Abstract
Atazanavir inhibits UDP-glucuronyl-transferase-1A1 (UGT1A1), which metabolizes raltegravir, but the magnitude of steady-state inhibition and role of the UGT1A1 genotype are unknown. Sufficient inhibition could lead to reduced-dose and -cost raltegravir regimens. Nineteen healthy volunteers, age 24 to 51 years, took raltegravir 400 mg twice daily (arm A) and 400 mg plus atazanavir 400 mg once daily (arm B), separated by ≥3 days, in a crossover design. After 1 week on each regimen, raltegravir and raltegravir-glucuronide plasma and urine concentrations were measured by liquid chromatography-tandem mass spectrometry in multiple samples obtained over 12 h (arm A) or 24 h (arm B) and analyzed by noncompartmental methods. UGT1A1 promoter variants were detected with a commercially available kit and published primers. The primary outcome was the ratio of plasma raltegravir Ctau, or concentration at the end of the dosing interval, for arm B (24 h) versus arm A (12 h). The arm B-to-arm A geometric mean ratios (95% confidence interval, P value) for plasma raltegravir Ctau, area under the concentration-time curve from 0 to 12 h (AUC0-12), and raltegravir-glucuronide/raltegravir AUC0-12 were 0.38 (0.22 to 0.65, 0.001), 1.32 (0.62 to 2.81, 0.45), and 0.47 (0.38 to 0.59, <0.001), respectively. Nine volunteers were heterozygous and one was homozygous for a UGT1A1 reduction-of-function allele, but these were not associated with metabolite formation. Although atazanavir significantly reduced the formation of the glucuronide metabolite, its steady-state boosting of plasma raltegravir did not render the Ctau with a once-daily raltegravir dose of 400 mg similar to the Ctau with the standard twice-daily dose. UGT1A1 promoter variants did not significantly influence this interaction.
In 2007, 4 million HIV-infected patients were taking antiretroviral medications in countries with low to middle income levels, according to the World Health Organization (WHO) (24). According to the 2009 update (23) of the WHO treatment guidelines (22), recommended first-line therapies should include two nucleoside reverse transcriptase inhibitors (NRTI) and either efavirenz or nevirapine. However, efavirenz is classified by the United States Food and Drug Administration (FDA) as a “D” drug during pregnancy, due to teratogenic potential demonstrated in monkeys and in retrospective human data, which means it is not recommended for women of child-bearing age who cannot practice reliable contraception. Nevirapine has a “black box” warning advising of increased risk of severe or fatal hepatotoxicity if used in women with CD4+ cell counts of >250 cells/ml. Therefore, there is room for additional first line and even salvage agents that are potentially affordable, safe, well-tolerated, and independent of current, widely used first- or second-line therapies. Raltegravir could be one of these new drugs, but its high cost, which is prohibitive for most of the world's HIV-infected patients (16), warrants investigation into strategies, such as boosting, to reduce the dose.
Raltegravir was approved by the FDA in October 2007. It is the first-in-class and, currently, sole FDA-approved inhibitor of viral integrase, an HIV-1-specific enzyme that is required for viral replication (9). Inhibition of integrase prevents the insertion of linear HIV-1 DNA into the host cell genome, a critical step in the life cycle of the virus. Raltegravir is primarily metabolized by UDP-glucuronosyl transferase 1A1 (UGT1A1) and not by the P450 cytochromes.
Atazanavir-mediated inhibition of UGT1A1, which also conjugates bilirubin, results in mild hyperbilirubinemia in most patients treated with atazanavir. When given as a single dose in addition to a steady-state regimen of atazanavir 400 mg daily in healthy volunteers, the maximum concentration (Cmax), 12-h postdose concentration (C12), and area under the time-concentration curve (AUC) of raltegravir were 53%, 95%, and 72% higher, respectively, than when raltegravir was given alone (11). With ritonavir-boosted atazanavir, these metrics were 10 to 20% lower, which is not surprising given the ability of ritonavir to induce drug-metabolizing enzymes, including glucuronyl transferase, in addition to its inhibitory effect on cytochrome P450 2D6 (CYP2D6), CYP3A4, or P-glycoprotein (6).
The combination of raltegravir and atazanavir has potential as an initial or salvage regimen. Both drugs are potent inhibitors of HIV-1 replication, have minimal impact on the lipid profile, are generally well tolerated, and do not require refrigeration. We hypothesized that at steady state, boosting of raltegravir plasma concentrations by atazanavir would be sufficient to permit a reduced daily dose of raltegravir by eliminating one of the two doses in the standard regimen of 400 mg twice daily. Such a dosing regimen could lower the cost of raltegravir and increase the feasibility of combination with atazanavir, which is routinely dosed once daily. We also hypothesized that the UGT1A1 genotype may influence the magnitude of a raltegravir-atazanavir interaction.
(Data from the investigation were presented in part at the 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, Cape Town, South Africa, July 2009.)
MATERIALS AND METHODS
Study design and population.
We conducted an open-label, crossover pharmacokinetic study in healthy adult volunteers over the age of 18 years. The University of Southern California (USC) Institutional Review Board approved the study, and all volunteers gave written informed consent to participate.
Volunteers were eligible if they were free from any chronic medical condition that would potentially alter the pharmacokinetics of either raltegravir or atazanavir or any psychiatric condition that might affect adherence to the study protocol. No concomitant medications except occasional ibuprofen or acetaminophen were allowed. Female participants using hormonal contraception were asked to refrain from use beginning 2 weeks prior to the study and to practice alternate forms of contraception. Avoidance of these disallowed medications was verified by participant self-report. Participants self-identified their racial category as Caucasian, Asian, or African American and their ethnicity as Hispanic or not.
Study procedures.
