demonstrated in these experiments supports the continued development of ACHN-490.
(These data were presented at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, September 2009 [1].)
MATERIALS AND METHODS
Synthesis of ACHN-490.
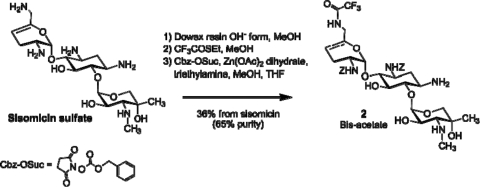
SIS was used to derive over 400 neoglycoside compounds. The lead compound, ACHN-490, was synthesized in an eight-step process as follows (Fig. 2). SIS sulfate (compound 1) was rendered basic by treatment with ion exchange resin, followed by reaction with ethyl trifluorothioacetate to selectively form the 6′-trifluoroacetamide (17). Treatment of the 6′-trifluoroacetamide with Zn(II) acetate and benzyloxycarbonyl (Z)-succinimide resulted in selective Z-blocking of both the 2′ and 3′ positions to form intermediate 2 (14, 18).
FIG. 2.
Conversion of SIS sulfate to intermediate 2.
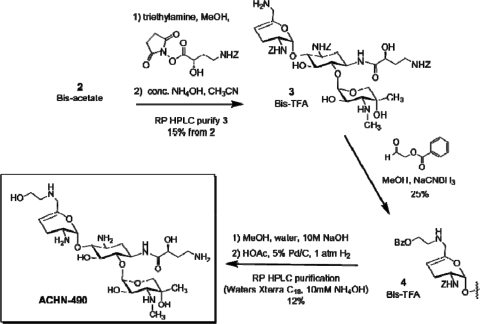
Reaction of intermediate 2 with the active ester of N-Z-(S)-hydroxy-aminobutyric acid [N-Z-(S)-HABA] resulted in selective N-1 acylation (Fig. 3). Subsequent treatment with concentrated ammonia liberated the 6′-amino functionality to produce compound 3. Reductive alkylation with O-benzoylglycolaldehyde appended the masked hydroxyethyl group to the 6′-position to give compound 4. Finally, basic hydrolysis of the O-benzoyl group followed by catalytic hydrogenolysis of the Z-groups and high-performance liquid chromatography purification gave ACHN-490. Key structural assumptions were confirmed by liquid chromatography-mass spectrometry fragmentation and two-dimensional nuclear magnetic resonance analysis.
FIG. 3.
Completion of the synthesis of ACHN-490.
In vitro activity of ACHN-490.
The antibacterial activities of ACHN-490 and comparator antibiotics were determined according to the broth microdilution method recommended by the Clinical and Laboratory Standards Institute (5). A screening panel composed of 25 strains with diverse AGRMs was used to compare the activities of the SIS derivatives generated during the discovery program. This panel included strains with key AMEs found in both Gram-negative bacteria and Staphylococcus aureus, as well as strains with changes in permeability or efflux and a representative ribosomal methyltransferase (armA).
Compounds that demonstrated increased activity against strains with AGRMs in the screening panel were tested against a larger panel of 461 clinical isolates from diverse geographic regions isolated in the years 2004 to 2006. The panel consisted primarily of Gram-negative bacteria, including multiple species of Enterobacteriaceae, P. aeruginosa, and Acinetobacter spp. Gram-positive bacteria included S. aureus and Staphylococcus epidermidis.
The collection was segregated and analyzed according to the resistance status against three AGs: AMK, GEN, and tobramycin (TOB). AG-susceptible (AG-S) isolates had AMK MICs of ≤16 μg/ml and GEN and TOB MICs of ≤4 μg/ml. AG-R isolates were those with AMK MICs of >16 μg/ml and/or GEN MICs of >4 μg/ml and/or TOB MICs of >4 μg/ml. To gauge the extent of antibiotic resistance present in AG-R isolates, we used the broth microdilution method to determine MICs for a panel of antibiotics from other classes (5). These included the β-lactams imipenem (IMI) and ceftobiprole (BPR), the fluoroquinolone ciprofloxacin (CIP), and the glycylcycline tigecycline (TGC).
