Abstract
Since glycopeptide-resistant enterococci (GRE) were reported in 1988, they have appeared in hospitals worldwide. Seven van gene cluster types (vanA, vanB, vanC, vanD, vanE, vanG, and vanL) are currently known. We investigated a clinical strain of Enterococcus faecium Efm-HS0661 that was isolated in 2006 from an inpatient with intra-abdominal infection in Shanghai. It was resistant to most antimicrobials, including vancomycin (MIC, >256 μg/ml) and teicoplanin (MIC, 96 μg/ml). Glycopeptide resistance could be transferred to E. faecium BM4105RF by conjugation. The donor and its transconjugant were negative by PCR for the known van genes. By cloning and primer walk sequencing, we discovered a novel van gene cluster, designated vanM. The vanM ligase gene was 1,032-bp in length and encoded a 343-amino-acid protein that shared 79.9, 70.8, 66.3, and 78.8% amino acid identity with VanA, VanB, VanD, and VanF, respectively. Although the vanM DNA sequence was closest to vanA, the organization of the vanM gene cluster was most similar to that of vanD. Upstream from the vanM cluster was an IS1216-like element, which may play a role in the dissemination of this resistance determinant. Liquid chromatography-mass spectrometry analysis of peptidoglycan precursors extracted from the VanM-type strain Efm-HS0661 treated with vancomycin or teicoplanin revealed a modified precursor (UDP-N-acetylmuramic acid [MurNAc]-tetrapeptide-d-Lac), indicating that VanM, like VanA, confers glycopeptide resistance by the inducible synthesis of precursor ending in d-Ala-d-Lac.
Glycopeptide-resistant enterococci have emerged as important nosocomial pathogens since the late 1980s (18, 25). The glycopeptides vancomycin and teicoplanin act by binding to the d-alanyl-d-alanine (d-Ala-d-Ala) terminus of intermediates in peptidoglycan formation, inhibiting cell wall cross-linking (8). Resistance to glycopeptide antibiotics in enterococci results from the synthesis of peptidoglycan precursors with low affinity for these antibiotics, mediated by different van gene clusters (4, 8). Seven types of gene clusters conferring glycopeptide resistance have been described in enterococci based on DNA sequence and organization. They are designated according to the name of the ligase gene, which encodes either a d-Ala:d-Lac (vanA, vanB, and vanD) or a d-Ala:d-Ser (vanC, vanE, vanG, and vanL) ligase for the synthesis of peptidoglycan precursors with low affinity for glycopeptides. The vanA, vanB, and vanD gene clusters contain genes for a two-component regulatory system (vanR and vanS), three resistance genes (vanH, encoding dehydrogenase; vanA, vanB, or vanD, encoding ligase; vanX, encoding dd-dipeptidase); an accessory gene (vanY); and the vanZ gene, which is present in the vanA gene cluster, whereas the vanW gene is found only in the vanB operon (8). Another van gene cluster, vanF, has been described in a biopesticide, Paenibacillus popilliae (20), but has not yet been found in enterococci.
Although vanA, vanB, and vanD gene clusters involve genes encoding similar enzymatic functions, they can be distinguished on the basis of the level and inducibility of resistance to glycopeptides and by the location of the genes (8, 11). The VanA type is characterized by acquired resistance to high levels of both vancomycin and teicoplanin, and it is induced by either vancomycin or teicoplanin. The VanB type is characterized by acquired resistance to various concentrations of vancomycin but not to teicoplanin and is induced only by vancomycin (1). VanA- and VanB-type resistances are the most common glycopeptide resistance phenotypes (23). The genes encoding the VanA and VanB phenotypes are carried on transposons, which may be found on plasmids or inserted into the chromosome (2).
The VanD type is characterized by resistance to intermediate levels of vancomycin and to low levels of teicoplanin and is expressed constitutively (10, 12, 21). vanD genes appear to be located on the chromosome and are not transferable to other enterococci (12). This could explain the scarcity of recognized VanD strains in contrast to the widespread and high prevalence of VanA and VanB strains.