Prior to receiving any study medication, all participants passed baseline screening with normal results for physical examination, complete blood cell count, serum electrolytes, liver enzymes (alanine and aspartate aminotransferase), serum creatine kinase, serum bilirubin, and 12-lead electrocardiogram (ECG), which was reviewed by a licensed cardiologist. HIV infection was excluded by establishing <50 HIV RNA copies/ml of blood (NucliSens; bioMerieux, United States) in order to avoid the scenario of administration of suboptimal antiretroviral therapy to a newly infected patient yet to seroconvert. Participants were then randomized to start one of two unblinded treatment arms according to a prespecified random number list with a block size of 2 to ensure equal allocation. In arm A, participants took one raltegravir 400-mg tablet orally twice daily; in arm B they took one raltegravir 400-mg tablet plus two atazanavir 200-mg capsules, both orally once daily.
Study medications for both arms A and B were obtained by open-market purchase, stored at recommended temperatures in the USC Investigational Drug Service (IDS) pharmacy, and dispensed by IDS pharmacists in bottles fitted with MEMS electronic caps which recorded the time and day each time the bottle was opened. Study staff instructed the participants on the nature, purpose, and use of the MEMS caps and asked them to only open the bottles when the medications were to be taken and to take the medications immediately, i.e., to not save them for later. Each participant's electronic dosing record was then uploaded into the MEMS software to obtain an accurate dosing record for pharmacokinetic modeling. As a backup, participants were also provided with a written medication log to record dates and times of all doses. Although participants were instructed to take all study medications in a semifasted state at least 1 h prior to or 2 h after eating, the medication log also contained space to record any food intake within this window.
After 7 days of dosing in arm A or B, participants were admitted to the USC General Clinical Research Center (GCRC) in the early morning, with no food consumption since the evening prior. A predose blood sample for pharmacokinetic and UGT1A1 genotypic analysis was obtained; the participant voided his/her bladder, and the urine was discarded. After a witnessed dose of study medications, further blood samples were obtained at 1, 2, 4, 8, and 12 h after the dose. Standardized meals were provided, beginning 1 h after study medication intake. All urine was collected and combined into 2-h aliquots. After the last 12-h sample, participants were discharged from the GCRC. For arm B, participants were given a container to collect the remaining 12-h production of urine as a single aliquot stored in the refrigerator at home. The next morning, 24 h after the last dose of study medications, they returned for a final blood sample, bringing the collected urine. Verification of complete urine collection was by participant self-report and analyzing the volume returned for outliers, defined as ml/h/kg of body weight of urine produced above or below 1.5 times the interquartile range. All participants were to complete both arms A and B, with a minimum 3-day period between arms.
Sample analysis.
After collection, samples were stored on ice, and within 30 min they were centrifuged at 1,500 × g (2,500 rpm) for 10 min. Plasma and urine aliquots were frozen at −70°C until batch analysis. Raltegravir and atazanavir concentrations in plasma and urine were determined by high-performance liquid chromatography coupled to tandem mass spectrometry (LC-MS-MS) after protein precipitation with acetonitrile using an adaptation of our previously reported methods (3, 5). We used the same LC-MS-MS methodology with modification for the assay of raltegravir-glucuronide. Briefly, aliquots (100 μl) of plasma or diluted urine (20-fold with ultrapure water) were mixed with a 100-μl volume of internal standard solution (2 μg/ml raltegravir-glucuronide-d3 [Toronto Research Chemicals, Inc., North York, Canada] in methanol/water [50:50]). The resulting sample was subjected to protein precipitation with acetonitrile (200 μl) and vortexed. The mixture was centrifuged at 4°C for 10 min at 20,000 × g (14,000 rpm). The supernatant (200 μl) was transferred into glass high-performance liquid chromatography (HPLC) vials and was diluted with 400 μl methanol-ammonium formate 20 mM (1:1). Chromatographic separations were done on an Atlantis dC18 column (2.1 by 50 mm, 3 μm; Waters, Milford, MA). The mobile phase used for chromatography was 2 mM ammonium acetate in ultrapure water (buffer A) and acetonitrile (solvent B), both containing 0.1% formic acid. The mobile phase was delivered using a stepwise gradient elution program: 2% acetonitrile (solvent B) at 0 min, 80% B at 6 min at a flow rate of 0.3 ml/min. The second part of the run included 1 min of rinsing with 80% B followed by a reequilibration step to the initial solvent up to 12 min. This chromatographic program allows an excellent separation of raltegravir-glucuronide and raltegravir, which were eluted at 4.28 min and 5.46 min, respectively. Raltegravir-glucuronide quantification was performed by selected reaction monitoring (SRM) in the negative mode, using the m/z transitions 619.1→316.0 and 622.1→319.0 for raltegravir-glucuronide and raltegravir-glucuronide-d3, respectively. The range of the calibration curve for raltegravir-glucuronide was established up to 10,000 ng/ml, with a lower limit of quantification of 10 ng/ml. The laboratory participates in an international external quality assurance program for antiretroviral drug analysis (Stichting Kwaliteitsbewaking Klinische Geneesmiddelanalyse en Toxicologie [KKGT; Association for Quality Assessment in TDM and Clinical Toxicology], The Hague, Netherlands).
For UGT1A1 genotyping, DNA was extracted from blood using a QIAamp DNA mini kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. Due to the small study population size, we focused on the most common reduction-of-function allele, UGT1A1*28, which is a TA insertion in the TATA box of the promoter region: A[TA]7TAA. The reference allele, *1, is A[TA]6TAA. UGT1A1*28 occurs with an allelic frequency of 0.39 in Caucasians, 0.43 in Africans, and 0.16 in Asians (1). Genotyping of UGT1A1*28 was performed by direct sequencing using forward (5′-AAGTGAACTCCCTGCTACCTT-3′) and reverse (5′-CCACTGGGATCAAC-AGTATCT-3′) primers as described by Monaghan et al. (17) This method also permitted the detection of UGT1A1*36 (A[TA]5TAA), UGT1A1*37 (A[TA]8TAA), and UGT1A1*81 (g.-64G>C), which have increased, decreased, and similar function to *1, respectively.
