Abstract
To date, there are few drugs licensed for the treatment of human cytomegalovirus (HCMV) infections, most of which target the viral DNA polymerase and suffer from many drawbacks. Thus, there is still a strong need for new anti-HCMV compounds with novel mechanisms of action. In this study, we investigated the anti-HCMV activity of chemically sulfated derivatives of Escherichia coli K5 capsular polysaccharide. These compounds are structurally related to cellular heparan sulfate and have been previously shown to be effective against some enveloped and nonenveloped viruses. We demonstrated that two derivatives, i.e., K5-N,OS(H) and K5-N,OS(L), are able to prevent cell infection by different strains of HCMV at concentrations in the nanomolar range while having no significant cytotoxicity. Studies performed to elucidate the mechanism of action of their anti-HCMV activity revealed that these compounds do not interact with either the host cell or the viral particle but need a virus-cell interaction to exert antiviral effects. Furthermore, these K5 derivatives were able to inhibit the attachment step of HCMV infection, as well as the viral cell-to-cell spread. Since the mode of inhibition of these compounds appears to differ from that of the available anti-HCMV drugs, sulfated K5 derivatives could represent the basis for the development of a novel class of potent anti-HCMV compounds. Interestingly, our studies highlight that small variations of the K5 derivatives structure can modulate the selectivity and potency of their activities against different viruses, including viruses belonging to the same family.
Human cytomegalovirus (HCMV) is a ubiquitous, opportunistic pathogen that infects the majority of world's population (22). HCMV infection in the immunocompetent host is usually benign and asymptomatic (except for occasional mononucleosislike outcomes); however, primary infection or reactivation in immunocompromised individuals such as AIDS patients or transplant recipients represents a major concern, since these conditions are often associated with high morbidity and mortality (1). HCMV also represents the most common cause of congenital defects in newborns.
To date, only few drugs have been approved for the management of HCMV infections (18). These are ganciclovir (GCV) and its oral prodrug valganciclovir (VGCV), cidofovir (CDV), and foscarnet (FOS). In addition, valacyclovir (VACV) and fomivirsen (ISIS 2922) are used in certain countries for prophylactic therapy of transplant recipients and for topical treatment of AIDS-related retinitis, respectively. All of these drugs are endowed with several drawbacks, including long-term toxicity, limited effectiveness, and poor bioavailability (except for VACV and VGCV), and most of them share the target. The latter feature can be a problem in the management of emerging drug-resistant HCMV strains, since clinical strains resistant to one of the anti-HCMV drugs may show cross-resistance to the others. Furthermore, none of the current anti-HCMV drugs has been approved for the treatment of congenital infection. For all of these reasons, the efforts spent in developing novel drugs to combat HCMV should be focused in identifying safer compounds with targets different from those of the currently licensed drugs.
The first events of the virus cycle, including attachment and entry, represent an attractive target for the development of novel antiviral compounds. In particular, in the case of HCMV such inhibitors would block not only the expression of viral immediate-early genes, whose products play a key role in the pathogenesis of HCMV infection, but also the host immunomodulation and the changes to cell physiology induced by the first events of virus infection (28).
In the last few years, many new drugs that act by blocking virus entry have been developed (14, 15, 25). Among these, some chemically sulfated derivatives of the K5 capsular polysaccharide from Escherichia coli have emerged as a promising novel class of antiviral compounds. The K5 polysaccharide has the same structure of N-acetyl-heparosan, the biosynthetic precursor of heparin. The addition of sulfate groups at the N or O position of the sugars led to the synthesis of several derivatives with different degrees of sulfation and charge distribution that are analogs of heparan sulfate proteoglycans (HSPGs). HSPGs are components of the cell membrane and play a key role in the entry process of a number of pathogenic viruses. Importantly, these sulfated K5 derivatives are devoid of toxicity and anticoagulant activity (24) and have already shown antiviral activity against both enveloped and nonenveloped viruses, i.e., human immunodeficiency virus (HIV), herpes simplex virus type 1 (HSV-1) and HSV-2, and human papillomaviruses (HPVs) (17, 23, 32).
In the present study, we investigated the antiviral properties of different K5 derivatives against HCMV and demonstrated that two N-/O-sulfated derivatives are able to inhibit potently virus attachment and cell-to-cell spread.
MATERIALS AND METHODS
Compounds.
K5 polysaccharide derivatives, i.e., K5-OS(L) (average molecular weight [MW], 14,000; sulfate/carboxyl [SO3−/COO−] ratio, 1.41), K5-OS(H) (MW, 11,000; sulfate/carboxyl ratio, 3.77), K5-NS (MW, 15,000; sulfate/carboxyl ratio, 1.00), K5-N,OS(L) (MW, 13,000; sulfate/carboxyl ratio, 1.70), and K5-N,OS(H) (MW, 15,000; sulfate/carboxyl ratio, 3.84) were obtained either by O sulfation (K5-OS) or by N deacetylation/N sulfation (K5-NS) and O sulfation (K5-N,OS) of a single batch of K5 polysaccharide as previously reported (16). The 13C nuclear magnetic resonance (NMR) spectrum analysis, the sulfate/carboxyl ratio analysis, and the MW determination of the different compounds were performed as described previously (2, 3, 9). Unmodified unfractionated beef mucosa heparin (MW, 15,000; sulfate/carboxyl ratio, 2.14) was obtained from Laboratori Derivati Organici, Milan, Italy. All of the sulfated K5 derivatives, as well as heparin, were solubilized in sterile water and stored at −20°C. GCV was purchased from Sigma (catalogue no. G2536).
Cells and viruses.