Glycopeptide-resistant enterococci (GRE) were rare in China a few years ago (22), although vancomycin has been used in clinical practice for multidrug-resistant Gram-positive infections in mainland China for decades. In recent years, GRE strains isolated from hospitalized patients in mainland China have been increasing (6, 22, 27). In the course of determining the genotypes of glycopeptide-resistant Enterococcus faecium strains isolated from a teaching hospital in Shanghai, we found that several strains were negative by PCR for vanA, vanB, and vanD genes. The responsible glycopeptide resistance gene was cloned and sequenced from strain Efm-HS0661 and found to be novel. This new glycopeptide resistance gene cluster has been termed the vanM cluster. Of 10 unique GRE clinical strains isolated at our teaching hospital from 2005 to 2008, 6 carried vanM, which plays a role in the increasing GRE prevalence in China.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Ten glycopeptide-resistant E. faecium clinical isolates and 100 glycopeptide-sensitive clinical strains (36 of Enterococcus faecalis and 64 of E. faecium) were collected from individual patients at the teaching hospital of Fudan University in Shanghai between 2005 and 2008. Escherichia coli DH5α (Invitrogen), E. faecium BM4105RF (22), and plasmid pRB473 (Gram+/Gram− shuttle vector; Ampr Chlr) (5) were used in cloning or mating experiments.
Conjugation and MIC determination.
To determine if the glycopeptide resistance in clinical strains was transferable, filter-mating experiments were carried out with E. faecium BM4105RF (Fusr, Rifr) as the recipient. Transconjugants were selected on brain heart infusion (BHI) agar (Oxoid, Basingstoke, England) plates containing rifampin (100 μg/ml) or fusidic acid (10 μg/ml) for counterselection and vancomycin (10 μg/ml) to select for plasmid-encoded resistance.
The MICs for all antimicrobial agents for the donor, recipient, and transconjugant strains were measured by Etest on Mueller-Hinton agar (Oxoid, Basingstoke, England) according to the manufacturer's instructions (AB Biodisk, Sweden) and interpreted according to recommendations of the Clinical and Laboratory Standards Institute (CLSI).
PFGE and MLST analysis.
Clinical strains and transconjugants were typed by the pulsed-field gel electrophoresis (PFGE) of genomic DNA using the CHEF mapper system (Bio-Rad). Agarose plugs were prepared with proteinase K (Merck, Germany) at 1 mg/ml and digested with SmaI, and the DNA was subjected to electrophoresis at 6 V/cm, 14°C, in a 1.0% agarose gel (Bio-Rad) with pulse times of 5 to 30 s for 22 h. Banding patterns were interpreted using the criteria devised by Tenover et al. (24).
Multilocus sequence typing (MLST) of E. faecium isolates was performed as previously reported (16). The alleles and sequence types (STs) were analyzed and determined through the MLST database (http://efaecium.mlst.net/).
van gene cluster sequencing.
To determine the van genotype present in the GRE clinical isolates, PCR with specific primers for vanA and vanB was used (4). A set of universal primers was designed in this study to detect d-Ala:d-Lac ligase genes in strains negative for vanA and vanB, NvanF (5′GTTTGGGGGTTGCTCAGAGG3′) and NvanR (5′TCACCCCTTTAACGCTAATACGATC3′), which selected a nucleotide region from position 27 to 1032 of the vanA gene (GenBank accession number AM296544) as the target to produce a PCR product of 1,006 bp. Purified PCR products were sequenced on both strands to determine the glycopeptide resistance genotype. The sequence of DNA adjacent to the vanM ligase gene was determined with a series of outward-facing primers starting from both sides of the vanM gene on plasmid DNA extracted from the transconjugant BM-HS0661 with the plasmid midi kit (Qiagen, Germany).
To confirm that the donor contained the same sequence as that found in the transconjugant, plasmid DNA extracted from Efm-HS0661 was digested with HindIII and PshAI, and the fragments were ligated into pRB473 digested with HindIII and SmaI (with blunt ends similar to those produced by PshAI). The recombinant plasmid pRB-0661 was transformed into E. coli DH5α, and the transformants were screened by PCR with primers NvanF and NvanR for the van gene. The plasmid DNA extracted from the van gene-positive transformant was sequenced.
Sequence analyses and comparisons to known sequences were performed with the BLAST programs at the National Center for Biotechnology Information (NCBI).
Extraction and analysis of peptidoglycan precursors.
