Abstract
We have determined the in vitro activities of amphotericin B (AMB), voriconazole, posaconazole (PSC), itraconazole (ITC), ravuconazole, terbinafine, and caspofungin against five strains of Cunninghamella bertholletiae and four of Cunninghamella echinulata. The best activity was shown by terbinafine against both species (MIC range = 0.3 to 0.6 μg/ml) and PSC against Cunninghamella bertholletiae (MIC = 0.5 μg/ml). We have also evaluated the efficacies of PSC, ITC, and AMB in neutropenic and diabetic murine models of disseminated infection by Cunninghamella bertholletiae. PSC at 40, 60, or 80 mg/kg of body weight/day was as effective as AMB at 0.8 mg/kg/day in prolonging survival and reducing the fungal tissue burden in neutropenic mice. PSC at 80 mg/kg/day was more effective than AMB at 0.8 mg/kg/day in reducing the fungal load in brain and lung of diabetic mice. Histological studies revealed an absence of fungal elements in organs of mice treated with either AMB at 0.8 mg/kg/day or PSC at 60 or 80 mg/kg/day in both models. ITC showed limited efficacy in both models. PSC could be a therapeutic option for the treatment of systemic infections caused by Cunninghamella bertholletiae.
The genus Cunninghamella in the order Mucorales encompasses filamentous fungi that are inhabitants of soil and other environments and are common laboratory contaminants. Cunninghamella bertholletiae is the only member of the genus documented to cause human infections (8), although recently the species C. echinulata was also found in clinical samples (3). Infections caused by Cunninghamella are less frequent than those produced by other genera of Mucorales, e.g., Rhizopus and Mucor, but the mortality rate is higher (76%) (5, 19). In general, infections caused by the members of Mucorales are life-threatening and devastating, requiring prompt and aggressive treatment. There are several simultaneous approaches recommended for patient management, including surgical debridement, antifungal therapy, and correction of the underlying predisposing factors, when possible (5, 20).
Amphotericin B (AMB) is the drug of choice, although very few data exist on the activity of this drug against some of the less frequent genera of Mucorales, such as Cunninghamella (1, 2). In a recent study in which a large set of clinical isolates of Mucorales was tested, C. bertholletiae demonstrated the highest resistance to AMB in vitro, with only 63% of the isolates tested showing MIC values under the working interpretative breakpoints described by the CLSI (2, 6). In addition, the failure of AMB therapy has been reported in clinical cases caused by Cunninghamella (4, 15, 18, 19). Posaconazole (PSC) appears to be a promising drug for the treatment of zygomycosis, having been successfully used as salvage therapy for patients who are refractory to or intolerant of AMB (10, 23). PSC is safer than AMB and shows good in vitro activity and in vivo efficacy against some zygomycetes (1, 2, 7, 22). There are only two clinical reports on the use of PSC for the management of Cunninghamella infection (9, 16), and there are no data on the use of this drug in animal models of C. bertholletiae infections. The aim of the present study, therefore, was to evaluate the in vitro activities of seven drugs against isolates of both C. bertholletiae and C. echinulata and to assess the in vivo efficacies of AMB, itraconazole (ITC), and PSC in neutropenic and diabetic murine models of disseminated infection by five strains of C. bertholletiae.
MATERIALS AND METHODS
Fungi.
Five clinical isolates of Cunninghamella bertholletiae and four of C. echinulata, identified molecularly (3), were used in this study. The isolates had been stored at −80°C in potato dextrose broth with glycerol, and before they were tested, they were subcultured onto potato dextrose agar (PDA) at 35°C. On the day of infection, 5-day PDA cultures were suspended in sterile saline and filtered through sterile gauze to remove hyphae. The resulting sporangiospore suspension was adjusted to the desired inoculum size by counting with a hemacytometer. Dilutions of the original suspension were subcultured on PDA plates to confirm the hemacytometer counts.
In vitro studies.
The MICs of AMB, voriconazole (VRC), PSC, ITC, ravuconazole (RVC), terbinafine (TBF), and caspofungin (CAS) against all strains of Cunninghamella used in this study were determined by a broth microdilution method, according to the CLSI guidelines for filamentous fungi (6). For azoles and AMB, the end-point criterion used was 100% inhibition of growth (MIC-0). For CAS, the MIC was defined as the lowest concentration that inhibited 50% of growth (MIC-2). For TBF, the MIC was defined as the lowest concentration that inhibited 80% of growth (MIC-1). Paecilomyces variotii ATCC 36257 was used as a quality control strain.
Animals.
Male OF1 mice (Charles River, Criffa S.A., Barcelona, Spain) with mean weight of 30 g were used. The animals were housed in standard boxes with corncob bedding and had free access to food and water. All animal experiments were approved by the Animal Welfare Committee of the Universitat Rovira i Virgili.