Human foreskin fibroblasts (HFFs), isolated at the Microbiology and Virology Unity of Padua University Hospital (Italy), and NIH 3T3 cells (ATCC CCL-92), purchased from the American Type Culture Collection (ATCC; Manassas, VA), were maintained in Dulbecco modified Eagle medium (DMEM; Life Biotechnologies, catalogue no. 41965) supplemented with 10% fetal bovine serum (FBS; Life Biotechnologies, catalogue no. 10270), 100 U of penicillin/ml, and 100 μg of streptomycin/ml (Sigma, catalogue no. P0781). HCMV strain AD169 (ATCC VR-538) was purchased from the ATCC. HCMV strain Towne (ATCC VR-2356) was kindly provided by L. Barzon, University of Padua, Padua, Italy. HCMV TB40-UL32-GFP was kindly provided by C. Sinzger, University of Tuebingen, Tuebingen, Germany. HCMV clinical isolates were collected at the Microbiology and Virology Unity of Padua University Hospital (Italy) from 2008 and 2009 and were all under passage 4 after primary isolation. HCMV strains resistant to antiviral drugs were obtained from the NIH AIDS Research and Reference Reagent Program (Rockville, MD). Murine cytomegalovirus (MCMV) strain Smith was purchased from the ATCC (ATCC VR-194).
Cytotoxicity assays.
The cytotoxicity of K5 derivatives and heparin in HFF cells was determined as described previously (19), with minor modifications. Briefly, HFFs were seeded at a density of 103 cells per well into 96-well plates, allowed to attach, and treated with various concentrations of each compound in triplicate for 120 h. Cell viability was then determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma, catalogue no. M2128) method.
Plaque reduction assays.
HFFs or NIH 3T3 cells were seeded at a density of 1.5 × 105 cells per well or 2 × 105 per well, respectively, in 24-well plates. The next day, the cells were infected at 37°C with HCMV or MCMV at 80 PFU per well in DMEM in the presence of different concentrations of test compounds. At 2 h postinfection, the inocula were removed, the cells were washed, and media containing various concentrations of each compound, 5% FBS, and 0.6% methylcellulose (MC) were added. All compound concentrations were tested at least in duplicate. After incubation for 10 to 11 days (for HCMV) or 5 days (for MCMV) at 37°C, the cell monolayers were stained with crystal violet, and viral plaques were counted.
Attachment assays.
The effects of K5 derivatives on HCMV attachment were analyzed as previously described (20). Briefly, 100 PFU of HCMV AD169 and various concentrations of K5 derivatives or heparin as a control were added to HFF monolayers prechilled at 4°C and incubated for either 2 or 4 h at 4°C. Cells were then washed to remove the compound and unattached virus, overlaid with compound-free medium containing 0.6% MC, and incubated for 10 days at 37°C. To confirm that incubation at 4°C allowed only viral attachment and not entry, cells to which virus had been preattached at 4°C were treated for 1 min with acidic citrate buffer (40 mM citrate, 10 mM KCl, 135 mM NaCl [pH 3.0]) to inactivate attached but not yet penetrated virus, and then incubated with medium containing 0.6% MC for 10 days at 37°C. This reproducibly resulted in 100% inhibition of plaque formation in untreated cells (data not shown), indicating that no virus had entered cells during the adsorption phase. Cells were then fixed and stained with crystal violet, and plaques were counted.
Entry assays.
To analyze the effects of K5 derivatives on the penetration of preattached virions, a procedure already described (20) was followed. Briefly, 100 PFU of HCMV AD169 were added to prechilled HFF monolayers and incubated for 2 h at 4°C to allow viral attachment. Cells were then washed to remove unattached virus. Various concentrations of K5 derivatives diluted in serum-free medium were added to the cells, and the temperature was shifted to 37°C for 2 h. The cells were then treated for 1 min with citrate buffer to inactivate the virus that had not entered the cells, washed three times with phosphate-buffered saline (PBS) to restore neutral pH, overlaid with medium containing 0.6% MC, and incubated for 10 days at 37°C. Plates were then fixed and stained with crystal violet, and the plaques were counted. The plaque number derived from cells incubated at 4°C with the virus but not treated was set as 100%.
Virus inactivation assays.
To evaluate the virucidal activity of sulfated K5 derivatives on HCMV virions, a procedure based on that of Shogan et al. (27) was followed, with minor modifications. Briefly, 2 × 105 PFU of HCMV AD169 were incubated with either K5-NS or K5-N,OS(H) derivative (concentrations ranging from 1,667 to 0.17 nM) at 37°C for 1, 2, or 4 h. After incubation, samples were five times 10-fold diluted to reach conditions well below the active concentrations, and then the amount of infectious virus in the lowest dilution was titrated on fresh monolayers of HFF cells. The virus titer determined in samples incubated at 37°C for 1, 2, or 4 h but not treated with compounds was set as 100%.
Cell pretreatment assays.
To investigate the effects of pretreatment of HFF cells with K5 derivatives on HCMV infectivity, HFFs were incubated with sulfated K5 derivatives at different concentrations for either 2 or 6 h at 37°C. At the end of incubation, cells were extensively washed with PBS to remove K5 derivatives and infected with HCMV AD169 according to the same protocol described for plaque reduction assays (except for the absence of compounds during and after infection). Cells were then incubated for 10 days at 37°C and successively fixed and stained with crystal violet. Plaques were then counted.
Cell-to-cell spread inhibition assays.