Sample size.
Our primary endpoint was the geometric mean ratio (GMR) of the concentrations for each dosing strategy at the end of the dosing interval (Ctau), i.e., the raltegravir 24-hour concentration (C24) when dosed once daily versus the 12-hour concentration (C12) when dosed twice daily. The relationship between raltegravir plasma concentrations and outcomes has not been established. Since the ratio of steady-state C12 with 100 mg twice daily to 400 mg twice daily is 0.35 (10) and the virologic efficacy of these two doses was similar in one study (15), we chose a sample size of 20 to ensure that if the mean primary endpoint GMR was 0.8, the two-sided 95% confidence interval (CI) about the mean GMR would be greater than or equal to 0.4, with an alpha of 0.05 and a power of 0.8, assuming a GMR standard deviation of 0.65.
Data analysis.
Summary statistics, comparative statistics, and noncompartmental raltegravir pharmacokinetic analyses were performed using R 2.9.2 (Vienna, Austria). The area under the concentration-time curve (AUC) was calculated using the linear trapezoidal algorithm. Apparent total plasma clearance of oral drug (CLt/F) was calculated using the formula CLt/F = dose/AUC, where the AUC is that in plasma during the dosing interval at steady state. Renal clearance (CLR) was calculated as CLR = RAL(urine)/AUC, where RAL(urine) is the amount of raltegravir excreted in the urine during the dosing interval and the AUC is that in plasma during the same time interval. Terminal elimination, β, was calculated by least-square fitting of the concentrations at the last sample time points for each arm. The apparent fraction of raltegravir eliminated unchanged in the urine after oral dosing (Fe/F) was calculated as the ratio of Ae/(dose/F), where Ae is the amount excreted in urine. Note the dependence of both total plasma clearance and fractional excretion of unchanged raltegravir on oral bioavailability, F, such that both will be underestimated relative to intravenous administration if F is less than 1. According to the package insert for raltegravir, absolute F has not been established.
Raltegravir and raltegravir-glucuronide concentrations in plasma and urine and AUCs were log10 transformed to satisfy assumptions of normality and first compared as ratios of sequence (i.e., arm A first or arm B). The GMRs for raltegravir Cmax (P = 0.97), C12 (P = 0.42), or 12-h AUC (P = 0.36) were similar whether participants started with arm A or B, indicating that the crossover design did not bias the results, for example, due to carryover effects from the first study arm to the second arm; hence, sequence effect was omitted from further ratios of arm B to arm A, which were tested using the single-sample Student's t test for the probability of difference from 0 or log10 (1).
A UGT1A1 diplotype score was generated for each participant by summing the function of both alleles, with −1 for reduced function (*28, *37), 0 for function similar to that of the reference allele (*1, *81), and +1 for increased function (*36). The association between the UGT1A1 score as an ordered categorical variable and the log-transformed raltegravir-glucuronide/raltegravir ratio at each sample time point was tested by linear mixed-effect modeling, with participants as the random effect and score and atazanavir use as the fixed effects. The association between score and the log-transformed ratio of AUCs for raltegravir-glucuronide and raltegravir was tested by multivariate linear regression, adjusting for atazanavir use. Fisher's exact test was used to test association between score and race/ethnicity.
RESULTS
Population characteristics.
Twenty-one adults were screened for participation. One was excluded due to an abnormal electrocardiogram. A second volunteer completed arm A but dropped out of the study before starting arm B due to a relapse of her chronic gastroesophageal reflux that developed during her washout period and was not judged to be study related. Therefore, of the 20 enrolled, 19 participants completed both study arms. Demographic characteristics of the study population are shown in Table 1 .
TABLE 1.
Population demographics
| Patient characteristic | Value |
|---|---|
| Age [median (range)] | 28.6 years (24.3-51.1) |
| Weight [median (range)] | 68.2 kg (49.3-86.0) |
| Race/ethnicity [no. (%)] | |
| Caucasian, non-Hispanic | 9 (45) |
| Caucasian, Hispanic | 3 (15) |
| Asian | 5 (25) |
| African American | 3 (15) |
| Female [no. (%)] | 13 (65) |
Adherence to the protocol was excellent. From MEMS logs and tablet or capsule counts, 524 (99.8%) of 525 prescribed doses were taken, with 478 (91.0%) at the prescribed time plus-or-minus 25% of the dosing interval. The MEMS logs were in good agreement with the written medication logs. As indicated on the written logs, 464 (88.3%) of the doses were taken in a semifasted state (not within 1 h before to 2 h after food), according to instructions. Study medications were well tolerated, with occasional mild nausea reported. Two participants developed rashes 1 and 2 days after completing the study (arm B in both cases) which were self-limited, did not interfere with daily activities, and judged as possibly study drug related. Both participants had tolerated arm A without incident. Repetition of the screening laboratories at the end of all study activities did not detect abnormalities in any participant.
Assay performance.
In plasma and urine, the raltegravir and raltegravir-glucuronide mean bias and percent coefficient of variation (CV%) were each <5%. Likewise, plasma atazanavir had a mean bias and CV% of <5%.
Plasma pharmacokinetics.