Mice were rendered neutropenic with a single intraperitoneal (i.p.) injection of 200 mg of cyclophosphamide per kg of body weight plus a single intravenous (i.v.) injection of 150 mg of 5-fluorouracil per kg 1 day before challenge (22).
Mice were rendered diabetic with a single i.p. injection of 210 mg of streptozocin per kg of body weight (12). Ten days after streptozocin administration, blood glucose levels were measured using a glucometer (Glucocard memory strips; A. Menarini Diagnostics, Florence, Italy). Mice with blood glucose levels greater than 250 mg/dl were considered diabetic (21). To prevent bacterial infection, the animals of both models received ceftazidime (5 mg/day subcutaneously) from days 1 to 7 after challenge.
Infection.
Neutropenic mice were challenged with 1 × 104 CFU and diabetic mice were challenged with 1 × 105 CFU in 0.2 ml of sterile saline, injected into the lateral tail vein. Preliminary experiments in both models using C. bertholletiae strain UTHSC 05-2275 demonstrated that these inocula were appropriate for producing an acute infection, with 100% of the animals dying within 8 days (data not shown).
The drugs tested were AMB (Fungizone; Bristol-Myers Squibb), PSC (Noxafil; Schering Plough), and ITC (Canadiol; Janssen Pharmaceutica).
Experiment 1.
Neutropenic mice were challenged with C. bertholletiae strain UTHSC 05-2275. Twenty-four hours after challenge, groups of 20 mice were randomly established for each treatment: 10 for the survival study and 10 for the tissue burden study. The different groups were treated as follows: AMB at 0.8 mg/kg of body weight/dose (i.v.) once daily; ITC at 25 or 50 mg/kg of body weight/dose given orally (p.o.) twice daily (BID); and PSC at 20, 40, 60, or 80 mg/kg of body weight/dose (p.o.) once daily. All treatments began 1 day after challenge, and the therapy lasted for 7 days. The control group received no treatment. Animals were checked daily for 20 days.
For tissue burden studies, animals were killed by CO2 inhalation at day 5 postinfection or at day 4 postinfection for those that met the criteria for euthanasia. Brain, lungs, and kidneys were removed aseptically, and approximately half of each organ was gently homogenized in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenates were plated onto potato dextrose agar and incubated for 24 h at 35°C. Colony count data were expressed as the log10 number of CFU per gram of tissue.
Experiment 2.
Since there were no significant differences observed between the two highest doses of PSC (60 or 80 mg/kg/day) and since the lung tissue burden with PSC at 60 mg/kg/day was lower than that for AMB in the neutropenic mice, only the efficacy of PSC at 60 mg/kg/day was compared with that of AMB. Groups of 20 neutropenic mice were challenged with C. bertholletiae strains FMR 9598, UTHSC 04-2581, UTHSC 03-2241, or UTHSC 06-1945. Twenty-four hours after the infection, the mice were treated with AMB at 0.8 mg/kg/day i.v. or PSC at 60 mg/kg/day p.o. Control groups received no treatment. Survival and tissue burden studies were performed following the same procedures described in experiment 1.
Experiment 3.
Diabetic mice were challenged with C. bertholletiae strain UTHSC 05-2275 and treated with the same antifungal drugs at the same doses as in experiment 1. The efficacies of the treatments were evaluated following the same procedures described in experiment 1.
Histological studies.
For the histopathology studies, half of each organ, obtained from tissue burden studies, was placed in 10% buffered formalin. Samples were dehydrated, paraffin embedded, and sliced into 2-μm sections, which were then stained with hematoxylin-eosin, Periodic acid-Schiff (PAS), or Gomori methenamine silver (GMS) and examined in a blinded fashion by light microscopy.
Statistics.
The mean survival time (MST) was estimated by the Kaplan-Meier method, and the MSTs among the groups were compared by using the log-rank test. In the tissue burden studies, the colony counts were analyzed by the Mann-Whitney U test. Calculations were made using SPSS, version 15.0.1, and GraphPad Prism, version 4.0, for Windows. A P value of ≤0.05 was considered statistically significant.
RESULTS
In vitro susceptibility.
The antifungal activities of the drugs tested are shown in Table 1. PSC and TBF were the most active drugs against C. bertholletiae. For C. echinulata, only TBF showed low MICs.
TABLE 1.