The ability of K5 derivatives to block HCMV cell-to-cell spread was evaluated as previously described (11), with minor modifications. Briefly, HFF cells seeded on coverslips in 24-well plates were infected at a low multiplicity of infection (MOI; 0.003 PFU/cell) with HCMV AD169 for 2 h at 37°C. After infection, virus that had not entered was inactivated with acidic citrate buffer, and either K5-N,OS(H) or K5-NS diluted in DMEM containing 2% FBS was added at different concentrations. Viral spread was detected by indirect immunofluorescence as follows. Cells were incubated for 5 days at 37°C and then fixed for 15 min with 4% paraformaldehyde in PBS. The samples were blocked with 4% FBS in PBS and reacted with a mouse anti-HCMV immediate-early antigens (IEA) monoclonal antibody (MAb; clone E13 [Argene Biosoft], diluted 1:500). After incubation for 1 h at 37°C, coverslips were washed thoroughly with PBS and incubated with a secondary goat anti-mouse immunoglobulin G MAb conjugated to fluorescein isothiocyanate (Argene Biosoft, diluted 1:500) for 1 h at 37°C. The coverslips were then counterstained with Evans blue dye (Sigma, catalogue no. 206364) prior to being washed with PBS, mounted on slides by using 70% glycerol in PBS, and examined by using a Leica TCS-NT/SP2 confocal microscope with a 20× objective. Images were then digitally analyzed with Leica software. Uninfected monolayers were stained in parallel to detect nonspecific reaction. The software ImageJ 1.43 (http://rsbweb.nih.gov/ij) was used to quantify the plaque size.
RESULTS
Chemical features of K5 sulfated derivatives.
K5 is a bacterial polysaccharide whose structure is the same as the heparin/heparan sulfate biosynthetic precursor, N-acetyl heparosan. Structurally, it is formed by the repetition of d-glucuronic acid α(1-4) linked to N-acetylglucosamine. Through the modification of this starting material, the synthesis of derivatives that differ in the number and position of the sulfate groups can be pursued, thus mimicking the structure of the nonanticoagulant heparan sulfates, the main components of proteoglycans. According to Casu et al. (4), different methods of sulfation can be applied to K5 polysaccharide to obtain derivatives with different structural characteristics (Table 1). In the present study, two classes of O-sulfated compounds were considered, i.e., the highly sulfated (H) and the low sulfated (L) derivatives, which can be either 100% N acetylated [K5-OS(H) and K5-OS(L)] or N sulfated [K5-N,OS(H) and K5-N,OS(L)]. In addition, through N deacetylation/N sulfation of the K5 polysaccharide the K5-NS derivative was obtained as previously described (16).
TABLE 1.
Chemical features of sulfated K5 derivatives
| Compound | MW | SO3−/COO− ratio | Chemical composition (%) |
||||
|---|---|---|---|---|---|---|---|
| Glc-NSO3− | Glc-6SO3− | GlcA-OSO3− | GlcA2,3SO3−a | Nonsulfated GlcA | |||
| K5 | 30,000 | 0 | 0 | 0 | 0 | 0 | 100 |
| K5-NS | 15,000 | 1.00 | 100 | 0 | 0 | 0 | 100 |
| K5-OS(L) | 14,000 | 1.41 | 0 | 90 | <10 | 0 | >90 |
| K5-OS(H) | 11,000 | 3.77 | 0 | 100 | 0 | 100 | 0 |
| K5-N,OS(L) | 13,000 | 1.70 | 100 | 90 | <10 | 0 | >90 |
| K5-N,OS(H) | 15,000 | 3.84 | 100 | 100 | 30 | 70 | 0 |
GlcA2SO3− or GlcA3SO3−.
Inhibition of HCMV replication by sulfated K5 derivatives.
The K5 derivatives were first examined for their effects on HCMV AD169 replication in HFF cells by a modified plaque reduction assay in which test compounds were present in cell medium both during and after virus adsorption. In these experiments, GCV was included as a control for inhibition, while the polysaccharide heparin, structurally related to HSPG and known to prevent HCMV adsorption (12), was included for a comparison. Consistent with previous results (12), heparin inhibited HCMV replication with a 50% effective concentration (EC50) in the nanomolar range (EC50, 28.1 nM, Table 2). No comparable effect on HCMV replication was observed when infected cells were treated with K5 polysaccharide as well as with its sulfated derivatives K5-NS, K5-OS(L), and K5-OS(H), which all possess sulfate groups only at either N or O positions (Table 2). In contrast, two derivatives characterized by sulfation at both N and O positions, i.e., K5-N,OS(L) and K5-N,OS(H), impaired HCMV infection with EC50s in the nanomolar range (EC50, 76.9 and 12.7 nM, respectively; Table 2), with the highly N-/O-sulfated derivative showing the greater inhibitory activity.
TABLE 2.
Antiviral activity against HCMV AD169 and cytotoxicity of K5 derivatives
| Compound | Antiviral activity (EC50, nM)a | Cytotoxicity (CC50, nM)b | SIc |
|---|---|---|---|
| K5 | >833 | >3,333 | >4 |
| K5-NS | >1,667 | >6,667 | >4 |
| K5-OS(L) | >1,785 | >7,142 | >4 |
| K5-OS(H) | >2,227 | >9,090 | >4 |
| K5-N,OS(L) | 76.9 ± 30.8 | >7,690 | >100 |
| K5-N,OS(H) | 12.7 ± 10.1 | >3,333 | >262 |
| Heparin | 28.1 ± 10.7 | >6,667 | >237 |
| GCV | 1,900 ± 200 | 550,000 ± 75,000 | 289 |
That is, the compound concentration that inhibits 50% of plaque formation, as determined by modified plaque reduction assays against HCMV AD169 in HFF cells as described in Materials and Methods. Reported values represent the means ± the SD of data derived from at least three independent experiments in performed duplicate.
That is, the compound concentration that produces 50% of cytotoxicity, as determined by MTT assays in HFF cells. Reported values represent the means ± the SD of data derived from at least three independent experiments performed in quadruplicate.
SI, selectivity index (determined as the ratio between CC50 and EC50).