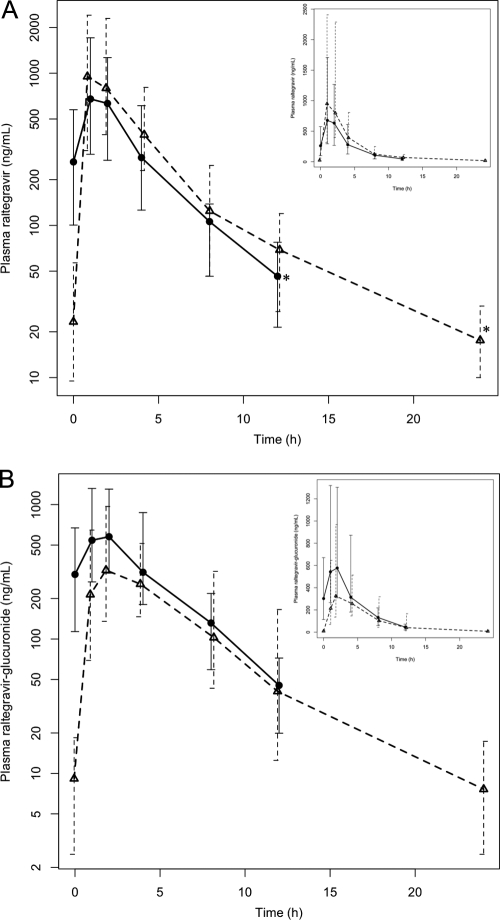
Plasma concentration-time profiles for raltegravir, raltegravir-glucuronide, and atazanavir are shown in Fig. 1. Pharmacokinetic characteristics of raltegravir and its metabolite in both arms A and B are shown in Table 2. With regard to the primary endpoint, the GMR of Ctau for arm B to arm A was 0.38, with a 95% confidence interval of 0.22 to 0.65, which did not meet the study definition of sufficient raltegravir boosting by atazanavir—a threshold of ≥0.4 for the lower boundary of the 95% confidence interval. The GM plasma raltegravir Cmax, C12, and 12-hour AUC were 37%, 49%, and 32% higher, respectively, in arm B, but none of these were statistically significant. When dosed with atazanavir, the GM total apparent plasma clearance of raltegravir was 42% lower than when dosed alone, but this difference was not significant due to nearly 50-fold intrasubject variability in both study arms. The terminal raltegravir concentration-time slope, β [ln(2)/β = half life], was significantly lower in arm B, indicating that atazanavir slowed the elimination of raltegravir, but the atazanavir AUC was not significantly associated with β in arm B (P = 0.86).
FIG. 1.
Observed concentration-time profiles for raltegravir with and without atazanavir (A) and raltegravir-glucuronide with and without atazanavir (B). Solid and dashed dark lines are the geometric mean concentrations at each time point for arm A (raltegravir 400 mg twice daily) and arm B (raltegravir 400 mg plus atazanavir 400 mg once daily), respectively. Insets are in linear scales. *, comparison of these two concentrations (Ctau) formed the primary endpoint. Whisker bars indicate the geometric interquartile range.
TABLE 2.
Comparison of raltegravir and raltegravir-glucuronide pharmacokinetics with and without coadministered atazanavir
| Type of sample, drug, and pharmacokinetic parametera | GM (range) of results for armb |
GMR for arm B/arm A (95% CI, P value) | |
|---|---|---|---|
| A | B | ||
| Plasma | |||
| Raltegravir | |||
| Ctau (ng/ml) | 46 (<10-257) | 18 (4-100) | 0.38 (0.22-0.65, 0.001) |
| Cmin (ng/ml) | 40 (<10-257) | 13 (4-89) | 0.32 (0.21-0.51, 0.001) |
| Cmax (ng/ml) | 1,016 (112-9,997) | 1,402 (219-8,696) | 1.37 (0.62-3.02, 0.40) |
| C12 (ng/ml) | 46 (<10-257) | 69 (11-1,812) | 1.49 (0.59-3.75, 0.38) |
| AUC (ng·h/ml) | |||
| 0-12 h | 3,532 (465-22,245) | 4,657 (610-35,236) | 1.32 (0.62-2.81, 0.45) |
| 12-24 h | NDc | 875 (177-16,517) | ND |
| 0-tau | 3,532 (465-22,245) | 6,085 (840-41,303) | 1.72 (0.79-3.75, 0.15) |
| a.m./p.m. trough | 5.64 (0.29-71.00) | 1.32 (0.30-34.50) | 0.23 (0.08-0.69, 0.01) |
| β | 0.26 (0.09-0.48) | 0.11 (0.05-0.30) | 0.45 (0.31-0.66, 0.001) |
| Raltegravir-glucuronide | |||
| Ctau (ng/ml) | 45 (<10-512) | 8 (3-92) | 0.16 (0.09-0.30, 0.001) |
| Cmin (ng/ml) | 39 (<10-512) | 5 (3-85) | 0.14 (0.08-0.22, 0.001) |
| Cmax (ng/ml) | 799 (157-3,264) | 458 (50-2,507) | 0.57 (0.29-1.12, 0.10) |
| C12 (ng/ml) | 45 (<10-511) | 41 (3-1,271) | 0.90 (0.31-2.61, 0.84) |
| AUC (ng·h/ml) | |||
| 0-12 h | 3,267 (562-12,466) | 2,036 (181-13,230) | 0.62 (0.33-1.20, 0.14) |
| 12-24 h | ND | 342 (0-5,361) | ND |
| Raltegravir-glucuronide AUC0-12/raltegravir AUC0-12 | 0.95 (0.39-2.14) | 0.43 (0.23-0.96) | 0.47 (0.38-0.59, 0.001) |
| β | 0.30 (0.16-0.46) | 0.14 (0.04-0.30) | 0.60 (0.43-0.84, 0.006) |
| Urine | |||
| Raltegravir | |||
| Amt (mg) | |||
| 0-12 h | 11.66 (1.80-102.03) | 10.81 (1.96-48.68) | 0.93 (0.43-2.00, 0.84) |
| 12-24 h | ND | 0.41 (0.046-4.08) | ND |
| Raltegravir-glucuronide | |||
| Amt (mg) | |||
| 0-12 h | 82.50 (18.57-239.65) | 84.51 (18.16-325.83) | 1.02 (0.53-1.99, 0.94) |
| 12-24 h | ND | 7.03 (2.02-59.74) | ND |
| Raltegravir-glucuronide/raltegravir [amt (mg) 0-12 h] | 7.08 (1.94-13.32) | 7.81 (3.68-24.73) | 1.10 (0.90-1.36, 0.33) |
| Raltegravir clearance | |||
| CLt/F (ml/min) | 1,887 (300-14,352) | 1,096 (161-7,940) | 0.58 (0.27-1.26, 0.16) |
| CLR (ml/min) | 55 (25-264) | 39 (11-84) | 0.70 (0.49-1.01, 0.06) |
| Fe/F (%) | 2.9 (0.5-25.5) | 3.5 (0.7-27.5) | 1.21 (0.53-2.77, 0.63) |
| Raltegravir-glucuronide CLR (ml/min) | 421 (151-1,559) | 692 (221-1,790) | 1.64 (1.11-2.44, 0.02) |