In vitro activities of seven antifungal agents against strains of Cunninghamella spp.
| Species and strain | MICa (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| AMB | VRC | PSC | ITC | RVC | TBF | CAS | |
| C. bertholletiae | |||||||
| UTHSC 05-2275 | 2 | 16 | 0.5 | 2 | >16 | 0.06 | >16 |
| FMR 9598 | 2 | >16 | 0.5 | 8 | >16 | 0.06 | >16 |
| UTHSC 04-2581 | 4 | 16 | 0.5 | 1 | >16 | 0.03 | >16 |
| UTHSC 03-2241 | 4 | 8 | 0.5 | 1 | >16 | 0.03 | >16 |
| UTHSC 06-1945 | 4 | 16 | 0.5 | 2 | >16 | 0.03 | >16 |
| C. echinulata | |||||||
| UTHSC 03-3725 | 8 | >16 | 1 | 2 | >16 | 0.03 | >16 |
| FMR 10974 | 8 | >16 | >16 | >16 | >16 | 0.06 | >16 |
| FMR 10973 | 4 | >16 | >16 | >16 | >16 | 0.06 | >16 |
| UTHSC 01-2298 | 16 | >16 | 16 | >16 | >16 | 0.06 | >16 |
MICs were determined after 24 h of incubation.
Experiment 1.
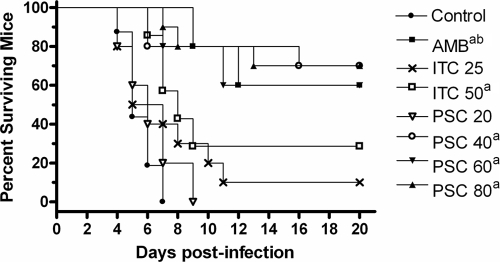
In the neutropenic model, all treatment regimens, with the exception of ITC at 25 mg/kg BID and PSC at 20 mg/kg/day, significantly prolonged survival compared with the length of survival for the control group (P < 0.05). In addition, AMB and PSC at 80 mg/kg/day significantly prolonged survival compared with that achieved with ITC at any dose administered (P < 0.05) (Fig. 1).
FIG. 1.
Cumulative mortality of neutropenic mice infected with Cunninghamella bertholletiae UTHSC 05-2275. AMB, amphotericin B at 0.8 mg/kg/day; ITC 25, itraconazole at 25 mg/kg BID; ITC 50, itraconazole at 50 mg/kg BID; PSC 20, posaconazole at 20 mg/kg/day; PSC 40, posaconazole at 40 mg/kg/day; PSC 60, posaconazole at 60 mg/kg/day; PSC 80, posaconazole at 80 mg/kg/day. a, P < 0.05 versus control; b, P < 0.05 versus ITC at 50 mg/kg.