We then identified the specific AGRMs present in AG-R isolates in an effort to establish the mechanisms to which ACHN-490 is immune and also those responsible for any observed decrease in ACHN-490 activity. The AMEs in the larger collection were initially assigned on the basis of antibiograms using standard AGs (21). Colony PCR was used to confirm the genes for six of the most common AMEs in Gram-negative bacteria and seven AMEs in Gram-positive bacteria, using the primers listed in Table 1.
TABLE 1.
Primer sequences used to confirm AMEs present in AG-R clinical isolates
| AME | Forward primer | Reverse primer |
|---|---|---|
| Gram-negative AMEs | ||
| AAC(3)-Ia | ACAAAGTTAGGTGGCTCAAGTATGGGCATC | TCACCGTAATCTGCTTGCACGTAGATCAC |
| AAC(3)-IIa | GCCGACTGGCACTGTGATGGGATAC | TGCAATGCGGTAACGGAGTTTAGCG |
| AAC(3)-IVa | CTCGAAGATGGGCCACTTGGACTGATC | AACTCGGCAAGATGCAGCGTCGTG |
| AAC(6′)-Ib/II | GTGACCAACAGCAACGATTCCGTCAC | GTGACCTCGGGATCATTGAACAGCAAC |
| ANT(2″)-Ia | GTGTAACACGCAAGCACGATGATATTGATCTG | CGAGCCTGTAGGACTCTATGTGCTTTGTAGG |
| APH(3)-VIa | CACATACAGTGTCTCTCGTGAAGCGAAAATG | CGTGATATCGCCATGAGAAAAAACCAATC |
| Gram-positive AMEs | ||
| AAC(6′)-Im | CGCCCGATGAATGAGGATGAT | TCCTGCCTTCTGATATGCTCG |
| ANT(4′)-Ia | ATGGACAACCGGTGAGTGGAA | CGTTCTGTCCACTCCTGAATC |
| APH(2″)-Ia + AAC(6′)-Ie | TTGAAATAATCGGTAGTGGTTATGATAGTGTGGCA | CCCTCAAAAACTGTTGTTGCATTTAGTCTTTCC |
| APH(2″)-Ib | GTCCGGCGATGATAGTGATAC | CGGCGCCTTATGCTGATAGTA |
| APH(2″)-Ic | GAGTCAACAAGGTGCAGACGA | CTCGCCTCTATAAGCCATCAC |
| APH(2″)-Id | CAGGAGAAGGCAATGACTGT | CGGGATCAGAAATAGCTGCA |
| APH(3′)-III | TGACGGACAGCCGGTATAAAG | GGCGCAGAAGGGAATGTCAT |
RESULTS
The in vitro activities of ACHN-490 and comparator AGs against 25 strains having diverse AGRMs are shown in Table 2. ACHN-490 showed potent in vitro activity (MIC, ≤8 μg/ml) against all but three strains tested; for 16 of 25 strains, the MIC was ≤2 μg/ml. ACHN-490 was more potent than comparator AGs against Escherichia coli, Acinetobacter baumannii, Acinetobacter calcoaceticus, and several strains of P. aeruginosa and S. aureus carrying one or more AMEs. However, ACHN-490 was not active against a strain expressing armA methylase or against Providencia stuartii carrying the AAC(2′)-I enzyme.
TABLE 2.