To exclude the possibility that the antiviral activity of K5-N,OS(L) and K5-N,OS(H) derivatives might be due to cytotoxicity, the effects on uninfected HFF cells of all K5 derivatives were investigated by MTT assays. As reported in Table 2, all compounds did not exhibit toxic effects, at least in the range of the examined concentrations, thus resulting in a very favorable selectivity index for the active compounds.
K5-N,OS(L) and K5-N,OS(H) affect the first events of HCMV replication cycle.
In order to elucidate the nature of the antiviral properties of the active K5 derivatives, we examined their effects on the early stages of viral infection, i.e., attachment and entry. First, we evaluated the inhibition of HCMV plaque formation when sulfated K5 derivatives were present for either 2 or 4 h only during viral attachment at 4°C (a condition that is known to allow virus adsorption only) and removed before virus entry. As a positive control, the susceptibility of viral attachment to heparin was tested. As previously reported (12), heparin was able to inhibit virus attachment potently (EC50, 8.7 nM; Table 3). Consistent with the results obtained in plaque reduction assays, K5 and some of the sulfated K5 derivatives tested [i.e., K5-NS, K5-OS(L) and K5-OS(H)] were determined to be inactive, whereas both K5-N,OS(L) and K5-N,OS(H) efficiently prevented HCMV attachment to HFF cells (EC50, 22.3 and 2 nM, respectively) after both 2 and 4 h of incubation (Table 3 and data not shown).
TABLE 3.
Effects of K5 derivatives on attachment and entry of HCMV
| Compound | Mean EC50 (nM) ± SD |
|
|---|---|---|
| Attachment inhibition assaya | Entry inhibition assayb | |
| K5 | ND | ND |
| K5-NS | 1,133 ± 707 | >1,667 |
| K5-OS(L) | 1,142 ± 100 | ND |
| K5-OS(H) | >2,272 | ND |
| K5-N,OS(L) | 22.3 ± 12.3 | 1,154 ± 138 |
| K5-N,OS(H) | 2.0 ± 0.7 | 333 ± 100 |
| Heparin | 8.7 ± 7.3 | >1,667 |
That is, a compound concentration that inhibits 50% of plaque formation, as determined by attachment inhibition assays against HCMV AD169 in HFF cells. The results presented here were obtained when test compounds were present for 2 h during virus attachment at 4°C; similar results were obtained when compounds were present for 4 h (data not shown). The reported values represent the means of data derived from at least three independent experiments performed in duplicate. ND, not determined.
That is, a compound concentration that inhibits 50% of plaque formation, as determined by entry inhibition assays against HCMV AD169 in HFF cells. Reported values represent the means of data derived from at least three independent experiments performed in duplicate. ND, not determined.
Next, to test the effects of the K5 derivatives on viral entry, experiments were performed in which HCMV was allowed to preattach to cells at 4°C in the absence of compound; various concentrations of test compounds were then added, and infected cells were shifted to 37°C for 2 h to permit virus entry. As shown in Table 3, under these experimental conditions the K5-N,OS(H) and K5-N,OS(L) derivatives exhibited EC50s ∼100-fold higher than those observed when the compounds were present only during virus adsorption.
The sulfated K5 derivatives do not exhibit virucidal activity against HCMV.
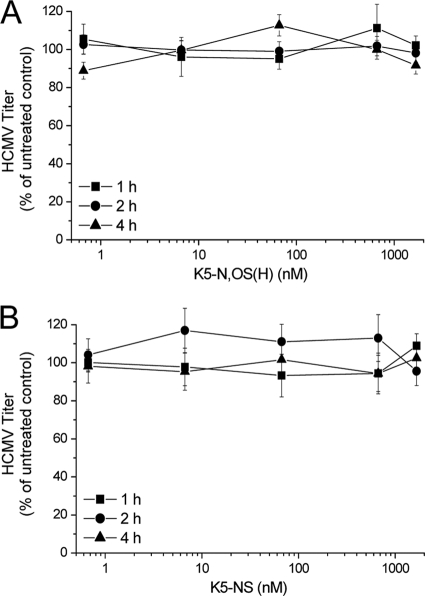
Next, we investigated whether K5 derivatives could exert their antiviral activity by inactivating HCMV particles. Therefore, virus inactivation assays with the most active derivative, i.e., K5-N,OS(H) and the inactive compound K5-NS as a control, were performed in which 2 × 105 PFU of HCMV AD169 were incubated at 37°C with different concentrations of test compounds for 1, 2, or 4 h. After incubation, samples were diluted well below the active concentrations and the infectivity of preincubated virions was assayed. As shown in Fig. 1, in the presence of either compound there was no detectable loss of infectivity, since virus titers of samples treated with either K5-N,OS(H) or K5-NS were comparable to those determined in untreated samples. Similar results were obtained incubating HCMV virions with the compounds at 4°C (data not shown).
FIG. 1.
Effects of pretreatment of HCMV virions with sulfated K5 derivatives on virus infectivity. HCMV AD169 was incubated at 37°C for 1, 2, or 4 h with either K5-N,OS(H) (A) or K5-NS (B). The virus (2.5 × 105 PFU) was then five times 10-fold diluted and titrated on fresh HFF monolayers. The data shown represent the means ± the standard deviations (SD) of data of three independent experiments performed in triplicate.
Cell pretreatment with sulfated K5 derivatives does not affect HCMV replication.