Results of the tissue burden studies are shown in Fig. 2. The brains, lungs, and kidneys of the control animals infected with strain UTHSC 05-2275 showed high fungal loads, with the lung being the most affected organ (P < 0.0001 versus brain and kidney). AMB and PSC at 60 or 80 mg/kg/day significantly reduced the fungal load in all the organs tested with respect to that for the control group, with no differences between them with the exception of lung, where PSC at 40 and 60 mg/kg/day was more active than AMB (P = 0.0003 and 0.035, respectively). In general, PSC at doses ≥40 mg/kg/day was as effective as AMB and better than ITC in kidney and lung. ITC at 25 or 50 mg/kg BID significantly reduced the fungal load in lung and brain but not in kidney with respect to that for the control group (P < 0.05). ITC at 50 mg/kg BID showed efficacy similar to the efficacies of AMB and PSC at ≥40 mg/kg/day in brain.
FIG. 2.
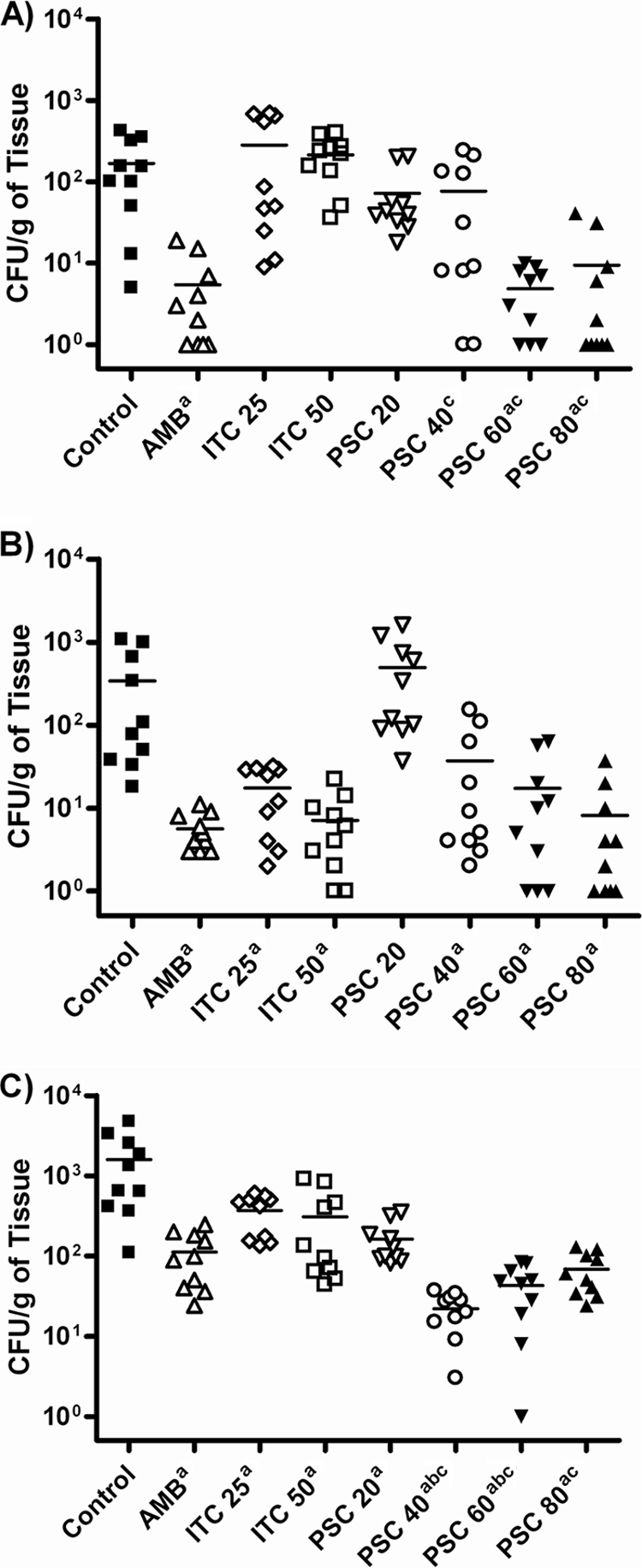
Effects of the antifungal treatments on colony counts of Cunninghamella bertholletiae UTHSC 05-2275 in kidney (A), brain (B), and lung (C) of neutropenic mice. AMB, amphotericin B at 0.8 mg/kg/day; ITC 25, itraconazole at 25 mg/kg BID; ITC 50, itraconazole at 50 mg/kg BID; PSC 20, posaconazole at 20 mg/kg/day; PSC 40, posaconazole at 40 mg/kg/day; PSC 60, posaconazole at 60 mg/kg/day; PSC 80, posaconazole at 80 mg/kg/day. a, P < 0.05 versus control; b, P < 0.05 versus AMB; c, P < 0.05 versus ITC at 25 and 50 mg/kg. The horizontal lines of the scatter plots indicate mean values.
Experiment 2.
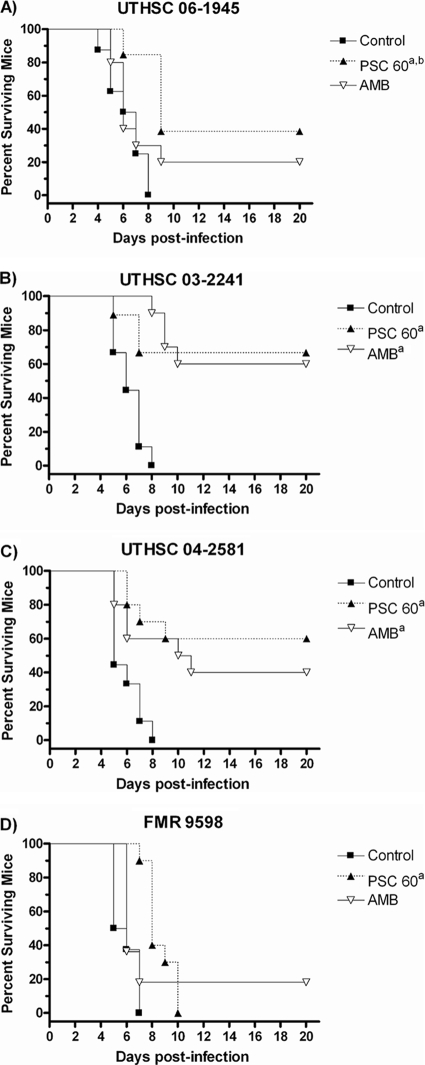
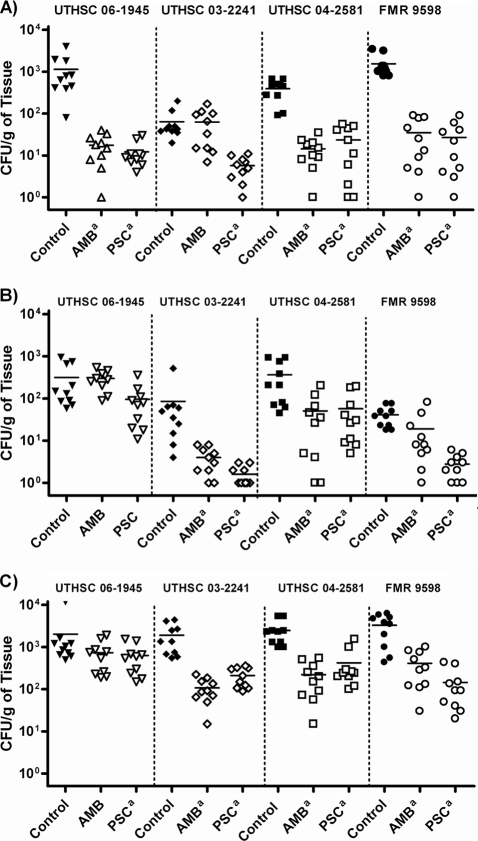
PSC significantly prolonged the rate of survival of mice infected with the four strains of C. bertholletiae (P < 0.05), while AMB was able to prolong the survival of mice infected with only two of the strains tested (P < 0.05) (Fig. 3). Against the four strains tested, both drugs reduced the fungal load in all the organs, with the exceptions of AMB in kidneys for strain UTHSC 03-2241 and lungs for strain UTHSC 06-1945 and of both drugs in the brains of mice infected with strain UTHSC 06-1945 (Fig. 4).