E. faecium Efm-HS0661 was grown in BHI broth (Oxoid, Basingstoke, England) to an optical density at 650 nm (OD650) of 0.4 and was treated with vancomycin (100 μg/ml) or teicoplanin (50 μg/ml) for an additional 120 min. Peptidoglycan precursors were extracted as previously described (3). The peptidoglycan precursors were analyzed by liquid chromatography-mass spectrometry (LC-MS). The experiments were performed on an LC-20AD system (Shimadzu, Japan) connected to an LTQ Orbitrap XL mass spectrometer (Thermo, Bremen, Germany) equipped with an online electrospray ion source (Michrom Bioresources, Auburn, GA). The samples were separated with a Zorbax 300SB-C18 column (1.0 by 250 mm) from Agilent. The peptides were eluted with a linear gradient, starting from 0 to 100% B in 18 min (eluent A, 10 mM ammonium acetate, pH 5; eluent B, 2% acetonitrile in 10 mM ammonium acetate, pH 5). The column flow rate was maintained at 30 μl/min. The electrospray voltage of 4.0 kV versus the inlet of the mass spectrometer was used. Survey full-scan MS spectra (m/z 400 to 1,800) were acquired in the orbitrap with a mass resolution of 100,000 at m/z 400. MS/MS spectra were acquired in the LTQ. The UDP-N-acetylmuramic acid (MurNAc) peptide structures were deduced from their molecular mass determined by MS/MS performed on singly charged protonated molecules. The quantification of the molecules under study was carried out by extracting selected ions (extracted ion chromatograms; XIC) from the respective full-scan MS chromatograms. The Xcalibur 2.0.7 software (Thermo Fisher Scientific) was used for acquiring and handling the data.
Nucleotide sequence accession numbers.
The sequences of the vanM gene cluster of vancomycin-resistant E. faecium Efm-HS0661 have been submitted to the GenBank database and assigned accession number FJ349556.
RESULTS AND DISCUSSION
Characterization of glycopeptide-resistant E. faecium.
The characteristics of the 10 isolates of glycopeptide-resistant E. faecium are listed in Table 1. All 10 patients had been administered glycopeptide antibiotics and/or broad-spectrum cephalosporins. All 10 E. faecium isolates were resistant to vancomycin, erythromycin (MIC > 256 μg/ml), ciprofloxacin (MIC > 32 μg/ml), ampicillin (MIC > 32 μg/ml), and gentamicin (MIC > 1,024 μg/ml), and 9 of them were resistant to teicoplanin, but none was resistant to linezolid (Table 1). The glycopeptide resistance of Efm-HS0661 could be transferred by conjugation to E. faecium BM4105 RF, but the resistance to other drugs could not.
TABLE 1.
Characteristics of the 10 isolates of glycopeptide-resistant E. faecium, recipient BM4105, and transconjugant BM-HS0661a
| Strain | Source | Date of isolation (yr/mo/day) | Ward | Gene cluster type | MLST sequence type | PFGE type | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAN | TEC | LNZ | RIF | NIT | DOX | |||||||
| Efm-HS05417 | Urine | 2005/12/6 | Neurology | vanA | ST17 | A | >256 | 96 | 1.5 | 0.25 | 128 | 0.19 |
| Efm-HS05446 | Urine | 2005/12/26 | Neurology | vanA | ST17 | B | >256 | 96 | 1.5 | 4 | 16 | 6 |
| Efm-HS0649 | Exudate | 2006/1/27 | General Surgery | vanA | ST17 | B | >256 | 64 | 1.5 | 4 | >512 | 6 |
| Efm-HS06188 | Urine | 2006/6/1 | ICU | vanA | ST78 | A | >256 | 64 | 1.5 | 0.19 | 128 | 0.125 |
| Efm-HS0661 | Exudate | 2006/2/4 | General Surgery | vanM | ST78 | C | >256 | 96 | 1.5 | 4 | 64 | 0.125 |
| Efm-HS0761 | Urine | 2007/3/9 | Neurosurgery | vanM | ST78 | C | >256 | 64 | 1.5 | 3 | 64 | 0.125 |
| Efm-HS07216 | Urine | 2007/7/9 | Neurosurgery | vanM | ST78 | D | >256 | 256 | 1.5 | >32 | 128 | 0.19 |
| Efm-HS0847 | Urine | 2008/2/5 | ICU | vanM | ST78 | E | >256 | >256 | 1.0 | >32 | 64 | 0.094 |
| Efm-HS08257 | Urine | 2008/8/5 | Neurosurgery | vanM | ST341 | F | >256 | 0.75 | 2 | 8 | 196 | 0.19 |
| Efm-HS08369 | Urine | 2008/10/22 | Neurology | vanM | ST18 | G | >256 | >256 | 2 | 16 | 32 | 32 |
| BM4105RF | NA | NA | NA | NA | NA | NA | 0.75 | 0.38 | 1.5 | >32 | 16 | 0.094 |
| BM-HS0661 | NA | NA | NA | vanM | NA | NA | >256 | 48 | 1.0 | >32 | 16 | 0.094 |
VAN, vancomycin; TEC, teicoplanin; LNZ, linezolid; RIF, rifampin; CIP, ciprofloxacin; NIT, nitrofurantoin; DOX, doxycycline; NA, not applicable; ICU, intensive care unit.