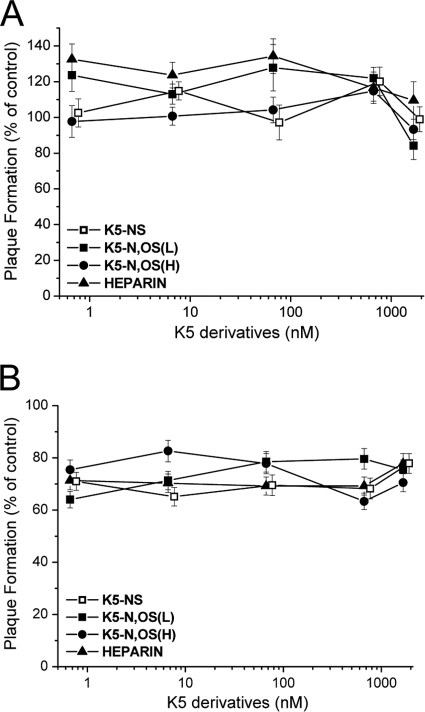
To gain further insights on K5 derivatives mode of action, we investigated whether they could exert antiviral activity by interacting with a cellular component(s). To do this, we preincubated HFFs for either 2 or 6 h with different concentrations of either the active compounds K5-N,OS(H) and K5-N,OS(L), the inactive compound K5-NS, or heparin as a control. After incubation, the cells were extensively washed to ensure the complete removal of test compounds from the media and infected with HCMV. As shown in Fig. 2, the pretreatment of cells with all compounds did not produce any inhibitory effect on the viral plaque formation, suggesting that, upon treatment, HFF cells remained susceptible to viral infection.
FIG. 2.
Effects of pretreatment of HFF cells with sulfated K5 derivatives on HCMV replication. HFFs were incubated with either sulfated K5 derivatives or heparin as a control at 37°C for either 2 (A) or 6 (B) h; the compounds were then removed from cell media, and cells were infected with HCMV AD169. The data shown are expressed as a percentage of the plaque number determined in treated samples with respect to the untreated mock-infected samples and represent the means ± the SD of three independent experiments done in duplicate.
K5-N,OS(H) inhibits cell-to-cell spread of HCMV.
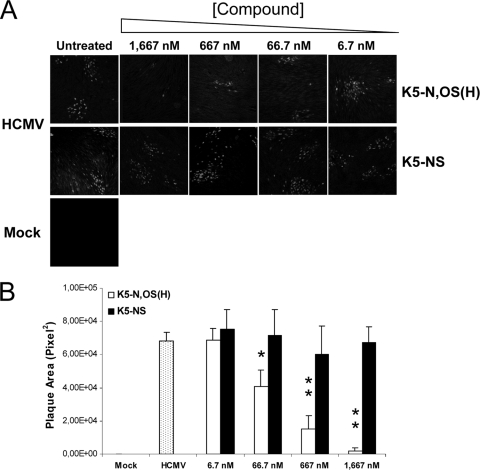
Then, we wanted to investigate whether K5-N,OS(H) was able to block the cell-to-cell transmission of HCMV. For this, HFFs infected with HCMV in the absence of compounds were incubated with medium containing either K5-N,OS(H) or the inactive derivative K5-NS as a negative control. After 5 days, virus spreading was evaluated by indirect immunofluorescence. As shown in Fig. 3A, K5-N,OS(H) was able to block the transmission of HCMV from a single originally infected cell to neighboring cells in a dose-dependent manner. In contrast, K5-NS, when added after the entry of HCMV, did not block virus spread, as demonstrated by the high number of IEA-positive cells per focus observed at all tested compound concentrations. To evaluate quantitatively these observations, the sizes of the infected foci were measured. A statistically significant reduction in the plaque size with respect to infected, untreated cells was observed in infected cells treated with K5-N,OS(H) from 66.7 nM upward (Fig. 3B); in contrast, the control compound K5-NS did not exert any effect on viral plaque formation at either tested concentration (Fig. 3B).
FIG. 3.
Effects of sulfated K5 derivatives on cell-to-cell spread of HCMV. (A) HFFs were infected with HCMV AD169 (MOI, 0.003 PFU/cell) in the absence of compounds (HCMV) or not infected (Mock). Either K5-N,OS(H) or K5-NS was then added at different concentrations, and cells were incubated for 5 days at 37°C. Viral cell-to-cell spread was assessed by indirect immunofluorescence with an anti-IEA MAb. (B) Fifteen representative infected foci per concentration were analyzed with ImageJ software to determine the viral plaques size in the presence of the test compounds. The data shown represent the means ± the SD. The asterisks denote a statistically significant difference (*, P < 0.05; **, P < 0.001).
K5-N,OS(H) inhibits the replication of different HCMV strains and also of MCMV.
Next, we wanted to evaluate the antiviral properties of the most active derivative against HCMV other than the laboratory AD169 strain. Thus, the antiviral activity of K5-N,OS(H) was evaluated against the HCMV strains Towne and TB40, as well as against a panel of clinical isolates recovered from infected patients. The EC50s of this K5 derivative against the selected viruses were in the same range of that obtained for the AD169 strain (compare Table 4 and Table 2), suggesting that the antiviral properties of this compound do not depend on virus strain. Furthermore, K5-N,OS(H) was also able to block MCMV replication in infected NIH 3T3 cells (Table 4) with EC50s similar to those observed for HCMV.
TABLE 4.
Antiviral activity of K5-N,OS(H) against different HCMV laboratory strains, HCMV clinical isolates, and MCMV
| Virus straina | Mean antiviral activity (EC50, nM) ± SDb |
|---|---|
| HCMV Towne | 18.5 ± 6.5 |
| HCMV TB40 | 17.2 ± 6.5 |
| HCMV 389025U* | 32.0 ± 2.5 |
| HCMV 395909A* | 20.4 ± 7.2 |
| HCMV 388438U* | 29.2 ± 3.8 |
| MCMV Smith | 31.1 ± 8.3 |
*, Clinical isolate.
That is, a compound concentration that inhibits 50% of plaque formation, as determined by modified plaque reduction assays against different HCMV strains or MCMV as described in Materials and Methods. The reported values represent the means of data derived from at least three independent experiments performed in duplicate.