Ctau, concentration at the end of the dosing interval, i.e., 12 h for arm A and 24 h for arm B; Cmin, minimum concentration(regardless of time, not always at time tau, the end of the dosing interval); Cmax, maximum concentration; C12, 12-h concentration; AUC, area under the concentration-time curve; β, terminal slope, such that ln(2)/β is half-life; CLt/F, apparent total plasma clearance of oral drug; CLR, renal clearance of plasma drug; Fe/F, apparent fraction of oral drug excreted unchanged.
Arm A, raltegravir 400 mg twice daily; arm B, raltegravir 400 mg + atazanavir 400 mg once daily.
ND, not determined.
Of note, in arm A the geometric mean (GM) predose (C0) morning concentration is over 5-fold higher than the evening C12, suggesting a circadian or diurnal variation in raltegravir absorption when dosed twice daily. For this reason, we did not try to extrapolate the 12-hour raltegravir AUC in arm A to 24 h by doubling it.
Just as the raltegravir-glucuronide Ctau and Cmin were lower in arm B than in arm A (Table 2) for raltegravir, the Cmax, C12, and 12-h AUC were similar between the two arms, and β was reduced in arm B. However, the 12-h AUC for raltegravir-glucuronide relative to that of raltegravir was 53% lower when coadministered with atazanavir than without atazanavir (P < 0.001).
Urinary data.
All participants reported full collection of urine from 12 to 24 h during the arm B pharmacokinetic study. The GM (range) volume collected during this interval was 1.0 (0.5 to 2.0) ml/kg of bodyweight/h, with an interquartile range of 0.8 to 1.4, indicating no outliers. As shown in Table 2, urinary clearance of unchanged raltegravir, consistent with extensive metabolism, was minimal relative to total clearance, with an apparent fractional excretion of about 3%, which was similar between the two study arms. Urinary clearance of raltegravir-glucuronide was higher when dosed with atazanavir, but the significance of this is unclear.
Glucuronidation and UGT1A1 genotypes.
UGT1A1 genotypes are shownin Table 3. All 20 enrolled participants were genotyped, but as mentioned, participant 8 did not complete the pharmacokinetic study. Eight (40%) of the 20 were homozygous for the reference allele (*1), with 6 TA repeats in the TATA box. Nine (45%) were heterozygous for promoter variants associated with reduced UGT1A1 function. All but one of these were carrying UGT1A1*28. The remaining reduced-function allele, which we have termed *X, had 9 TA repeats in the TATA box (8) and occurs with such low frequency that it has not received an official designation. One participant (5%) was homozygous for UGT1A1*28, which is associated with Gilbert's syndrome. All patients had normal bilirubin at screening and at completion of the study. There were no increased-function alleles in this small population. By Fisher's exact test, there was no association between genotype and race (P = 0.16) or ethnicity (P = 0.19).
TABLE 3.
Participant's UGT1A1 genotype, functionality score, race, ethnicity, and plasma raltegravir-glucuronide/raltegravir geometric mean ratio by study arm
| Participant | Race | Hispanic | Genotypea | Scoreb | Plasma raltegravir- glucuronide/ raltegravir GMR in armc |
|
|---|---|---|---|---|---|---|
| A | B | |||||
| 1 | Caucasian | No | *1/*1 | 0 | 1.00 | 0.45 |
| 2 | Caucasian | Yes | *1/*1 | 0 | 0.97 | 0.68 |
| 3 | Caucasian | No | *1/*28 | −1 | 0.81 | 0.53 |
| 4 | Caucasian | No | *1/*28 | −1 | 1.99 | 1.04 |
| 5 | Asian | No | *1/*28 | −1 | 1.09 | 0.60 |
| 6 | Caucasian | No | *1/*1 | 0 | 0.98 | 0.25 |
| 7 | Asian | No | *1/*1 | 0 | 2.16 | 0.54 |
| 8 | Caucasian | Yes | *1/*1 | 0 | ||
| 9 | Caucasian | No | *28/*28 | −2 | 0.47 | 0.27 |
| 10 | Caucasian | No | *1/*28 | −1 | 1.50 | 0.62 |
| 11 | Asian | No | *1/*1 | 0 | 1.25 | 0.51 |
| 12 | Caucasian | No | *1/*1 | 0 | 1.76 | 0.42 |
| 13 | Asian | No | *1/*81 | 0 | 0.51 | 0.33 |
| 14 | Asian | No | *1/*81 | 0 | 0.70 | 0.52 |
| 15 | African American | No | *1/*28 | −1 | 1.04 | 0.40 |
| 16 | Caucasian | Yes | *1/*X | −1 | 1.02 | 0.52 |
| 17 | African American | No | *1/*28 | −1 | 0.77 | 0.43 |
| 18 | Caucasian | No | *1/*28 | −1 | 1.02 | 0.45 |
| 19 | African American | No | *1/*28 | −1 | 1.00 | 0.69 |
| 20 | Caucasian | No | *1/*1 | 0 | 0.93 | 0.31 |
| Avg | 1.02† | 0.47† | ||||
*1, reference sequence A(TA)6TAA; *28, A(TA)7TAA; *81, g.-64G>C; *X A(TA)9TAA.