Activities of ACHN-490 and comparator aminoglycosides against strains with diverse AGRMs
| Species | Phenotype | MIC (μg/ml) |
|||
|---|---|---|---|---|---|
| SIS | AMK | GEN | ACHN-490a | ||
| Escherichia coli | ATCC 25922 (normal) | 0.5 | 2 | 1 | 1 |
| ANT(2″)-I | 64 | 4 | >64 | 1 | |
| AAC(6′)-I | 32 | 32 | 4 | 0.25 | |
| AAC(3)-II | >64 | 4 | >64 | 2 | |
| APH(3′)-Ib | 0.25 | 0.5 | 0.25 | 0.25 | |
| AAC(3)-IVa | 32 | 4 | 32 | 1 | |
| armA methylase | >64 | >64 | >64 | >64 | |
| Klebsiella pneumoniae | ATCC 10031 | 0.25 | 0.5 | 0.125 | 0.25 |
| Providencia stuartii | AAC(2′)-I | 64 | 8 | >64 | >64 |
| Serratia marcescens | ANT(2″), AAC(6′) | 8 | 8 | 4 | 2 |
| Staphylococcus aureus | ATCC 29213 (normal) | 0.5 | 4 | 0.5 | 1 |
| ANT(4′)-I | 1 | >64 | 0.5 | 1 | |
| APH(3′)-III | 0.25 | 2 | 0.5 | 0.5 | |
| APH(2″) + AAC(6′) | >64 | 64 | >64 | 4 | |
| Acinetobacter spp. | APH(3′)-VI | 0.5 | >64 | 1 | 1 |
| Acinetobacter baumannii | Susceptible | 0.5 | 2 | 1 | 4 |
| ATCC 19606 | 8 | 16 | 16 | 8 | |
| Acinetobacter calcoaceticus | AAC(6′)-I | 32 | 32 | 8 | 2 |
| Pseudomonas aeruginosa | ATCC 27853 (normal) | 0.5 | 2 | 1 | 2 |
| Wild-type pump | 1 | 4 | 1 | 4 | |
| ΔEfflux pumps | 0.125 | 0.5 | 0.125 | 0.125 | |
| MexXY upb | 1 | 4 | 2 | 8 | |
| ANT(4′)-II | 1 | 32 | 4 | 4 | |
| AAC(3)-I | 32 | 4 | 64 | 8 | |
| AAC(6′)-II | 32 | 4 | 32 | 2 | |
ACHN-490 showed improved activity over SIS, AMK, and GEN against Gram-negative strains and staphylococci with defined AGRMs. ACHN-490 activity was not altered by the presence of AMEs. Values in boldface indicate ACHN-490 MICs that were improved ≥4-fold relative to SIS.
MexXY up, upregulation of mexXY operon.
The activity of ACHN-490 was further assessed against a collection of 461 clinical isolates consisting of representative nosocomial and community pathogens. ACHN-490 displayed potent activity against a broad range of Gram-negative and Staphylococcus spp. isolates, including those showing AG resistance (Table 3). It is notable that many AG-R isolates were also resistant to other classes of antibiotics in vitro (Table 4). Although ACHN-490 was broadly active against these bacteria, the modal MICs of the AG-R isolates of Acinetobacter spp. and P. aeruginosa were 4- to 8-fold higher than the corresponding values for the AG-S populations of these species. No AMEs were identified that correlated with the increased MICs observed in some isolates of these species. The presence of AMEs alone did not alter the activity of ACHN-490 in several species backgrounds (Table 5).
TABLE 3.