FIG. 3.
Cumulative mortality of neutropenic mice infected with Cunninghamella bertholletiae UTHSC 06-1945 (A), UTHSC 03-2241 (B), UTHSC 04-2581 (C), and FMR 9598 (D). AMB, amphotericin B at 0.8 mg/kg/day; PSC, posaconazole at 60 mg/kg/day. a, P < 0.05 versus control; b, P < 0.05 versus AMB.
FIG. 4.
Effects of the antifungal treatment on colony counts of Cunninghamella bertholletiae in kidney (A), brain (B), and lung (C) of neutropenic mice. AMB, amphotericin B at 0.8 mg/kg/day; PSC, posaconazole at 60 mg/kg/day. a, P < 0.05 versus control. The horizontal lines of the scatter plots indicate mean values.
Experiment 3.
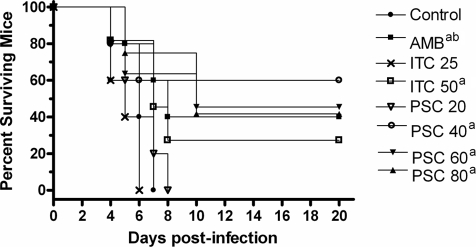
In diabetic mice, all treatment regimens, with the exceptions of ITC at 25 mg/kg BID and PSC at 20 mg/kg/day, significantly prolonged survival versus the length of survival for the control group (P < 0.05) (Fig. 5). AMB and PSC at 40 mg/kg/day reduced the tissue burden only in kidney (Fig. 6). PSC at 60 or 80 mg/kg/day significantly reduced the fungal load in most of the organs tested with respect to that for the control group. In brain and lung, PSC at 80 mg/kg/day was more effective than AMB (P ≤ 0.043). ITC at 50 mg/kg BID significantly reduced the fungal load in lung and brain (both P ≤ 0.014) but not in kidney (P = 0.063).
FIG. 5.
Cumulative mortality of diabetic mice infected with Cunninghamella bertholletiae UTSCH 05-2275. AMB, amphotericin B at 0.8 mg/kg/day; ITC 25, itraconazole at 25 mg/kg BID; ITC 50, itraconazole at 50 mg/kg BID; PSC 20, posaconazole at 20 mg/kg/day; PSC 40, posaconazole at 40 mg/kg/day; PSC 60, posaconazole at 60 mg/kg/day; PSC 80, posaconazole at 80 mg/kg/day. a, P < 0.05 versus control; b, P < 0.05 versus PSC at 20 mg/kg/day and ITC at 25 mg/kg BID.
FIG. 6.