Sequencing and characterization of vanM.
Efm-HS0661, a GRE clinical isolate, was negative for vanA and vanB by PCR amplification. The strain amplified with primers NvanF and NvanR yielded a 1,006-bp product, which was sequenced on both strands. Using outward-facing primers that matched both ends of this sequence, primer walk sequencing was carried out on the original unmodified plasmid DNA extracted from a transconjugant, BM-HS0661. The analysis of the sequence and its putative protein indicated the presence of a novel van gene, designated vanM. vanM was 1,032 bp in length and encoded a 343-amino-acid protein. vanM had 81.8, 72.5, 67.7, and 78.2% nucleotide identity with vanA, vanB, vanD, and vanF, respectively. The VanM protein had the highest identity (79.9%) to VanA and the lowest similarity (66.3%) to VanD (Fig. 1). The PEKG motif specifically found in the ω loop of d-Ala:d-Lac ligases in VanA, VanB, and VanD also was present in VanM between positions 249 and 252 (7, 13).
FIG. 1.
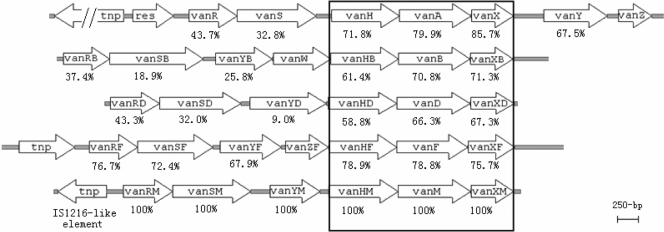
Alignment of vancomycin resistance gene clusters of vanA (M97297), vanB (EFU35369), vanD (AF130997), vanF (AF155139), and vanM (FJ349556). The percent amino acid identities to vanRM, vanSM, vanYM, vanHM, vanM, and vanXM products are shown below the respective genes.
Adjacent sequence analysis.
By sequencing with a series of outward-facing primers from both sides of the vanM gene, a 6,592-bp DNA fragment was obtained. Cleavage site analysis showed that there were two unique restriction sites, HindIII and PshAI, at the ends of the fragment. Plasmid DNA extracted from clinical isolate Efm-HS0661 was digested with HindIII and PshAI, and the 6,280-bp fragment was ligated into pRB473 digested with HindIII and SmaI. The vanM-positive recombinant plasmid was sequenced. The sequences from Efm-HS0661 and BM-HS0661 were identical, confirming that the vanM-bearing plasmid had been transferred from donor to transconjugant.
The analysis of the 6,592-bp fragment revealed seven open reading frames (ORFs). Based on the identity of the deduced amino acid sequences to those of the proteins encoded by the vanA, vanB, and vanD gene clusters, six ORFs were assigned to vanRM, vanSM, vanYM, vanHM, vanM, and vanXM, respectively. VanRM and VanSM were similar to VanR and VanS (Fig. 1), which are part of a two-component regulatory system in the vanA gene cluster. VanYM was similar to VanY, displaying dd-carboxypeptidase activity (26). The identities among the VanHM, VanH, VanHB, and VanHD dehydrogenases; the VanM, VanA, VanB, and VanD ligases; and the VanXM, VanXA, VanXB, and VanXD dd-dipeptidases were from 58.8 to 85.7% (Fig. 1). The three conserved residues, Arg, Glu, and His, which were predicted to participate in substrate binding and catalysis of d-Lac dehydrogenases (7), were present in VanHM at positions 235, 264, and 296, respectively.