Activities of ACHN-490 against 461 AG-S and AG-R isolates
| Species | AG susceptibility (n) | No. of isolates with indicated MIC (μg/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | >64 | ||
| Citrobacter spp. | S (15) | 3 | 10 | 2 | |||||||
| R (9) | 3 | 5 | 1 | ||||||||
| Escherichia coli | S (15) | 1 | 5 | 6 | 3 | ||||||
| R (24) | 3 | 14 | 6 | 1 | |||||||
| Enterobacter spp. | S (15) | 2 | 6 | 5 | 1 | 1 | |||||
| R (20) | 2 | 10 | 6 | 1 | 1 | ||||||
| Klebsiella spp. | S (15) | 1 | 12 | 2 | |||||||
| R (45) | 1 | 23 | 18 | 2 | 1 | ||||||
| Proteus mirabilis | S (7) | 3 | 2 | 1 | 1 | ||||||
| R (16) | 1 | 7 | 7 | 1 | |||||||
| Proteae, indole+ | S (23) | 2 | 12 | 7 | 1 | 1 | |||||
| R (8) | 2 | 4 | 2 | ||||||||
| Salmonella and Shigella spp. | S (13) | 1 | 8 | 2 | 2 | ||||||
| R (1) | 1 | ||||||||||
| Serratia spp. | S (20) | 5 | 14 | 1 | |||||||
| R (8) | 2 | 4 | 1 | 1 | |||||||
| Staphylococcus spp. | S (10) | 2 | 5 | 2 | 1 | ||||||
| R (49) | 3 | 21 | 18 | 6 | 1 | ||||||
| Acinetobacter spp. | S (15) | 1 | 3 | 10 | 1 | ||||||
| R (67) | 1 | 2 | 16 | 8 | 19 | 15 | 5 | 1 | |||
| Pseudomonas aeruginosa | S (15) | 3 | 11 | 1 | |||||||
| R (51) | 1 | 3 | 3 | 19 | 10 | 8 | 3 | 4 | |||
TABLE 4.
In vitro activities of ACHN-490 and comparators against AG-R isolatesa
| Species (n) | AG | MIC50 (μg/ml) | MIC90 (μg/ml) | MIC range |
|---|---|---|---|---|
| Citrobacter spp. (9) | ACHN-490 | 0.5 | —b | 0.25-1 |
| AMK | 32 | — | 2-64 | |
| GEN | >64 | — | 2 to >64 | |
| BPR | 1 | — | <0.03 to >32 | |
| CIP | 8 | — | <0.015 to >16 | |
| IMI | 0.5 | — | 0.125-4 | |
| TGC | 0.5 | — | 0.25-1 | |
| Escherichia coli (24) | ACHN-490 | 1 | 2 | 0.5-16 |
| AMK | 16 | 32 | 2-64 | |
| GEN | 64 | >64 | 1 to >64 | |
| BPR | >32 | >32 | <0.03 to >32 | |
| CIP | >16 | >16 | <0.015 to >16 | |
| IMI | 0.125 | 0.25 | 0.06-0.5 | |
| TGC | 0.25 | 0.5 | 0.06-1 | |
| Enterobacter spp. (20) | ACHN-490 | 0.5 | 1 | 0.25-8 |
| AMK | 8 | 64 | 1 to >64 | |
| GEN | 32 | >64 | 0.5 to >64 | |
| BPR | >32 | >32 | 0.06 to >32 | |
| CIP | 1 | >16 | <0.015 to >16 | |
| IMI | 1 | 4 | 0.125 to >16 | |
| TGC | 0.5 | 4 | 0.125-8 | |
| Klebsiella spp. (45) | ACHN-490 | 0.5 | 1 | 0.25-8 |
| AMK | 32 | >64 | 2 to >64 | |
| GEN | 32 | >64 | 0.5 to >64 | |
| BPR | >32 | >32 | 0.125 to >32 | |
| CIP | 16 | >16 | <0.015 to >16 | |
| IMI | 0.25 | >16 | 0.06 to >16 | |
| TGC | 1 | 2 | 0.125-8 | |
| Proteus mirabilis (16) | ACHN-490 | 4 | 8 | 1-16 |
| AMK | 8 | 64 | 8 to >64 | |
| GEN | 32 | >64 | 1 to >64 | |
| BPR | 0.125 | >32 | <0.03 to >32 | |
| CIP | 2 | >16 | 0.03 to >16 | |
| IMI | 4 | 8 | 0.25 to >16 | |
| TGC | 4 | 8 | 1-8 | |
| Proteae, indole+ (8) | ACHN-490 | 8 | — | 4-16 |
| AMK | 8 | — | 4-32 | |
| GEN | >64 | — | 16 to >64 | |
| BPR | >32 | — | 0.06 to >32 | |
| CIP | 16 | — | 1 to >16 | |
| IMI | 1 | — | 1-2 | |
