Abstract
The objective of the present retrospective observational study carried out in patients receiving a standard dosage of linezolid and undergoing routine therapeutic drug monitoring (TDM) was to assess the interindividual variability in plasma exposure, to identify the prevalence of attainment of optimal pharmacodynamics, and to define if an intensive program of TDM may be warranted in some categories of patients. Linezolid plasma concentrations (trough [Cmin] and peak [Cmax] levels) were analyzed by means of a high-performance liquid chromatography (HPLC) method, and daily drug exposure was estimated (daily area under the plasma concentration-time curve [AUC24]). The final database included 280 Cmin and 223 Cmax measurements performed in 92 patients who were treated with the fixed 600-mg dose every 12 h (q12h) intravenously (n = 58) or orally (n = 34). A wide variability was observed (median values [interquartile range]: 3.80 mg/liter [1.75 to 7.53 mg/liter] for Cmin, 14.70 mg/liter [10.57 to 19.64] for Cmax, and 196.08 mg·h/liter [144.02 to 312.10 mg·h/liter] for estimated AUC24). Linezolid Cmin was linearly correlated with estimated AUC24 (r2 = 0.85). Optimal pharmacodynamic target attainment (defined as Cmin of ≥2 mg/liter and/or AUC24/MIC90 ratio of >80) was obtained in about 60 to 70% of cases, but potential overexposure (defined as Cmin of ≥10 mg/liter and/or AUC24 of ≥400 mg·h/liter) was documented in about 12% of cases. A significantly higher proportion of cases with potential overexposure received cotreatment with omeprazole, amiodarone, or amlodipine. Our study suggests that the application of TDM might be especially worthwhile in about 30% of cases with the intent of avoiding either the risk of dose-dependent toxicity or that of treatment failure.
Linezolid is the first commercially available oxazolidinone, whose use in the treatment of infection caused by multidrug-resistant (MDR) Gram-positive bacteria is continuously increasing (37).
Among the several reasons for this, two appear to be the most clinically relevant. First, linezolid retains its efficacy even against bacterial strains which become tolerant of glycopeptides. This fact may justify even its empirical use in settings with high incidences of bacterial strains with borderline susceptibility to vancomycin (23) or its use for salvage therapy (13). Second, it presents very favorable rates of penetration into tissues (33), even in the presence of intact anatomical barriers (18). This suggests a potential pharmacokinetic advantage over glycopeptides in the treatment of deep-seated infections, such as pneumonia (1, 33) and/or central nervous system infections (27).
Based on the drug's intrinsic chemicophysical and pharmacokinetic characteristics, it is expected that dosing adjustment and intensive therapeutic drug monitoring (TDM) may be unnecessary in most cases. Linezolid is a moderately lipophilic drug whose pharmacokinetic behavior is not expected to vary significantly according to renal and/or hepatic failure and/or to polytherapy (32). The oxidative metabolism of linezolid is nonenzymatic and does not involve the hepatic microsomal oxidative system CYP450. Nonrenal clearance accounts for 65% of an administered linezolid dose, with roughly 30% of the dose appearing unchanged in the urine (31). Additionally, in contrast to those of more hydrophilic antimicrobial agents such as the beta-lactams, linezolid pharmacokinetics did not appear to be substantially affected by the pathophysiological changes occurring during sepsis and/or septic shock (36).
However, some recent studies suggest that TDM of linezolid could be especially helpful for dosage adjustment in some settings. Significant underexposure with increased risk of therapeutic failure was documented in patients with major thermal injuries (10, 17) and with cystic fibrosis (4), whereas, conversely, significant overexposure with increased toxicity risk was observed in some critically ill patients (21, 25).
To the best of our knowledge, no observational data in daily clinical practice on linezolid plasma exposure during routine use at standard fixed doses are available to date.
On the basis of an institutional program devoted to improving knowledge of the pharmacokinetic behavior of newly commercially available antimicrobial agents in the clinical setting, in 2003 we started to measure plasma levels of linezolid in patients treated because of documented and/or suspected MDR Gram-positive bacterium-related infections.
The present retrospective observational study aimed to assess the interindividual pharmacokinetic variability in plasma exposure to linezolid, to identify the prevalence of optimal pharmacodynamic exposure enabled by the standard dosing regimen according to the pharmacokinetic/pharmacodynamic principles and to the pattern of susceptibility to this antibiotic, and to define if an intensive program of TDM may be warranted in some categories of patients.