Score, UGT1A1 score as described in Materials and Methods.
Arm A, raltegravir alone; arm B, raltegravir with atazanavir. Participant 8 did not complete the pharmacokinetic study but was genotyped. †, P < 0.0001.
Although the only participant who was homozygous for 2 reduced-function alleles had the lowest raltegravir-glucuronide/raltegravir ratio regardless of atazanavir dosing, overall by linear mixed-effect modeling, participants with 1 or 2 reduced-function alleles had on average a nearly identical ratio compared to those with the reference UGT1A1*1 (1.08-fold versus the reference; 95% CI 0.80 to 1.44, P = 0.90) when adjusted for the effect of atazanavir exposure (0.46-fold versus no atazanavir; 95% CI 0.39 to 0.54, P < 0.001). By linear regression, again adjusting for atazanavir, the ratio of the raltegravir-glucuronide AUC to the raltegravir AUC was no different in those with reduced-function alleles than in those with the reference allele (1.19-fold change; 95% CI 0.62 to 2.30, P = 0.59).
Atazanavir plasma levels.
The atazanavir GM (range) Cmax was 3,439 (290 to 6,299) ng/ml, C24 was 83 (13 to 495) ng/ml, and AUC was 18.2 (2.8 to 47.2) μg·h/ml. Given that participants took atazanavir on an empty stomach, these observations agree closely with those reported in the Reyataz package insert (http://www.bms.com/products/Pages/prescribing.aspx) for healthy volunteers at steady state with 400 mg once daily, when corrected for fasting conditions that lower Cmax and AUC by 64% and 58%, respectively, to values of 3,412 ng/ml and 17.2 μg·h/ml.
DISCUSSION
In this healthy adult volunteer population, despite reduced formation of plasma raltegravir-glucuronide, there were only small (and nonsignificant) increases in raltegravir plasma concentrations when raltegravir was dosed once daily with atazanavir. We found that the degree of raltegravir boosting in plasma by atazanavir was insufficient to ensure that raltegravir 400 mg once daily would result in concentrations at the end of the dosing interval that would be at least similar to those with current standard dosing. Overall, at steady state, the boosting effect of atazanavir (without ritonavir) on raltegravir was less than in a previously reported single-dose study (11).
Does the lack of boosting by atazanavir mean that once-daily raltegravir dosing is out of the question? We do not believe that our data force this conclusion, for three reasons. The first reason is that a minimally effective plasma concentration relative to in vitro viral susceptibility has not been established for raltegravir. In early dose-ranging studies, 100 mg twice daily in treatment-naïve patients had the same 48-week virologic and immunologic efficacy as doses up to 600 mg twice daily (15). In triple-class experienced patients, the 24-week virologic efficacy was the same in the 200-mg twice-daily group as in the 600-mg twice-daily group, although the CD4 cell count gains in the 200-mg group were less than in the other groups (7).
The second reason to consider once-daily raltegravir dosing as a still-viable strategy is the persistent binding of the drug to its intracellular target, the preintegration complex. Although there are as yet few published data on the intracellular pharmacokinetics of raltegravir, it is known in vitro to disassociate from the preintegration complex with a half-time that is longer than the half-time of the complex itself, effectively making the binding irreversible (9). This may be analogous to zidovudine: recognition that the active moiety had a prolonged intracellular half-life enabled a decrease in dosing frequency from five times to twice daily (20). We did not measure intracellular concentrations in this study.
The third reason that we cannot exclude the possibility of 400-mg once-daily dosing is simply that this was a healthy-volunteer study that cannot provide any efficacy data. Without a clearly defined pharmacokinetic-pharmacodynamic target, extrapolation to an effect in HIV-infected patients is impossible.
Although 400-mg once-daily raltegravir (50% of the currently recommended dose for both naïve and experienced patients) is attractive from cost and adherence perspectives, and despite the previous arguments, data from this study nonetheless highlight concerns with this dosing strategy. First, as is obvious from the results in Fig. 1 and the wide concentration ranges shown in Table 2, absorption of this drug is clearly erratic, even in the relatively controlled circumstances of this clinical study, and this raises the worrisome possibility that some patients with once-daily dosing may have particularly low concentrations, which may compromise efficacy if and when a concentration-response relationship can be described.
Second, our overall observed plasma concentrations were lower than those reported by the manufacturer in phase I studies of the same dose in young, healthy, adult participants. The AUC from 0 to 12 h (AUC0-12) for arm A was only 31% (10) or 46% (11) of the reported AUC0-12, depending on the study. However, our AUC0-12 is very similar to that found in other pharmacokinetic studies that included a group receiving raltegravir 400 mg twice daily (2, 12, 13, 19). Despite the lack of food-drug interaction reported in the package insert (http://www.isentress.com), food has subsequently been shown to have a major effect on the absorption profile (18), and this may have caused the diminished concentrations relative to the more stringent fasting conditions in the manufacturer's studies, since participants in our study were allowed to eat 1 h after a dose. These lower concentrations cause one to question what concentrations were achieved in the large phase III efficacy studies, with less regulation of food intake, since none have actually reported raltegravir concentrations in participants with or without virologic suppression.