K5-N,OS(H) retains activity against viruses resistant to anti-HCMV drugs.
Finally, since the emergence of drug-resistant HCMV strains is becoming a problem of major concern in the management of HCMV-infected patients, we tested the activity of the most active derivative, i.e., K5-N,OS(H), against viruses resistant to the current licensed anti-HCMV drugs GCV, CDV, and FOS. As reported in Table 5, K5-N,OS(H) retained full antiviral activity against both GCV- and FOS-resistant viruses, as well as against the multiple drug-resistant virus GDGrP53 (29).
TABLE 5.
Antiviral activity of K5-N,OS(H) against drug-resistant HCMV strains
| HCMV strain | Reference | Drug resistance | Antiviral activity (EC50, nM)a ± SD |
|---|---|---|---|
| AD169 | None | 12.7 ± 10.1 | |
| 759rD100 | 29 | GCV | 16.5 ± 6.2 |
| PFArD100 | 30 | PFA, ACV | 21.3 ± 7.5 |
| GDGrK17 | 31 | GCV | 18.9 ± 9.2 |
| GDGrP53 | 29 | CDV, GCV | 20.1 ± 4.3 |
That is, a compound concentration that inhibits 50% of plaque formation as determined by modified plaque reduction assays against drug-resistant HCMVs as described in Materials and Methods. Reported values represent the means of data derived from at least three independent experiments in performed duplicate.
DISCUSSION
HCMV remains one of the major causes of life-threatening diseases in immunocompromised individuals; furthermore, it also represents one of the most common causes of congenital defects. The few drugs approved for the treatment of HCMV infections are becoming insufficient due to their toxicity in the context of prolonged antiviral therapy and to the emergence of drug-resistant strains. Furthermore, none of the currently available anti-HCMV drugs is suitable for use during pregnancy and for treatment of congenital infection. Therefore, the development of new anti-HCMV drugs with improved efficacy and novel mechanisms of action is still a priority.
In the present study, we report the identification of two compounds structurally related to the heparan sulfate moiety of cellular HSPG, i.e., K5-N,OS(H) and K5-N,OS(L), that prevent the entry process and cell-to-cell spread of HCMV. Importantly, the most active of these compounds, K5-N,OS(H), is able to inhibit potently the replication not only of laboratory HCMV strains but also of a panel of clinical viral isolates recovered from infected patients. Furthermore, thanks to the different mechanism of action, K5-N,OS(H) retains antiviral activity also against HCMV strains resistant to the anti-HCMV drugs currently used for therapy, thus suggesting that it may represent an alternative therapeutic option in the case of resistance outcomes.
These compounds arise from a novel class of antiviral substances, the sulfated derivatives of E. coli capsular polysaccharide K5, characterized by broad-spectrum activity against a number of enveloped and nonenveloped viruses (25). In line with this, the K5-N,OS(H) derivative, which potently blocks HCMV infection, is also an efficient inhibitor of other viruses such as HSV-1 and -2, genital HPVs, and HIV-1 (17, 23, 32). Thus, K5-N,OS(H) may represent a new, broad-spectrum antiviral compound. On the other hand, structural variations of antiviral sulfated polysaccharides, including K5 derivatives, have been shown to modulate their potency and specificity (25). Consistently, the anti-HCMV activity of sulfated K5 derivatives appears to depend both on sulfation degree [the amount of sulfate groups on the molecule correlated with anti-HCMV activity, since the highly sulfated K5-N,OS(H) was more efficient in preventing HCMV infection than the less sulfated derivative K5-N,OS(L)], and on distribution of sulfate groups. In fact, our results indicate that the presence of sulfate groups at both the N and the O position of the sugar backbone is required for potent anti-HCMV activity, because compounds with O-sulfate groups only [i.e., (K5-OS(H) and K5-OS(L)] or N-sulfate groups only (i.e., K5-NS) showed an anti-HCMV activity much lower than that of the N,O-sulfated compounds (see Table 2). In addition, a certain selectivity of K5 derivatives is evident; in fact, in contrast to the results obtained for HCMV, the O-sulfated K5-OS(H) derivative prevented the replication of HIV and HPVs as efficiently as K5-N,OS(H) (17, 32). Remarkably, our work also highlights that differences can exist in the activity of K5 derivatives against viruses more closely related. In fact, while the low sulfated compound K5-N,OS(L) exhibited an EC50 against HCMV in the nanomolar range, its EC50 against HSV-1 was in the micromolar range (23).
Studies to gain deeper insights into the nature of the anti-HCMV activity of sulfated K5 derivatives suggested that they might affect a step in the attachment/entry process of HCMV in which viral and cellular components are interacting and then undergoing either a conformational modification or the transfer from HSPGs to a more stable, still unknown, cellular receptor, events which have been proposed to take place during HCMV entry (5). This hypothesis is supported by several lines of evidence. First, K5-N,OS(L) and K5-N,OS(H) appeared to interfere with the adsorption of HCMV virions in attachment inhibition assays with a potency similar to that of heparin, which has been shown to prevent HCMV infection by competing with cellular HSPGs for binding to virion components. It is known that HSPGs mediate HCMV attachment to the host cell and provide the initial binding site for components of the viral envelope (5). Thanks to the structural resemblance to HSPGs, sulfated K5 polysaccharide derivatives could likely exert antiviral activity by interfering with the interactions between viral glycoproteins and HSPGs that occur during attachment and consequently preventing the virus penetration into the host cell. Second, HFFs pretreated with different K5 derivatives remained fully susceptible to HCMV infection, thus excluding that these compounds could act by stably interacting with a cellular component(s) and preventing its/their interaction with viral glycoproteins, as it has been reported for other anti-HCMV compounds (21, 26). Third, preincubation of HCMV virions with the active sulfated K5 derivatives did not result in loss of infectivity, suggesting that the antiviral activity of the compounds does not rely on inactivation of virion component(s), as it has been reported for some antiherpetic compounds endowed with virucidal activity (8, 10, 27). Thus, K5-N,OS(H) and K5-N,OS(L) seem not to interact with either cellular or viral components alone, but rather a virus-cell interaction appears to be required for their antiviral activity.