MATERIALS AND METHODS
Study design.
Plasma TDM of linezolid carried out in the period between December 2003 and May 2009 at the Institute of Clinical Pharmacology and Toxicology, Azienda Ospedaliero-Universitaria, University of Udine, represented the starting database. Patients included in this retrospective observational study were those admitted in intensive care units (ICUs) or medical or surgical wards who were treated intravenously or orally with linezolid at the standard daily dosage of 600 mg every 12 h (q12h) because of documented or suspected MDR Gram-positive bacterial infections and who underwent TDM. At our institution linezolid TDM is performed three times a week (on Monday, Wednesday, and Friday) and the feedback to the clinician is given in real time (within the same day). However, since the aim of this study was to assess the degree of interindividual variability in plasma exposure to linezolid observed during the standard fixed-dosing regimen, only the TDM carried out before the eventual application of TDM-guided dosage adjustments was considered in this analysis.
The study was approved by the Ethical Committee.
Measurement of linezolid plasma concentrations.
Venous blood samples were drawn just before the next administration to assess trough plasma concentration (Cmin) and 30 min after a 1-h intravenous infusion or 2 h after oral administration to assess peak plasma concentration (Cmax). Times for blood collections were carefully checked and recorded in each single case, and whenever doubts on appropriate sampling arose, samples were excluded from this analysis. Linezolid concentrations in plasma were analyzed by means of a validated high-performance liquid chromatography (HPLC) analysis method, as previously described (25, 26). Precision and accuracy were assessed by performing replicate analyses of quality control samples against calibration standards, intra- and interassay coefficients of variation always being less than 10%. The low limit of detection was 0.2 mg/liter.
Estimation of CLCR.
Creatinine clearance (CLCR) was estimated by means of the Cockcroft and Gault formula (5).
Pharmacokinetic analysis.
Pharmacokinetic analysis of linezolid was performed using the Abbottbase Pharmacokinetic System (PKS) program using Bayesian forecasting (16). Intravenous administration data were fitted to a standard one-compartment model with zero-order input (1-h drug infusion) and first-order elimination. For oral administration, the concentration data were fitted to a standard one-compartment model with first-order absorption and first-order elimination. The calculated pharmacokinetic parameters were clearance and volume of distribution. Daily area under the plasma concentration-time curve (AUC24) was estimated using the pharmacokinetic parameters. Only patients having at least one complete set of Cmin and Cmax measurement samples drawn at steady state were included for AUC24 estimation.
Assessment of pharmacodynamics. (i) Efficacy thresholds.
Optimal theoretical pharmacodynamic determinants of efficacy for the time-dependent antibacterial activity of linezolid against methicillin-resistant (MR) staphylococci and against vancomycin-resistant (VR) enterococci were defined as plasma Cmins of ≥2 mg/liter and/or AUC/MIC ratios of >80. The rationale behind these choices derives from the notion that the MIC90 for linezolid against both MR staphylococci and VR enterococci is 2 mg/liter (14) and from the finding that, in one study conducted in seriously ill adult patients, higher success rates were achieved when the cumulative percentage of a 24-h period that the drug concentration exceeded the MIC under steady-state pharmacokinetic conditions (%TMIC) exceeded 85% of the dosing interval and AUC/MIC ratios were between 80 and 120 (28). Indeed, the achievement of these targets may become especially relevant for critically ill septic patients, who may often be immunocompromised (24, 28).
(ii) Toxicity thresholds.
Conversely, the thresholds of potential overexposure to linezolid were arbitrarily defined as Cmins of ≥10 mg/liter and/or AUC24 of ≥400 mg·h/liter. The reason for this choice is that exposures higher than these, being more than 2-fold higher than those normally observed with similar dosages in healthy volunteers (12, 32), might significantly increase the risk of dose-dependent toxicity with linezolid (37). Concomitant drug treatments were reviewed in order to identify the frequency of recurrence.
Statistical analysis.
The Kolmogorov-Smirnov test was performed to assess whether data were normally or nonnormally distributed. Accordingly, descriptive data were expressed as means ± standard deviations (SD) or as medians and interquartile (IQ) ranges. Categorical variables were compared by the χ2 test with Yates' correction or Fisher's exact test when necessary, and continuous variables were compared using Student's t test. A P value of <0.05 was required to achieve statistical significance. The statistical analysis was carried out with SigmaStat version 3.1.