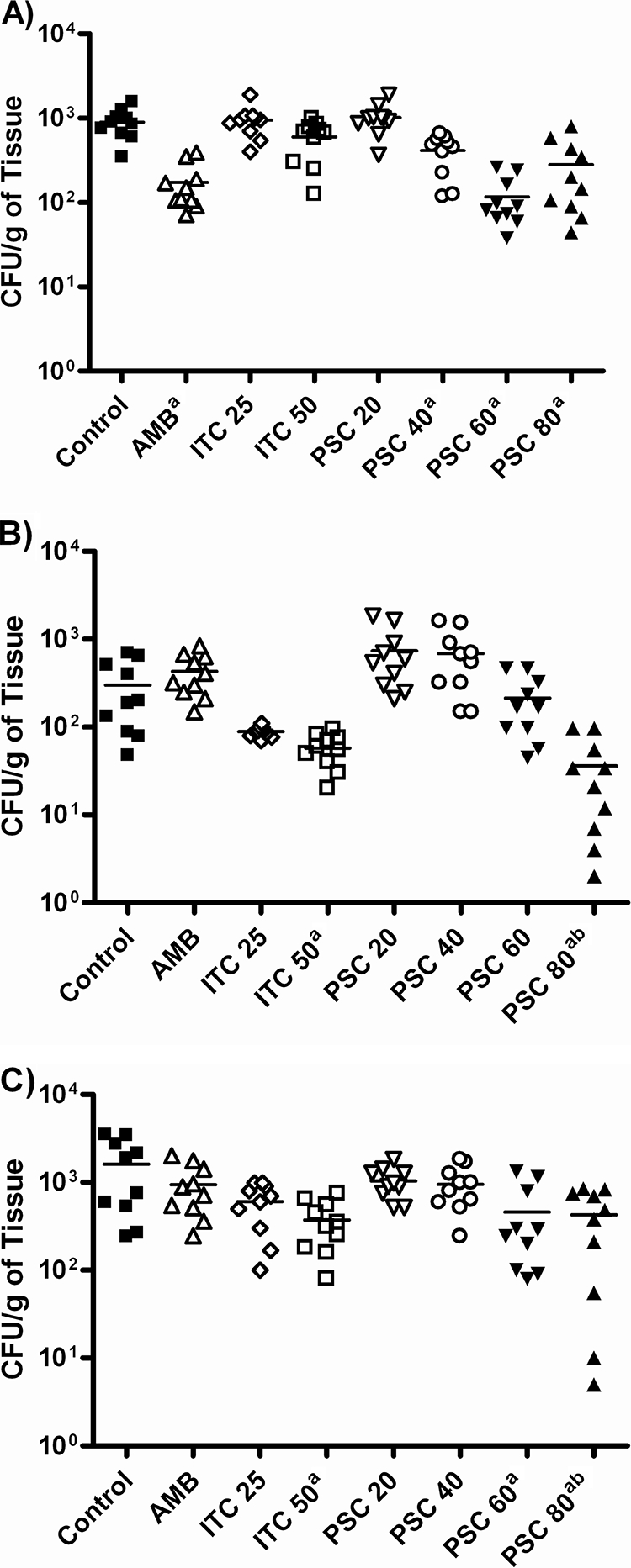
Effects of the antifungal treatments on colony counts of Cunninghamella bertholletiae UTSCH 05-2275 in kidney (A), brain (B), and lung (C) of diabetic mice. AMB, amphotericin B at 0.8 mg/kg/day; ITC 25, itraconazole at 25 mg/kg BID; ITC 50, itraconazole at 50 mg/kg BID; PSC 20, posaconazole at 20 mg/kg/day; PSC 40, posaconazole at 40 mg/kg/day; PSC 60, posaconazole at 60 mg/kg/day; PSC 80, posaconazole at 80 mg/kg/day. a, P < 0.05 versus control; b, P < 0.05 versus AMB. The horizontal lines of the scatter plots indicate mean values.
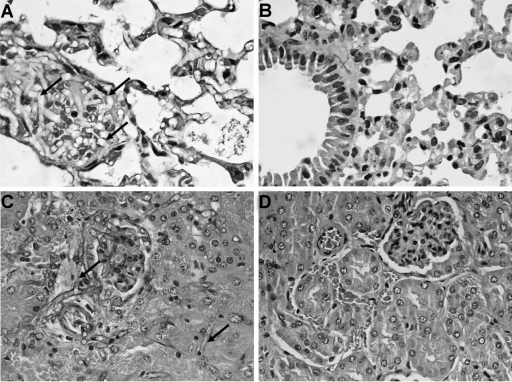
In untreated control groups of both models infected with any of the strains of C. bertholletiae tested, histological studies showed infiltration of fungal elements without an inflammatory response (Fig. 7). No fungal elements were observed in the sections of the organs of mice treated with AMB or PSC at 60 or 80 mg/kg, with the exceptions of those infected with strains UTHSC 05-2275 and UTHSC 06-1945 treated with AMB and with AMB or PSC, respectively, in which few fungal elements and occasional polymorphonuclear cells were seen in lung.
FIG. 7.
Histopathology of lung and kidney tissues from neutropenic mice infected with Cunninghamella bertholletiae strain UTHSC 05-2275 and treated with posaconazole at 60 mg/kg/day. (A) Lung section of control group showing numerous fungal elements (black arrows) (hematoxylin-eosin); (B) lung section of PSC-treated groups, showing normal tissue and no fungal elements (hematoxylin-eosin); (C) kidney section of control group showing numerous fungal cells (black arrows) (PAS); (D) kidney section of PSC-treated groups, showing normal tissue and no fungal elements (hematoxylin-eosin). Magnifications, ×400.