Interestingly, the K5-N,OS(H) derivative is not only able to interfere with the virus entry process, but it also efficiently prevents cell-to-cell spread of HCMV in a dose-dependent manner at nontoxic concentrations. Such antiviral properties might be useful in the clinical setting, where K5-N,OS(H) might be able to prevent both cell-to-cell spread, which represents the predominant route of transmission for HCMV in vivo (1), and the transmission of cell-free virus, which is often present in body fluids at high titers. Pharmacokinetic and bioavailability studies are necessary to understand whether K5 derivatives could be administered parenterally and/or perorally. Taken as a group, sulfated polysaccharides are poorly bioavailable, although some of them can be adsorbed after oral administration (6). The parenteral route seems more promising for the K5 derivatives. We do not expect high toxicity since their structure is similar to that of heparin but, unlike heparin, they are devoid of anticoagulant activity (24). Moreover, for one of them, K5-OS(H), a preliminary toxicology study was performed that evaluated acute toxicity in mice and a 50% lethal dose of >1 g/kg was found (P. Oreste, unpublished results). Finally, a previous study reported that intravenous administration of a sulfated polysaccharide (i.e., curdlan sulfate) to HCMV-infected individuals resulted in anti-HCMV activity with low toxicity (7). For all this, K5 derivatives are promising candidates for the development of a novel class of potent anti-HCMV compounds. However, the species specificity of HCMV infection often makes it difficult to determine the efficacy of an anti-HCMV compound in an animal model. MCMV infection of mice is a well-established model for preclinical evaluation of anti-CMV agents (13). Our finding that K5 derivatives are active against MCMV infection in vitro paves the way for further studies aimed at investigating the antiviral efficacy of some K5 derivatives in MCMV-infected mice.
Acknowledgments
We thank L. Barzon for kindly providing HCMV Towne, C. Singer for HCMV TBA40-UL32-GFP, and R. Cusinato for HCMV clinical isolates.
This study was supported by MURST EX60%, Progetto di Ricerca di Ateneo 2007 (grant CPDA074945), and PRIN 2008 (grant 20085FF4J4) to A.L; by Regione Veneto to G.P.; and by Regione Piemonte (Ricerca Finalizzata 2008-bis) to D.L.
Footnotes
Published ahead of print on 16 August 2010.
REFERENCES
- 1.Britt, W. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr. Top. Microbiol. Immunol. 325:417-470. [DOI] [PubMed] [Google Scholar]
- 2.Casu, B., G. Diamantini, G. Fedeli, M. Mantovani, P. Oreste, R. Pescador, R. Porta, G. Prino, G. Torri, and G. Zoppetti. 1986. Retention of antilipemic activity by periodate-oxidized non-anticoagulant heparins. Arzneimittelforschung 36:637-642. [PubMed] [Google Scholar]
- 3.Casu, B., and U. Gennaro. 1975. A conductimetric method for the determination of sulphate and carboxyl groups in heparin and other mucopolysaccharides. Carbohydr. Res. 39:168-176. [DOI] [PubMed] [Google Scholar]
- 4.Casu, B., G. Grazioli, N. Razi, M. Guerrini, A. Naggi, G. Torri, P. Oreste, F. Tursi, G. Zoppetti, and U. Lindahl. 1994. Heparin-like compounds prepared by chemical modification of capsular polysaccharide from E. coli K5. Carbohydr. Res. 263:271-284. [DOI] [PubMed] [Google Scholar]
- 5.Compton, T. 2004. Receptors and immune sensors: the complex entry path of human cytomegalovirus. Trends Cell. Biol. 14:5-8. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh, T., K. Chattopadhyay, M. Marschall, P. Karmakar, P. Mandal, and B. Ray. 2009. Focus on antivirally active sulfated polysaccharides: from structure-activity analysis to clinical evaluation. Glycobiology 19:2-15. [DOI] [PubMed] [Google Scholar]
- 7.Gordon, M., S. Deeks, C. De Marzo, J. Goodgame, M. Guralnik, W. Lang, T. Mimura, D. Pearce, and Y. Kaneko. 1997. Curdlan sulfate (CRDS) in a 21-day intravenous tolerance study in human immunodeficiency virus (HIV)- and cytomegalovirus (CMV)-infected patients: indication of anti-CMV activity with low toxicity. J. Med. 28:108-128. [PubMed] [Google Scholar]
- 8.Harden, E. A., R. Falshaw, S. M. Carnachan, E. R. Kern, and M. N. Prichard. 2009. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 83:282-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harenberg, J., and J. X. De Vries. 1983. Characterization of heparins by high performance size exclusion liquid chromatography. J. Chromatogr. 261:287-292. [Google Scholar]
- 10.Hayashi, K., T. Hayashi, and A. Tomoda. 2008. Phenoxazine derivatives inactivate human cytomegalovirus, herpes simplex virus-1, and herpes simplex virus-2 in vitro. J. Pharmacol. Sci. 106:369-375. [DOI] [PubMed] [Google Scholar]
- 11.Jones, T. R., S. W. Lee, S. V. Johann, V. Razinkov, R. J. Visalli, B. Feld, J. D. Bloom, and J. O'Connell. 2004. Specific inhibition of human cytomegalovirus glycoprotein B-mediated fusion by a novel thiourea small molecule. J. Virol. 78:1289-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kari, B., and R. Gehrz. 1992. A human cytomegalovirus glycoprotein complex designated gC-II is a major heparin-binding component of the envelope. J. Virol. 66:1761-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern, E. R. 1999. Animal models for cytomegalovirus infections: murine CMV, p. 927-934. In O. Zak, M. Sande, C. Carbon, R. Kaninsky, T. O'Reilly, and E. R. Kern (ed.), Handbook on animal models for antimicrobial therapy. Academic Press, London, United Kingdom.