RESULTS
Patient characteristics are shown in Table 1. The most frequent hospital admission was surgical (43.5%), and the main reasons for linezolid therapy were bloodstream infections (19.6%) and central nervous system infections (19.6%).
TABLE 1.
Patient characteristicsa
| Characteristic | Value |
|---|---|
| No. of patients | 92 |
| Age (yr) | 57.2 ± 14.3 |
| Gender (no. M/no. F) | 67/25 |
| Body wt (kg) | 75.2 ± 18.2 |
| CLCR (ml/min)b | 84.8 (6.1-349.5) |
| Normalized dose of linezolid (mg/kg/12 h) | 8.37 ± 1.74 |
| Hospital admission (no. [%] of patients) | |
| Surgical ward | 40 (43.5) |
| ICU | 28 (30.4) |
| Medical ward | 24 (26.1) |
| Main reason for linezolid treatment (no. [%] of patients) | |
| Bloodstream infections | 18 (19.6) |
| CNS infections | 18 (19.6) |
| Empirical use for severe sepsis | 16 (17.4) |
| Intra-abdominal infections | 11 (12.0) |
| Hospital-acquired pneumonia | 10 (10.9) |
| Bone/joint infections | 10 (10.9) |
| Cardiosurgical infections/endocarditis | 8 (8.7) |
| SST infections | 1 (0.9) |
Values are expressed as means ± standard deviations, median (range), or numbers (percentages). Abbreviations: CLCR, estimated creatinine clearance by means of the Cockcroft and Gault formula; CNS, central nervous system; ICU, intensive care unit; SST, skin and soft tissue; TDM, therapeutic drug monitoring; M, male; F, female.
At time of first TDM.
The database included 280 Cmin (median per patient = 3; IQ range, 1 to 4) and 223 Cmax measurements performed in 92 patients, who were treated with the fixed 600-mg q12h dosing regimen of linezolid intravenously in 58 cases and orally in the other 34.
Median pharmacokinetic values (IQ ranges) were 3.80 mg/liter (1.75 to 7.53 mg/liter) for Cmin, 14.70 mg/liter (10.57 to 19.64 mg/liter) for Cmax, and 196.08 mg/liter·h (144.02 to 312.10 mg·h/liter) for estimated AUC24.
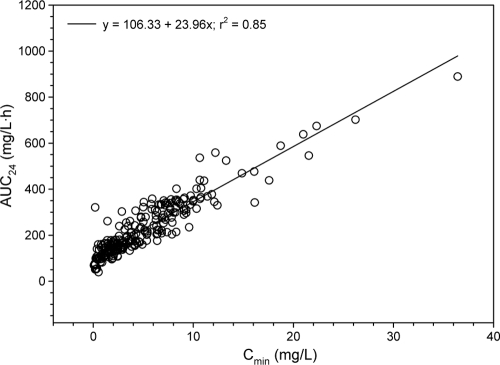
No significant linear relationships between linezolid Cmin and either estimated CLCR (r2 = 0.01) (Fig. 1) or body weight (r2 = 0.06) (Fig. 2) were found. Conversely, linezolid Cmin was linearly correlated with estimated AUC24 (r2 = 0.85) (Fig. 3).
FIG. 1.
Relationship between linezolid trough levels (Cmin) and patients' estimated creatinine clearance (CLCR).
FIG. 2.
Relationship between linezolid trough levels (Cmin) and patients' weight.
FIG. 3.
Relationship between linezolid trough levels (Cmin) and estimated daily area under the plasma concentration-versus-time curve (AUC24) (n = 223).
For linezolid trough levels sorted according to patients' hospital admissions (Fig. 4), medians (IQ ranges) of Cmin were 4.78 mg/liter (2.12 to 7.68 mg/liter) in surgical patients, 2.94 mg/liter (1.10 to 6.93 mg/liter) in intensive care unit (ICU) patients, and 2.94 mg/liter (1.66 to 7.25 mg/liter) in medical patients. Although a trend toward higher exposure was observed among surgical patients, these differences were not statistically significant.
FIG. 4.
Box (median and 25th to 75th percentile) and whisker (5th and 95th percentile) plots of trough plasma concentrations (Cmin) of linezolid observed according to the type of ward of admission (surgical wards [Surg], intensive care units [ICU], and medical wards [Med]). Filled circles are outliers.