DISCUSSION
While human infections by Cunninghamella bertholletiae are rare, they are particularly severe and have a high mortality (19). Therefore, some effort to obtain a better understanding of the infection and its treatment is justified.
In general, our in vitro results agree with those reported by other authors. The in vitro resistance of some isolates to AMB and the low MICs for PSC and TRB against C. bertholletiae have been described previously (1, 2). However, there are no previous in vitro data on the use of those antifungal agents against C. echinulata. This is the first report on the antifungal susceptibility testing of this potential pathogenic species (3), which demonstrated its resistance, in vitro, to all available antifungal drugs, with the exception of TRB. In previous studies in which Cunninghamella spp. were tested, the two species were not differentiated (1, 2).
To our knowledge, this is the first study that has evaluated the efficacy of PSC in the treatment of experimental infections by C. bertholletiae. We have established both neutropenic and diabetic murine models of disseminated infection by C. bertholletiae in order to evaluate the effectiveness of different treatments against this infection, since both conditions are the major risk factors associated with zygomycosis (17, 19). The present study is the second experimental study on the efficacies of antifungal drugs against this fungus. Previously, Honda et al. (11) described a murine model of disseminated infection by C. bertholletiae in which the efficacy of AMB at 0.25 mg/kg/day was evaluated, obtaining a minimal improvement in the survival rate. Our study differs from that one in many aspects, such as the route of infection, type of immunosuppression, number of fungal strains tested, and antifungals evaluated, making it difficult to compare the results. Using an intravenous route of inoculation in our study, we were able to provoke a disseminated infection that resulted in a marked invasion of brain, kidney, and lung, with the lung being the organ with the highest fungal load (P < 0.0001). These data agree with the increased frequency of pulmonary involvement produced by C. bertholletiae in relation to that produced by other agents of zygomycosis (19). To obtain similar mortality rates in neutropenic and diabetic mice, we had to use different inocula. In a previous study that evaluated the efficacy of PSC in the treatment of disseminated infection caused by Rhizopus oryzae, Ibrahim et al. (13) also had to use a higher inoculum for diabetic mice than for neutropenic mice to achieve similar mortality rates. The marked neutropenia induced by the immunosuppressive regimen used in our model (polymorphonuclear leukocyte count, <100/μl for 9 to 10 days) (14) probably contributed to the development of an acute infection in mice challenged with a lower inoculum than was needed to obtain similar mortality in diabetic mice. This would correlate with the clinical experience, since a higher frequency of pulmonary and disseminated zygomycosis was reported in neutropenic patients than in diabetic ones, in which sinus involvement predominated (19). Some differences in the efficacies of the treatments were observed between the two animal models developed here, probably due to the higher inoculum used for diabetic mice. PSC was effective in both experimental models, although in the diabetic model, only PSC at 80 mg/kg/day was more effective than AMB in reducing the tissue burden in brain and lung. Up to now there have been no data published regarding the use of antifungal agents in diabetic mice infected with Cunninghamella bertholletiae.
In the clinical setting, PSC as salvage therapy or combined with AMB showed efficacy in the treatment of refractory zygomycosis in general (10, 23), but data concerning C. bertholletiae infections are scarce. In two recent cases of pulmonary infection by C. bertholletiae, PSC showed efficacy. The first case was refractory to liposomal amphotericin B and VRC, and the cure was obtained using a combination of surgical resection and PSC therapy (16). The other one was resolved with a sequential treatment with liposomal amphotericin B and PSC (9). Although low doses of PSC were not effective in our study, the highest ones prolonged the survival rate, reduced the tissue burden, and cleared noticeable fungal elements, as seen in the histological sections. ITC has shown limited efficacy in our study. However, there are no data on the use of this compound in clinical cases in which this species was involved in order to be able to confirm these findings.
In our murine models, PSC showed efficacy similar to that of AMB. Although further experimental and clinical studies are needed to ascertain the clinical relevance of PSC in the treatment of infections produced by C. bertholletiae, our results suggest that this drug could be an alternative to AMB in the therapy of these infections.
Footnotes
Published ahead of print on 30 August 2010.