The organization of the vanM gene cluster was most similar to that of the vanD gene cluster, although proteins encoded by genes in the vanM gene cluster shared higher deduced amino acid identity with those encoded by the vanA, vanB, and vanF gene clusters than with those of the vanD gene cluster (Fig. 1). No genes similar to vanZ or vanW present in the vanA and vanB gene clusters were found. Therefore, the vanM gene cluster may have a common ancestral origin with the vanA, vanB, and vanD gene clusters, but each has evolved independently. Meanwhile, VanRM and VanSM have more identity with VanRF and VanSF, which indicate that the genes in Paenibacillus popilliae are a precursor to the vancomycin resistance genes in VanM-type enterococci. The use of P. popilliae biopesticidal preparations in agricultural practice may have an impact on bacterial resistance in human pathogens (20).
Upstream from the vanM gene cluster was an insertion sequence that was identical to an IS1216-like element (Fig. 1). A similar IS element, IS1216V, is widespread among VanA-type GRE and may play an important role in the dissemination of resistance determinants by the transposon-mediated fusion of vanA plasmids with other plasmids (9, 15, 19). In this study, we observed that the plasmids bearing vanM in donor and transconjugant were of different sizes (data not shown), which may have resulted from an IS1216-mediated transposition event (17). Another interesting phenomenon is that a vanM-type strain, Efm-HS08257, had a VanB phenotype (vancomycin MIC, >256 μg/ml; teicoplanin MIC, 0.75 μg/ml). Sequence analysis showed that there was a 173-base insertion between the IS1216-like element and vanRM of the vanM gene cluster in this strain (data not shown). This insertion might induce the change of phenotype, and we are carrying out a further study to confirm the relationship.
Distribution of vanM.
By PCR with primers NvanF and NvanR and sequencing, vanM was found in six glycopeptide-resistant E. faecium strains, and vanA was found in the other four strains (Table 1). No van gene (vanA, vanB, vanD, vanF, or vanM) was found in the 100 glycopeptide-sensitive Enterococcus spp. clinical isolates.
Of the 10 vancomycin-resistant GRE strains, the PFGE profiles of SmaI-digested chromosomal DNA divided them into seven types (A to G), while MLST revealed four different sequence types. One ST78 isolate and three ST17 isolates harbored vanA genes. Four ST78 isolates, one ST18 isolate, and one ST341 isolate harbored vanM genes (Table 1).
Analysis of peptidoglycan precursors.
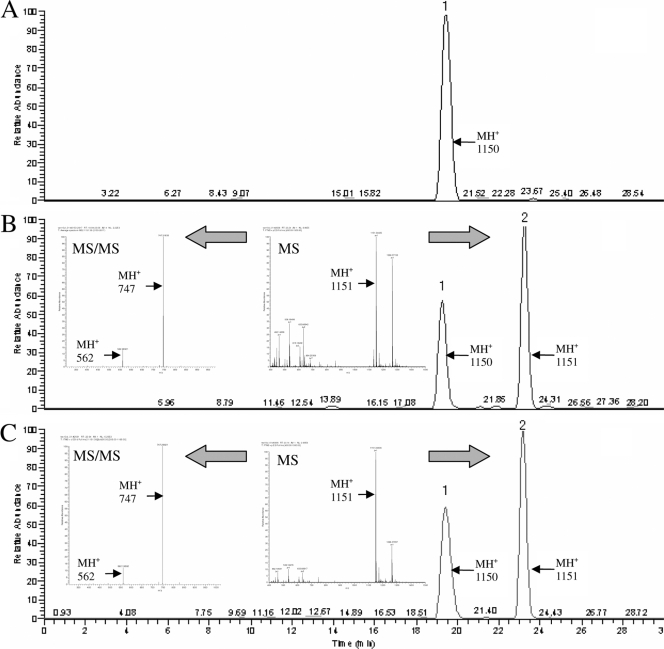
The peptidoglycan precursors in E. faecium Efm-HS0661 and vancomycin- or teicoplanin-treated Efm-HS0661 were analyzed by LC-MS. The results are presented in Fig. 2. The full-scan mass spectrum of the peptidoglycan precursors extracted from vancomycin or teicoplanin treated Efm-HS0661 showed a protonated molecule at m/z 1,151 (MH+), which indicates a molecular weight of 1,150, which is consistent with UDP-MurNAc-tetrapeptide-d-lactate (1,150 Da). This protonated molecule was not detected in the peptidoglycan precursors extracted from Efm-HS0661 not treated with glycopeptide. MS-MS analysis showed that the protonated molecule at m/z 1,151 (MH+) could produce two protonated molecules at m/z 562 and 747, which also were consistent with the UDP-MurNAc-tetrapeptide-d-lactate fragments tetrapeptide-d-lactate and MurNAc-tetrapeptide-d-lactate, respectively (14). These data indicate that the vanM gene cluster confers glycopeptide resistance by the production of peptidoglycan precursors ending in d-Ala-d-Lac, which could be induced by either vancomycin or teicoplanin.