Our data suggested a circadian rhythm to raltegravir pharmacokinetics, evidenced by the arm A morning raltegravir trough concentration that was 5-fold higher than in the evening. We are the first to report this, but careful inspection of the previous pharmacokinetic study (see their Fig. 1B, inset) with atazanavir from the manufacturer shows a 3-fold higher morning trough compared to the evening trough (11). In our study, when dosed once daily, this trough-to-trough variation was not noted. Because of the similar rate of raltegravir decline in both arms, as shown in Fig. 1, this circadian rhythm is likely due to differential absorption patterns of morning versus evening doses. However, without overnight data, we are unable to verify this hypothesis. The significance is that one should use caution if extrapolating 12-h to 24-h raltegravir exposures for a regimen with two daily doses.
Especially when one considers the recent, disappointing results of the SWITCHMRK study, in which treatment-experienced, virologically suppressed patients who switched their boosted lopinavir to raltegravir were more likely to lose virologic control than those who continued lopinavir (4), it would seem that if a once-daily 400-mg raltegravir strategy were to have any application, efficacy from the ongoing 800-mg once-daily study (http://www.clinicaltrials.gov/ct2/show/NCT00745823) must first be demonstrated and the relationship of plasma and/or intracellular raltegravir concentrations to efficacy should be established.
UGT1A1 genetic variants, while common even in this small population, did not significantly influence the degree of raltegravir glucuronidation or raltegravir plasma exposure. This is in contrast to the modest effect on plasma exposure demonstrated in a previous study (21). It is likely that the high degree of variability in raltegravir plasma concentrations in our study obscured any pharmacogenomic effects on either overall raltegravir exposure or the degree of glucuronidation. Furthermore, only one of the 19 patients with genomic and pharmacokinetic data had a homozygous reduced-function diplotype (*28/*28). Lastly, it is possible that there were polymorphisms in other regions of the gene that we did not sequence but that are associated with changes in function (e.g., *6 and *60) (1). However, these are present at a much lower frequency in the racial/ethnic groups in this study (1, 14) and, therefore, would be unlikely to contribute significantly to the substantial observed variation in raltegravir pharmacokinetics.
In summary, although atazanavir reduced the formation of the glucuronide metabolite, its steady-state boosting did not render a once-daily raltegravir dose of 400 mg pharmacokinetically similar to the standard twice-daily dose in terms of the concentration at the end of the dosing interval. Conversely, the observed concentrations of atazanavir when dosed with raltegravir were similar to those in the package insert, when adjusted for fed state, indicating that raltegravir did not influence the pharmacokinetics of atazanavir. As the relationship between raltegravir plasma exposure and efficacy is unclear, there may still be a rationale to test a simpler and cheaper single 400-mg daily dose of raltegravir, but because of highly variable interindividual plasma concentrations, it should be tested in a pilot study that includes measurement of extra- and intracellular drug concentrations. Atazanavir in such a trial would appear to add no pharmacological benefit. Results from the ongoing study of 800 mg once daily and better characterization of the relationship between plasma/intracellular concentrations and efficacy are crucial prerequisites of any study of reduced-dose raltegravir.
Acknowledgments
We thank Andrew Hill for his advice and the study volunteers for their time and effort.
This work was supported by National Institute of Allergy and Infectious Diseases grant no. K23 AI076106 (M.N.), National Institute of Biomedical Imaging and Bioengineering grant no. R01 EB005803 (R.J. and M.N.), Swiss National Science Foundation (SNF) grants no. 324700-112655 and 32430-124943 (A.F. and J.D.), SNF REQUIP grant no. 326000-121314/1 (L.D.), and a grant from Médecins Sans Frontières.
Footnotes
Published ahead of print on 7 September 2010.
REFERENCES
- 1.Beutler, E., T. Gelbart, and A. Demina. 1998. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc. Natl. Acad. Sci. U. S. A. 95:8170-8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco, J., M. Martinez-Rebollar, M. Calvo, I. Perez, M. Larrousse, M. Martin, R. Lopez, M. Brunet, J. Gatell, and J. Mallolas. 2009. Pharmacokinetic interaction between raltegravir and tipranavir in HIV-1 infected patients, abstr. P-23. 10th Int. Workshop Clin. Pharmacol. HIV Ther.
- 3.Colombo, S., A. Beguin, A. Telenti, J. Biollaz, T. Buclin, B. Rochat, and L. Decosterd. 2005. Intracellular measurements of anti-HIV drugs indinavir, amprenavir, saquinavir, ritonavir, nelfinavir, lopinavir, atazanavir, efavirenz and nevirapine in peripheral blood mononuclear cells by liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B. 819:259-276. [DOI] [PubMed] [Google Scholar]
- 4.Eron, J., J. Andrade, R. Zajdenverg, C. Workman, D. Cooper, B. Young, X. Xu, B. Nguyen, R. Leavitt, and P. Sklar. 2009. Switching from stable lopinavir/ritonavir-based to raltegravir-based combination ART resulted in a superior lipid profile at week 12 but did not demonstrate non-inferior virologic efficacy at week 24, abstr. 70aLB. 16th Conf. Retrovir. Opportunistic Infect.