| TGC | 2 | — | 2-8 | |
| Serratia spp. (9) | ACHN-490 | 1 | — | 0.5-4 |
| AMK | 8 | — | 0.5 to >64 | |
| GEN | 64 | — | 1 to >64 | |
| BPR | >32 | — | 0.5 to >32 | |
| CIP | 4 | — | 0.06-16 | |
| IMI | 1 | — | 0.125-2 | |
| TGC | 2 | — | 1-8 | |
| Staphylococcus spp. (49) | ACHN-490 | 1 | 2 | 0.25-4 |
| AMK | 16 | >64 | 0.5 to >64 | |
| GEN | 32 | >64 | 0.25 to >64 | |
| BPR | NDc | ND | ND | |
| CIP | 16 | >16 | 0.125 to >16 | |
| IMI | ND | ND | ND | |
| TGC | 0.25 | 0.5 | 0.03-0.5 | |
| Acinetobacter spp. (67) | ACHN-490 | 16 | 32 | 1 to >64 |
| AMK | >64 | >64 | 4 to >64 | |
| GEN | >64 | >64 | 2 to >64 | |
| BPR | >32 | >32 | 0.5 to >32 | |
| CIP | >16 | >16 | 0.25 to >16 | |
| IMI | 2 | >16 | 0.125 to >16 | |
| TGC | 2 | 16 | 0.5 to >16 | |
| Pseudomonas aeruginosa (51) | ACHN-490 | 8 | 64 | 0.5 to >64 |
| AMK | 16 | >64 | 1 to >64 | |
| GEN | >64 | >64 | 8 to >64 | |
| BPR | 16 | >32 | 4 to >32 | |
| CIP | 8 | >16 | 0.03 to >16 | |
| IMI | 4 | 16 | 0.125 to >16 | |
| TGC | 16 | >16 | 1 to >16 |
ACHN-490 retains activity in the presence of AGRMs that inactivate AMK and GEN and in strains that are resistant to several other antibiotic classes.
The MIC90 was not calculated for groups for which n was <10.
ND, not determined.
TABLE 5.
Activities of ACHN-490 against AG-R clinical isolates with a single AMEa
| Species | Phenotype | n | Geometric mean MIC (μg/ml) of: |
||
|---|---|---|---|---|---|
| AMK | GEN | ACHN-490 | |||
| Enterobacteriaceae | |||||
| E. coli | ATCC 25922 (normal) | 1 | 3 | 1 | 1 |
| K. pneumoniae | AAC(3)-I | 1 | 4 | 32 | 1 |
| Citrobacter freundii, Enterobacter aerogenes, Enterobacter cloacae, E. coli, Klebsiella oxytoca, K. pneumoniae, Serratia liquefaciens | AAC(3)-II | 22 | 4 | 62 | 1 |
| E. coli, K. pneumoniae | AAC(3)-IV | 2 | 2 | 16 | 1 |
| C. freundii, E. aerogenes, E. cloacae, E. coli, K. oxytoca, K. pneumoniae, S. marcescens | AAC(6′)-I | 26 | 30 | 1 | 1 |
| Enterobacter gergoviae, K. pneumoniae, E. cloacae, E. coli, S. marcescens | ANT(2″)-I | 7 | 2 | 35 | 1 |
| Staphylococci | |||||
| S. aureus | ATCC 29213 (normal) | 1 | 3 | 1 | 1 |
| S. aureus, S. epidermidis | ANT(4′,4″)-I | 9 | 13 | 1 | 1 |
| S. aureus, S. epidermidis | APH(2″) + AAC(6′)-I | 22 | 18 | 50 | 1 |
| S. aureus, S. epidermidis | APH(3′)-III | 14 | 4 | 1 | 1 |
| A. baumannii | (Normal) | 1 | 4 | 1 | 2 |
| AAC(3′)-I | 2 | 4 | 16 | 4 | |
| ANT(2″)-I | 1 | 16 | >64 | 2 | |
| APH(3′)-VI | 3 | >64 | 4 | 3 | |
| P. aeruginosa | ATCC 27853 (normal) | 1 | 2 | 1 | 3 |
| AAC(6′)-II | 5 | 14 | 64 | 6 | |
| ANT(2″)-I | 5 | 8 | 56 | 3 | |
ACHN-490 activity was not altered by the presence of various AMEs. Only isolates with a single AME are reported here; those with combinations of AMEs (other than APH(3′)-I/II, which generates kanamycin/neomycin resistance) were excluded for the sake of clarity.