As far as pharmacokinetic/pharmacodynamic relationships are concerned, optimal pharmacodynamic exposure in terms of Cmin being higher than MIC90 was documented in 71.4% of cases and AUC/MIC90 ratios were estimated to be >80 in as many as 61.9% of the 223 evaluable cases.
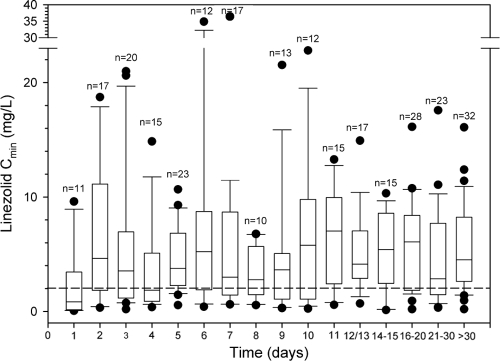
The trend over time of linezolid Cmin showed that median values persisted above the pharmacodynamic threshold of efficacy from day 2 onward (Fig. 5). Potential overexposure in terms of Cmins of ≥10 mg/liter was found in 11.8% of instances (33 instances of TDM in 23 patients; median, 12.40 mg/liter; range, 11.0 to 19.2 mg/liter), and AUC24 was estimated to be >400 mg·h/liter in 8.1% of cases.
FIG. 5.
Box (median and 25th to 75th percentile) and whisker (5th and 95th percentile) plots of trough plasma concentrations (Cmin) of linezolid observed over time. Filled circles are outliers; n is the number of observations on each day of treatment. The dashed line refers to 2 mg/liter, namely, the MIC90 of linezolid for staphylococci and enterococci. Median Cmin was almost always above the MIC90 of linezolid for staphylococci and enterococci, regardless of the day of treatment.
When patients with potential overexposure were compared with those having trough levels within the lower range (<10 mg/liter), normalized daily doses per kg of body weight and route of administration of linezolid were similar (Table 2). However, a significantly higher proportion of the patients with linezolid Cmins of ≥10 mg/liter received cotreatment with omeprazole (78.8 versus 27.5%, P < 0.001), amiodarone (21.2 versus 2.4%, P < 0.001), or amlodipine (21.2 versus 5.2, P < 0.003).
TABLE 2.
Comparison of linezolid dosages, administration routes, and most frequent drug cotreatments in cases with trough levels (Cmin) of ≥10 mg/liter versus those with trough levels of <10 mg/liter
| Parameter | No. (%) of cases by linezolid Cmin: |
P value | |
|---|---|---|---|
| ≥10 mg/liter (n = 33) | <10 mg/liter (n = 247) | ||
| Linezolid administration route | |||
| Intravenous | 21 (63.6) | 157 (63.6) | 0.847 |
| Oral | 12 (36.4) | 90 (36.4) | 0.845 |
| Linezolid dosage, median (IQ range) (mg/kg/q12h) | |||
| Overall | 9.3 (7.5-10.2) | 8.0 (7.1-10.0) | 0.067 |
| Intravenous | 10.0 (7.9-10.0) | 8.0 (7.1-10.0) | 0.071 |
| Oral | 7.9 (7.9-10.3) | 8.5 (7.1-10.0) | 0.876 |
| Cotreatments | |||
| Omeprazole | 26 (78.8) | 68 (27.5) | <0.001 |
| Amiodarone | 7 (21.2) | 6 (2.4) | <0.001 |
| Amlodipine | 7 (21.1) | 13 (5.2) | 0.003 |
DISCUSSION
Our findings suggest that during routine clinical use linezolid exposure after standard fixed daily dosages may vary among patients, irrespective of the type of ward of admission.
Indeed, although median values for Cmin, Cmax, and AUC24 were similar to those observed in healthy volunteers (17, 32), their ranges were significantly wider.
From this perspective, it would be of interest to clarify if some patient characteristics, pathophysiological conditions, and/or drug cotreatments might account for this variability.
Indeed, in our study neither estimated CLCR nor body weight was found to be a useful predictor of linezolid exposure. The absence of a significant relationship between linezolid Cmin and estimated CLCR is attributable to the mainly nonrenal clearance of linezolid (32). This finding allows us to confirm that no major dosage adjustments need to be recommended in patients with impaired renal function. Likewise, linezolid Cmin did not show a clear relationship with patients' body weight. In our study very low drug exposures in terms of Cmin, Cmax, and AUC24 among obese patients were observed only in some cases, even if the limited number of instances of TDM carried out in this subpopulation did not enable any definite conclusion. Indeed, we recognize that linezolid pharmacokinetics may be influenced by the degree of obesity, but considering that existing data for this patient demographic are quite sparse and variable (11, 19), no empirical alterations of linezolid dosage in obese patients can be suggested to date (19).