REFERENCES
- 1.Alastruey-Izquierdo, A., M. V. Castelli, I. Cuesta, A. Monzon, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2009. Activity of posaconazole and other antifungal agents against Mucorales identified by means of ITS sequencing. Antimicrob. Agents Chemother. 53:1686-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almyroudis, N. G., D. A. Sutton, A. W. Fothergill, M. G. Rinaldi, and S. Kusne. 2007. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob. Agents Chemother. 51:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez, E., D. A. Sutton, J. Cano, A. W. Fothergill, A. Stchigel, M. G. Rinaldi, and J. Guarro. 2009. Spectrum of zygomycete species identified from clinically significant specimens in the United States. J. Clin. Microbiol. 47:1650-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibashi, E., V. Sidi, M. Kotsiou, E. Makrigiannaki, and D. Koliouskas. 2008. Pulmonary zygomycosis caused by Cunninghamella bertholletiae in a child with acute lymphoblastic leukemia. Hippokratia 12:43-45. [PMC free article] [PubMed] [Google Scholar]
- 5.Chayakulkeeree, M., M. A. Ghannoum, and J. R. Perfect. 2006. Zygomycosis: the re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 25:215-229. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed. CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Dannaoui, E., J. F. G. M. Meis, D. Loebenberg, and P. E. Verweij. 2003. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob. Agents Chemother. 47:3647-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands.
- 9.Garbino, J., C. Myers, J. Ambrosioni, and F. Gumy-Pause. 2010. Report of a successful treatment of pulmonary Cunninghamella bertholletiae infection with liposomal amphotericin and posaconazole in a child with GvHD and review of the literature. J. Pediatr. Hematol. Oncol. 32:83-84. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg, R. N., K. Mullane, J. A. van Burik, I. Raad, M. J. Abzug, G. Anstead, R. Herbrecht, A. Langston, K. A. Marr, G. Schiller, M. Schuster, J. R. Wingard, C. E. Gonzalez, S. G. Revankar, G. Corcoran, R. J. Kryscio, and R. Hare. 2006. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 50:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda, A., K. Kamei, H. Unno, K. Hiroshima, T. Kuriyama, and M. Miyaji. 1999. A murine model of zygomycosis by Cunninghamella bertholletiae. Mycopathologia 144:141-146. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim, A. S., V. Avanessian, B. Spellberg, and J. E. Edwards, Jr. 2003. Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob. Agents Chemother. 47:3343-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim, A. S., T. Gebremariam, J. A. Schwartz, J. E. Edwards, Jr., and B. Spellberg. 2009. Posaconazole mono- or combination therapy for treatment of murine zygomycosis. Antimicrob. Agents Chemother. 53:772-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortoneda, M., J. Capilla, F. J. Pastor, C. Serena, and J. Guarro. 2004. Interaction of granulocyte colony-stimulating factor and high doses of liposomal amphotericin B in the treatment of systemic murine scedosporiosis. Diagn. Microbiol. Infect. Dis. 50:247-251. [DOI] [PubMed] [Google Scholar]
- 15.Passos, X. S., W. S. Sales, P. J. Maciel, C. R. Costa, D. M. Ferreira, and M. R. do Silva. 2006. Nosocomial invasive infection caused by Cunninghamella bertholletiae: case report. Mycopathologia 161:33-35. [DOI] [PubMed] [Google Scholar]
- 16.Paul, S., F. M. Marty, and Y. L. Colson. 2006. Treatment of cavitary pulmonary zygomycosis with surgical resection and posaconazole. Ann. Thorac. Surg. 82:338-340. [DOI] [PubMed] [Google Scholar]
- 17.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Righi, E., C. G. Giacomazzi, V. Lindstrom, A. Albarello, O. Soro, M. Miglino, M. Perotti, O. E. Varnier, M. Gobbi, C. Viscoli, and M. Bassetti. 2008. A case of Cunninghamella bertholettiae rhino-cerebral infection in a leukaemic patient and review of recent published studies. Mycopathologia 165:407-410. [DOI] [PubMed] [Google Scholar]
- 19.Roden, M. M., T. E. Zaoutis, W. L. Buchanan, T. A. Knudsen, T. A. Sarkisova, R. L. Schaufele, M. Sein, T. Sein, C. C. Chiou, J. H. Chu, D. P. Kontoyiannis, and T. J. Walsh. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634-653. [DOI] [PubMed] [Google Scholar]
- 20.Rogers, T. R. 2008. Treatment of zygomycosis: current and new options. J. Antimicrob. Chemother. 61(Suppl. 1):i35-i40. [DOI] [PubMed] [Google Scholar]
- 21.Rosen, D. A., C. S. Hung, K. A. Kline, and S. J. Hultgren. 2008. Streptozocin-induced diabetic mouse model of urinary tract infection. Infect. Immun. 76:4290-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun, Q., L. Najvar, R. Bocanegra, D. Loebenberg, and J. R. Graybill. 2002. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed-mouse model. Antimicrob. Agents Chemother. 46:2310-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Burik, J. A., R. S. Hare, H. F. Solomon, M. L. Corrado, and D. P. Kontoyiannis. 2006. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin. Infect. Dis. 42:61-65. [DOI] [PubMed] [Google Scholar]