DISCUSSION
Compared to the activities of SIS, AMK, and GEN, the specific structural modifications incorporated in ACHN-490 resulted in equivalent or improved activity against bacterial strains carrying common AGRMs. In Enterobacteriaceae, resistance to AGs is primarily caused by AMEs, either alone or in combination with other AMEs (16). These AMEs are diverse with respect to the positions on the AG scaffold that they attack and the number of different genes that encode them. The enzymes may be present on transferable elements or chromosomally, as in the AAC(6′)-Ic enzyme found in Serratia marcescens (24). Many of these enzymes cause the MICs of widely used AGs such as AMK and GEN to increase beyond a clinically useful level.
As shown in Tables 2 and 5, virtually all AMEs that modify AMK or GEN have no impact on the activity of ACHN-490 across different species backgrounds. ACHN-490 and its parent molecule, SIS, naturally lack the 3′- and 4′-OH groups, which protects them from the APH(3′) and ANT(4′) enzymes that generate resistance to AMK (Fig. 1). The introduction of the HABA substituent provides protection from the AAC(3), ANT(2″), and APH(2″) AMEs. The hydroxyethyl substituent at the 6′ position blocks the multitude of AAC(6′) AMEs without reducing potency, as had been found with other efforts to shield this position (17). The prevalence of each AME varies geographically, but by far the three most prevalent enzymes that contribute to AG resistance in Enterobacteriaceae are AAC(3)-II, AAC(6′)-I, and ANT(2″)-I (2, 3). As observed in the experiments we report here, ACHN-490 remains highly potent against isolates with each of these three resistance mechanisms.
There are specific resistance mechanisms in Enterobacteriaceae that were effective against ACHN-490 but are of limited clinical significance. ACHN-490 was found to be inactive (MIC, >64 μg/ml) against a P. stuartii isolate with the AAC(2′)-I enzyme, which also inactivates GEN. This is a chromosomal AME restricted to P. stuartii and is therefore unlikely to spread to organisms of broader clinical importance. The Proteae utilize AMEs to provide AG resistance but, in addition, have intrinsic outer membrane characteristics that form a barrier to AG uptake (23). Changes in membrane permeability that decrease AG uptake are only rarely observed in other Enterobacteriaceae. However, one such isolate was included in the expanded panel, and ACHN-490 and the two legacy AGs were similarly affected. One example of a ribosomal methyltransferase, armA, was included in the screening panel and was associated with high MICs for ACHN-490 and each of the legacy AGs. armA methylates a key guanine at the AG ribosomal target site and prevents binding of 4,6-disubstituted AGs. Although this mechanism is very rare at this time, it is being monitored closely for changes in prevalence (6).
In staphylococci, there are fewer types of AMEs, but these tend to be widespread and clonal (16). None of them altered the activity of ACHN-490 in our experiments, whereas the MICs of the legacy AGs were increased. For ACHN-490, the MIC distribution against Staphylococcus spp. isolates was found to be the same regardless of AG resistance status. We therefore concluded that the presence of resistance to AMK, GEN, and/or TOB had no impact on the activity of ACHN-490 against clinical isolates in these species regardless of the type or number of AGRMs present.
AG-R P. aeruginosa and Acinetobacter isolates were found to have higher ACHN-490 MICs than their AG-S counterparts. Increased efflux is a common mechanism of resistance to the legacy AGs in P. aeruginosa, usually through the upregulation of the MexXY efflux pump (22), and may have a similar impact on ACHN-490. MICs in A. baumannii isolates that appear to have increased efflux based on their AG-R phenotype were similarly elevated for ACHN-490. It is important to note that efflux is rarely the sole AGRM present in either species (16). Underlying AMEs are frequently present in strains with increased efflux. It is clear from our results that the activity of ACHN-490 is not altered by the AMEs in P. aeruginosa and A. baumannii, even though the MICs of AMK and GEN are elevated in the presence of such AMEs.