FIG. 2.
Analysis of peptidoglycan precursors by liquid chromatography-mass spectrometry. Shown is the XIC of protonated molecules at m/z 1,150 (MH+) and 1,151 (MH+), which correspond to UDP-MurNAc-tetrapeptide-d-alanine (peak 1) and UDP-MurNAc-tetrapeptide-d-lactate (peak 2), in the peptidoglycan precursors (A to C). The m/z value of the selected precursor ions for the MS/MS analysis is 1,151 (MH+). The protonated molecule could produce two protonated molecules at m/z 562 and 747, which is consistent with the UDP-MurNAc-tetrapeptide-d-lactate fragments tetrapeptide-d-lactate and MurNAc-tetrapeptide-d-lactate, respectively. The precursors were extracted from untreated E. faecium Efm-HS0661 (A), Efm-HS0661 treated with vancomycin (B), and Efm-HS0661 treated with teicoplanin (C).
In conclusion, a new glycopeptide resistance determinant, the vanM gene cluster, was characterized from a glycopeptide-resistant clinical isolate of E. faecium. The vanM gene encoded a d-Ala:d-Lac ligase related to VanA, VanB, and VanD and could be transferred by conjugation.
Acknowledgments
This work was supported by grant 2005CB0523101 (to M.W.) from the National Basic Research Program of China from the Ministry of Science and Technology, China, grant LJ06052 (to M.W.) from the Shanghai Municipal Health Bureau, and grant 09411967900 (to X.X.) from the Shanghai Municipal Science and Technology Commission.
Footnotes
Published ahead of print on 23 August 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Arthur, M., F. Depardieu, P. Reynolds, and P. Courvalin. 1996. Quantitative analysis of the metabolism of soluble cytoplasmic peptidoglycan precursors of glycopeptide-resistant enterococci. Mol. Microbiol. 21:33-44. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, M., P. Reynolds, and P. Courvalin. 1996. Glycopeptide resistance in enterococci. Trends Microbiol. 4:401-407. [DOI] [PubMed] [Google Scholar]
- 3.Billot-Klein, D., L. Gutmann, E. Collatz, and J. Van Hejenoort. 1992. Analysis of peptidoglycan precursors in vancomycin resistant enterococci. Antimicrob. Agents Chemother. 36:1487-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd, D. A., B. M. Willey, D. Fawcett, N. Gillani, and M. R. Mulvey. 2008. Molecular characterization of Enterococcus faecalis N06-0364 with low-level vancomycin resistance harboring a novel D-Ala-D-Ser gene cluster, vanL. Antimicrob. Agents Chemother. 52:2667-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brückner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. [DOI] [PubMed] [Google Scholar]
- 6.Cao, B., Y. Liu, S. Song, R. Li, H. Wang, and C. Wang. 2008. First report of clinical and epidemiological characterisation of vancomycin-resistant enterococci from mainland China. Int. J. Antimicrob. Agents. 32:279-281. [DOI] [PubMed] [Google Scholar]
- 7.Casadewall, B., and P. Courvalin. 1999. Characterization of the vanD glycopeptide resistance gene cluster from Enterococcus faecium BM4339. J. Bacteriol. 181:3644-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courvalin, P. 2006. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 42(Suppl. 1):S25-S34. [DOI] [PubMed] [Google Scholar]