- 5.Fayet, A., A. Béguin, B. Zanolari, S. Cruchon, N. Guignard, A. Telenti, M. Cavassini, H. F. Günthard, T. Buclin, J. Biollaz, B. Rochat, and L. A. Decosterd. 2009. A LC-tandem MS assay for the simultaneous measurement of new antiretroviral agents: raltegravir, maraviroc, darunavir, and etravirine. J. Chromatogr. B. 877:1057-1069. [DOI] [PubMed] [Google Scholar]
- 6.Foisy, M. M., E. M. Yakiwchuk, and C. A. Hughes. 2008. Induction effects of ritonavir: implications for drug interactions. Ann. Pharmacother. 42:1048-1059. [DOI] [PubMed] [Google Scholar]
- 7.Grinsztejn, B., B. Nguyen, C. Katlama, J. M. Gatell, A. Lazzarin, D. Vittecoq, C. J. Gonzalez, J. Chen, C. M. Harvey, and R. D. Isaacs. 2007. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 369:1261-1269. [DOI] [PubMed] [Google Scholar]
- 8.Guillemette, C., R. C. Millikan, B. Newman, and D. E. Housman. 2000. Genetic polymorphisms in uridine diphospho-glucuronosyltransferase 1A1 and association with breast cancer among African Americans. Cancer Res. 60:950-956. [PubMed] [Google Scholar]
- 9.Hazuda, D., M. Iwamoto, and L. Wenning. 2009. Emerging pharmacology: inhibitors of human immunodeficiency virus integration. Annu. Rev. Pharmacol. Toxicol. 49:377-394. [DOI] [PubMed] [Google Scholar]
- 10.Iwamoto, M., L. Wenning, A. Petry, M. Laethem, M. De Smet, J. Kost, S. Merschman, K. Strohmaier, S. Ramael, K. Lasseter, J. Stone, K. Gottesdiener, and J. Wagner. 2008. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin. Pharmacol. Ther. 83:293-299. [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto, M., L. A. Wenning, G. C. Mistry, A. S. Petry, S. Y. Liou, K. Ghosh, S. Breidinger, N. Azrolan, M. J. Gutierrez, W. E. Bridson, J. A. Stone, K. M. Gottesdiener, and J. A. Wagner. 2008. Atazanavir modestly increases plasma levels of raltegravir in healthy subjects. Clin. Infect. Dis. 47:137-140. [DOI] [PubMed] [Google Scholar]
- 12.Jackson, A., G. Moyle, V. Watson, D. Back, S. Khoo, C. Higgs, K. Armenis, A. Pozniak, B. Gazzard, and M. Boffito. 2009. Variability in steady-state raltegravir pharmacokinetics, impact of ezatimibe? abstr. P-25. 10th Int. Workshop Clin. Pharmacol. HIV Ther.
- 13.Jones, A., J. Talameh, K. Patterson, N. Rezk, H. Prince, and A. Kashuba. 2009. First-dose and steady-state pharmacokinetics of raltegravir in the genital tract of HIV-uninfected women, abstr. O-86. 10th Int. Workshop Clin. Pharmacol. HIV Ther.
- 14.Kaniwa, N., K. Kurose, H. Jinno, T. Tanaka-Kagawa, Y. Saito, M. Saeki, J. Sawada, M. Tohkin, and R. Hasegawa. 2005. Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C> T (P229L) found in an African-American. Drug Metab. Dispos. 33:458-465. [DOI] [PubMed] [Google Scholar]
- 15.Markowitz, M., B. Nguyen, E. Gotuzzo, F. Mendo, W. Ratanasuwan, C. Kovacs, G. Prada, J. O. Morales-Ramirez, C. S. Crumpacker, R. D. Isaacs, L. R. Gilde, H. Wan, M. D. Miller, L. A. Wenning, and H. Teppler. 2007. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J. Acquir. Immune Defic. Syndr. 46:125-133. [DOI] [PubMed] [Google Scholar]
- 16.Medecins Sans Frontieres. 2009. Untangling the Web of antiretroviral price reductions. Campaign for Access to Essential Medicines, Medecins Sans Frontieres, Geneva, Switzerland.
- 17.Monaghan, G., M. Ryan, R. Seddon, R. Hume, and B. Burchell. 1996. Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert's syndrome. Lancet 347:578-581. [DOI] [PubMed] [Google Scholar]
- 18.Nachman, S., E. Acosta, A. Wiznia, H. Teppler, M. Long, B. Homony, B. Graham, C. Worrell, P. Samson, E. Handelsman, and P1066 Protocol Team. 2008. Raltegravir pharmacokinetics and safety in adolescents: preliminary results from IMPAACT P1066, abstr. H-4059a. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 19.Rhame, F., M. Long, and E. Acosta. 2008. Pharmacokinetics (PK) of coadministered raltegravir (RAL) and lopinavir-ritonavir (KAL) in healthy adults, abstr. TUPE0075. XVII Int. AIDS Conf., Mexico City, Mexico.
- 20.Stretcher, B. N., A. J. Pesce, J. A. Murray, P. E. Hurtubise, W. H. Vine, and P. T. Frame. 1991. Concentrations of phosphorylated zidovudine (ZDV) in patient leukocytes do not correlate with ZDV dose or plasma concentrations. Ther. Drug Monit. 13:325-331. [DOI] [PubMed] [Google Scholar]
- 21.Wenning, L., A. Petry, J. Kost, B. Jin, S. Breidinger, I. DeLepeleire, E. Carlini, S. Young, T. Rushmore, F. Wagner, N. Lunde, F. Bieberdorf, H. Greenberg, J. Stone, J. Wagner, and M. Iwamoto. 2009. Pharmacokinetics of raltegravir in individuals with UGT1A1 polymorphisms. Clin. Pharmacol. Ther. 85:623-627. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. 1 January 2006. Antiretroviral therapy for HIV infection in adults and adolescents. World Health Organization, Geneva, Switzerland.
- 23.World Health Organization. 30 November 2009. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. World Health Organization, Geneva, Switzerland.
- 24.World Health Organization. 30 September 2009. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. September 2009 progress report. World Health Organization, Geneva, Switzerland.