AGs are often used in combination with other agents in an effort to prevent the emergence of resistance, to treat polymicrobial infections empirically, and to benefit from synergistic activity against organisms that are otherwise nonsusceptible (13, 20). We plan to explore the in vitro synergy between ACHN-490 and other antipseudomonal agents against clinical isolates of Pseudomonas. We anticipate that many of these isolates will have the MexXY pump upregulated in addition to AMEs that cause resistance to existing AGs. The imperviousness of ACHN-490 to the AMEs present in P. aeruginosa may serve to bolster its utility in this setting.
The broader collection of clinical isolates was compiled in an effort to ascertain the activity of ACHN-490 against the diversity of AGRMs present in multiple combinations and genetic backgrounds. However, AGRMs are commonly found in combination with resistance mechanisms such as β-lactamases and gyrase mutations, which generate resistance to other antibiotic classes (8). This was evident from an analysis of the activity of comparator antibiotics against the AG-R collection. The MIC90 for CIP is above the breakpoint in each species group. Newer and investigational agents, such as TGC and BPR, display elevated MIC90s against many of the Gram-negative organisms. IMI remains a reliable drug of last resort; however, IMI activity is diminished against some isolates of Klebsiella spp., P. aeruginosa, and Acinetobacter spp. Given that this collection was assembled to represent the AG-R population, it is not surprising that AMK and GEN showed limited activity, but it is disturbing that the activities of unrelated drugs were so altered. This observation makes it all the more striking that ACHN-490 was not similarly affected.
ACHN-490 has emerged as a promising new antibacterial agent with the potential to rejuvenate the AG class of antibiotics, and thus it has been advanced into clinical development.
Acknowledgments
All authors are employees of Achaogen, Inc. Melanie Watson, at AlphaBioCom, LLC, provided editorial assistance, which was funded by Achaogen, Inc.
Footnotes
Published ahead of print on 30 August 2010.
REFERENCES
- 1.Aggen, J., E. Armstrong, A. Goldblum, P. Dozzo, M. Linsell, M. Gliedt, et al. 2009. Synthesis, structure, and in vitro activity of the neoglycoside ACHN-490, poster F1-840. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 2.Armstrong, E., D. Biedenbach, R. Jones, and G. Miller. 2009. Surveying aminoglycoside resistance mechanisms: a tool for the development of neoglycosides, poster P-643. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland.
- 3.Biedenbach, D., R. Jones, G. Miller, and E. Armstrong. 2009. Ten-year trend in aminoglycoside resistance from a worldwide collection of gram-negative pathogens (1998-2007), poster P-636. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland.
- 4.Chambers, H. F. 2006. Aminoglycosides, p. 1155-1171. In L. Brunton, J. Lazo, and K. Parker (ed.), Goodman & Gilman's the pharmacological basis of therapeutics, 11th ed. McGraw-Hill, New York, NY.