- 9.Darini, A. L., M. F. Palepou, and N. Woodford. 2000. Effects of the movement of insertion sequences on the structure of VanA glycopeptide resistance elements in Enterococcus faecium. Antimicrob. Agents Chemother. 44:1362-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Depardieu, F., M. Kolbert, H. Pruul, J. Bell, and P. Courvalin. 2004. VanD-type vancomycin-resistant Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 48:3892-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depardieu, F., I. Podglajen, R. Leclercq, E. Collatz, and P. Courvalin. 2007. Modes and modulations of antibiotic resistance gene expression. Clin. Microbiol. Rev. 20:79-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Depardieu, F., P. E. Reynolds, and P. Courvalin. 2003. VanD-type vancomycin-resistant Enterococcus faecium 10/96A. Antimicrob. Agents Chemother. 47:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evers, S., B. Casadewall, M. Charles, S. Dutka-Malen, M. Galimand, and P. Courvalin. 1996. Evolution of structure and substrate specificity in D-alanine:D-alanine ligases and related enzymes. J. Mol. Evol. 42:706-712. [DOI] [PubMed] [Google Scholar]
- 14.Handwerger, S., M. J. Pucci, K. J. Volk, J. Liu, and M. S. Lee. 1992. The cytoplasmic peptidoglycan precursor of vancomycin-resistant Enterococcus faecalis terminates in lactate. J. Bacteriol. 174:5982-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heaton, M. P., L. F. Discotto, M. J. Pucci, and S. Handwerger. 1996. Mobilization of vancomycin resistance by transposon-mediated fusion of a VanA plasmid with an Enterococcus faecium sex pheromone-response plasmid. Gene 171:9-17. [DOI] [PubMed] [Google Scholar]
- 16.Homan, W. L., D. Tribe, S. Poznanski, M. Li, G. Hogg, E. Spalburg, J. D. Van Embden, and R. J. Willems. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen, L. B. 1998. Internal size variations in Tn1546-like elements due to the presence of IS1216V. FEMS Microbiol. Lett. 169:349-354. [DOI] [PubMed] [Google Scholar]
- 18.Leclercq, R., E. Derlot, J. Duval, and P. Courvalin. 1988. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 319:157-161. [DOI] [PubMed] [Google Scholar]
- 19.Palepou, M. F., A. M. Adebiyi, C. H. Tremlett, L. B. Jensen, and N. Woodford. 1998. Molecular analysis of diverse elements mediating VanA glycopeptide resistance in enterococci. J. Antimicrob. Chemother. 42:605-612. [DOI] [PubMed] [Google Scholar]
- 20.Patel, R., K. Piper, F. R. Cockerill III, J. M. Steckelberg, and A. A. Yousten. 2000. The biopesticide Paenibacillus popilliae has a vancomycin resistance gene cluster homologous to the enterococcal VanA vancomycin resistance gene cluster. Antimicrob. Agents Chemother. 44:705-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perichon, B., P. Reynolds, and P. Courvalin. 1997. VanD-type glycopeptide-resistant Enterococcus faecium BM4339. Antimicrob. Agents Chemother. 41:2016-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu, T. T., Y. G. Chen, Y. S. Yu, H. X. Lv, X. Q. Dong, Z. Xiao, H. Q. Gu, and L. J. Li. 2007. Molecular characterization of vancomycin-resistant enterococci in Hangzhou, China. J. Antimicrob. Chemother. 60:1403-1405. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro, T., M. Abrantes, M. F. Lopes, and M. T. Crespo. 2007. Vancomycin-susceptible dairy and clinical enterococcal isolates carry vanA and vanB genes. Int. J. Food Microbiol. 113:289-295. [DOI] [PubMed] [Google Scholar]
- 24.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uttley, A. H., C. H. Collins, J. Naidoo, and R. C. George. 1988. Vancomycin-resistant enterococci. Lancet i:57-58. [DOI] [PubMed] [Google Scholar]
- 26.Wright, G. D., C. Molinas, M. Arthur, P. Courvalln, and C. T. WaLsh. 1992. Characterization of VanY: a DD-carboxypeptidase from vancomycin resistant Enterococcus faecium BM4147. Antimicrob. Agents Chemother. 36:1514-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng, B., H. Tomita, Y. H. Xiao, S. Wang, Y. Li, and Y. Ike. 2007. Molecular characterization of vancomycin-resistant Enterococcus faecium isolates from mainland China. J. Clin. Microbiol. 45:2813-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]