- 14.Klibanov, O. M. 2009. Vicriviroc, a CCR5 receptor antagonist for the potential treatment of HIV infection. Curr. Opin. Invest. Drugs. 10:845-859. [PubMed] [Google Scholar]
- 15.Kuritzkes, D. R. 2009. HIV-1 entry inhibitors: an overview. Curr. Opin. HIV AIDS 4:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leali, D., M. Belleri, C. Urbinati, D. Coltrini, P. Oreste, G. Zoppetti, D. Ribatti, M. Rusnati, and M. Presta. 2001. Fibroblast growth factor-2 antagonist activity and angiostatic capacity of sulfated Escherichia coli K5 polysaccharide derivatives. J. Biol. Chem. 276:37900-37908. [DOI] [PubMed] [Google Scholar]
- 17.Lembo, D., M. Donalisio, M. Rusnati, A. Bugatti, M. Cornaglia, P. Cappello, M. Giovarelli, P. Oreste, and S. Landolfo. 2008. Sulfated K5 Escherichia coli polysaccharide derivatives as wide-range inhibitors of genital types of human papillomavirus. Antimicrob. Agents Chemother. 52:1374-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lischka, P., and H. Zimmermann. 2008. Antiviral strategies to combat cytomegalovirus infections in transplant recipients. Curr. Opin. Pharmacol. 8:541-548. [DOI] [PubMed] [Google Scholar]
- 19.Loregian, A., and D. M. Coen. 2006. Selective anti-cytomegalovirus compounds discovered by screening for inhibitors of subunit interactions of the viral polymerase. Chem. Biol. 13:191-200. [DOI] [PubMed] [Google Scholar]
- 20.Loregian, A., B. Mercorelli, G. Muratore, E. Sinigalia, S. Pagni, S. Massari, G. Gribaudo, B. Gatto, M. Palumbo, O. Tabarrini, V. Cecchetti, and G. Palu. The 6-aminoquinolone WC5 inhibits human cytomegalovirus replication at an early stage by interfering with the transactivating activity of viral immediate-early 2 protein. Antimicrob. Agents Chemother. 54:1930-1940. [DOI] [PMC free article] [PubMed]
- 21.Luganini, A., A. Giuliani, G. Pirri, L. Pizzuto, S. Landolfo, and G. Gribaudo. Peptide-derivatized dendrimers inhibit human cytomegalovirus infection by blocking virus binding to cell surface heparan sulfate. Antivir. Res. 85:532-540. [DOI] [PubMed]
- 22.Mocarski, E. S., T. Shenk, and R. F. Pass. 2007. Cytomegaloviruses, p. 2701-2772. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 23.Pinna, D., P. Oreste, T. Coradin, A. Kajaste-Rudnitski, S. Ghezzi, G. Zoppetti, A. Rotola, R. Argnani, G. Poli, R. Manservigi, and E. Vicenzi. 2008. Inhibition of herpes simplex virus types 1 and 2 in vitro infection by sulfated derivatives of Escherichia coli K5 polysaccharide. Antimicrob. Agents Chemother. 52:3078-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rusnati, M., P. Oreste, G. Zoppetti, and M. Presta. 2005. Biotechnological engineering of heparin/heparan sulphate: a novel area of multi-target drug discovery. Curr. Pharm. Des. 11:2489-2499. [DOI] [PubMed] [Google Scholar]
- 25.Rusnati, M., E. Vicenzi, M. Donalisio, P. Oreste, S. Landolfo, and D. Lembo. 2009. Sulfated K5 Escherichia coli polysaccharide derivatives: a novel class of candidate antiviral microbicides. Pharmacol. Ther. 123:310-322. [DOI] [PubMed] [Google Scholar]
- 26.Schmidtke, M., A. Karger, A. Meerbach, R. Egerer, A. Stelzner, and V. Makarov. 2003. Binding of a N,N′-bisheteryl derivative of dispirotripiperazine to heparan sulfate residues on the cell surface specifically prevents infection of viruses from different families. Virology 311:134-143. [DOI] [PubMed] [Google Scholar]
- 27.Shogan, B., L. Kruse, G. B. Mulamba, A. Hu, and D. M. Coen. 2006. Virucidal activity of a GT-rich oligonucleotide against herpes simplex virus mediated by glycoprotein B. J. Virol. 80:4740-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stinski, M. F., and D. T. Petrik. 2008. Functional roles of the human cytomegalovirus essential IE86 protein. Curr. Top. Microbiol. Immunol. 325:133-152. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan, V., K. K. Biron, C. Talarico, S. C. Stanat, M. Davis, L. M. Pozzi, and D. M. Coen. 1993. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob. Agents Chemother. 37:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan, V., and D. M. Coen. 1991. Isolation of foscarnet-resistant human cytomegalovirus patterns of resistance and sensitivity to other antiviral drugs. J. Infect. Dis. 164:781-784. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 359:85. [DOI] [PubMed] [Google Scholar]
- 32.Vicenzi, E., A. Gatti, S. Ghezzi, P. Oreste, G. Zoppetti, and G. Poli. 2003. Broad spectrum inhibition of HIV-1 infection by sulfated K5 Escherichia coli polysaccharide derivatives. AIDS 17:177-181. [DOI] [PubMed] [Google Scholar]