- 5.Clinical and Laboratory Standards Institute. 2009. M100-S19. Performance standards for antimicrobial susceptibility testing; nineteenth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Doi, Y., and Y. Arakawa. 2007. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45:88-94. [DOI] [PubMed] [Google Scholar]
- 7.Drusano, G. L., P. G. Ambrose, S. M. Bhavnani, J. S. Bertino, A. N. Nafziger, and A. Louie. 2007. Back to the future: using aminoglycosides again and how to dose them optimally. Clin. Infect. Dis. 45:753-760. [DOI] [PubMed] [Google Scholar]
- 8.Hawkey, P. M., and A. M. Jones. 2009. The changing epidemiology of resistance. J. Antimicrob. Chemother. 64(Suppl. 1):i3-i10. [DOI] [PubMed] [Google Scholar]
- 9.Hawser, S. P., S. K. Bouchillon, D. J. Hoban, and R. E. Badal. 2009. In vitro susceptibilities of aerobic and facultative anaerobic gram-negative bacilli from patients with intra-abdominal infections worldwide from 2005-2007: results from the SMART study. Int. J. Antimicrob. Agents 34:585-588. [DOI] [PubMed] [Google Scholar]
- 10.Hermann, T. 2007. Aminoglycoside antibiotics: old drugs and new therapeutic approaches. Cell. Mol. Life Sci. 64:1841-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jana, S., and J. K. Deb. 2006. Molecular understanding of aminoglycoside action and resistance. Appl. Microbiol. Biotechnol. 70:140-150. [DOI] [PubMed] [Google Scholar]
- 12.Jones, R. N., M. L. Beach, and M. A. Pfaller. 2001. Spectrum and activity of three contemporary fluoroquinolones tested against Pseudomonas aeruginosa isolates from urinary tract infections in the SENTRY Antimicrobial Surveillance Program (Europe and the Americas; 2000): more alike than different! Diagn. Microbiol. Infect. Dis. 41:161-163. [DOI] [PubMed] [Google Scholar]
- 13.Klastersky, J., R. Cappel, and D. Daneau. 1972. Clinical significance of in vitro synergism between antibiotics in gram-negative infections. Antimicrob. Agents Chemother. 2:470-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, S., and C. Cheong. 2001. Selective reactions of reactive amino groups in polyamino compounds by metal-chelated or -mediated methods. Tetrahedron 57:4801-4815. [Google Scholar]
- 15.Miller, G. H., F. J. Sabatelli, R. S. Hare, Y. Glupczynski, P. Mackey, D. Shlaes, et al. 1997. The most frequent aminoglycoside resistance mechanisms. Changes with time and geographic area: a reflection of aminoglycoside usage patterns? Aminoglycoside Resistance Study Groups. Clin. Infect. Dis. 24(Suppl. 1):S46-S62. [DOI] [PubMed] [Google Scholar]
- 16.Miller, G. H., F. J. Sabatelli, L. Naples, R. S. Hare, K. J. Shaw, et al. 1995. The most frequently occurring aminoglycoside resistance mechanisms: combined results of surveys in eight regions of the world. Aminoglycoside Resistance Study Groups. J. Chemother. 7(Suppl. 2):17-30. [PubMed] [Google Scholar]
- 17.Nagabhushan, T. L. 14 December 1976. U.S. patent 3,997,524.
- 18.Nam, G., S. H. Kim, J.-H. Kim, J.-H. Shin, and E.-S. Jang. 2002. An efficient and selective 1-N-monoethylation of sisomicin: process development of netilmicin. Org. Proc. Res. Dev. 6:78-81. [Google Scholar]
- 19.Rossi, F., F. Baquero, P. R. Hsueh, D. L. Paterson, G. V. Bochicchio, T. A. Snyder, et al. 2006. In vitro susceptibilities of aerobic and facultatively anaerobic gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2004 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). J. Antimicrob. Chemother. 58:205-210. [DOI] [PubMed] [Google Scholar]
- 20.Rybak, M. J., and B. J. McGrath. 1996. Combination antimicrobial therapy for bacterial infections. Guidelines for the clinician. Drugs 52:390-405. [DOI] [PubMed] [Google Scholar]
- 21.Shaw, K. J., P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobel, M. L., G. A. McKay, and K. Poole. 2003. Contribution of the MexXY multidrug transporter to aminoglycoside resistance in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 47:3202-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waitz, J. A., G. H. Miller, E. Moss, Jr., and P. J. Chiu. 1978. Chemotherapeutic evaluation of 5-episisomicin (Sch 22591), a new semisynthetic aminoglycoside. Antimicrob. Agents Chemother. 13:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright, G. D. 1999. Aminoglycoside-modifying enzymes. Curr. Opin. Microbiol. 2:499-503. [DOI] [PubMed] [Google Scholar]