Overall, these observations and the wide interpatient variability seen in this study suggest that it would be difficult to accurately predict the pharmacokinetic disposition of linezolid in all patients, so that the application of TDM, at least in some cases, seems logical.
The good linear relationship between Cmin and estimated AUC24 (r2 = 0.85) suggests that Cmin may represent a useful predictor of linezolid total body exposure in daily clinical practice. Interestingly, our hypothesis is in agreement with a recent population pharmacokinetic study carried out in patients with MDR tuberculosis treated with linezolid which showed that the estimation of AUC based on a trough concentration did not differ significantly from that based on multiple samples (3). Accordingly, it may be speculated that TDM of Cmin could be used to guide dosage adjustment with linezolid in individual patients with the intent of avoiding either the risk of toxicity or that of therapeutic failure.
In a pharmacodynamic study with linezolid, it was shown that the two parameters most strongly associated with linezolid efficacy were AUC/MIC ratios of >80 to 100 and %TMIC of 85 (28). Additionally, it has been postulated that a Cmin higher than the MIC may be especially useful for linezolid efficacy in immunocompromised patients (24, 28). Considering that 2 mg/liter is the MIC90 of linezolid against staphylococci and enterococci (14) and that from our estimates the achievable AUC24 in the presence of Cmin of >2 mg/liter is >160 mg·h/liter, this means that for a Cmin of linezolid greater than 2 mg/liter both the pharmacodynamic targets of efficacy (Cmin higher than MIC90 and AUC/MIC90 ratio of >80) may be attained. Accordingly, this plasma trough value could be proposed as the lower threshold for efficacy with the standard dosage of linezolid.
In our study standard dosages of linezolid ensured the attainment of these pharmacodynamic targets in the majority of cases (almost 70 to 75%). Interestingly, median Cmin was >2 mg/liter just on day 2 and then it was maintained over this threshold throughout the entire treatment period, even if the range of variability was consistent. This fact seems to suggest that perhaps the role of TDM in order to avoid potential underexposure with linezolid could be limited if the susceptibility breakpoint for staphylococci was lower than that currently recommended (i.e., 2 mg/liter rather than 4 mg/liter).
The role of TDM might be especially valuable in lowering the risk of exposure-dependent toxicity with linezolid, which in our study could have related to the 12% of cases with potential overexposure. It was shown that some adverse events with linezolid, namely, hematological alterations and hyperlactacidemia, are dose dependent (37) and may be related to a reversible inhibition of the mitochondrial protein synthesis (8). In our study potential overexposure was not correlated with the use of higher dosage per kg of body weight or with different administration routes and, of note, its onset seemed to be unrelated to the length of treatment. Some years ago we reported the case of a liver transplant patient who experienced an unexpected early onset of hyperlactacidemia due to a consistent linezolid overexposure (Cmin, 26.99 mg/liter; AUC12, 412.55 mg·h/liter) (21). Among the various hypotheses, we postulated that this overexposure could have been due to drug-drug interaction.
Of note, in the present study patients experiencing linezolid overexposure were found to be more frequently cotreated with omeprazole, amiodarone, and/or amlodipine than were those with Cmins of <10 mg/liter, and all of these drugs are potent inhibitors of P-glycoprotein (P-gp) (7, 15, 20). These findings seem to suggest that linezolid overexposure might occur especially in patients cotreated with some drugs which may act as P-gp inhibitors, who might especially benefit from TDM in the prevention of linezolid-related toxicity. Unfortunately, this study was not set up to verify this hypothesis and its retrospective nature did not allow us to assess the incidence of toxicity in these cases.
Likewise, TDM could be relevant for avoiding potential underexposure, which may have occurred in 28% of cases. Conflicting opinions exist about the role that severe sepsis and septic shock may have in affecting linezolid pharmacokinetics. Some authors found no major influence (36), whereas others found a very wide interindividual variability (2, 35) and recommended the use of TDM (35). In our study, the lack of relevant differences in linezolid exposure among patients according to the type of ward of admission suggests that the pharmacokinetic variability in ICU patients does not seem to be significantly higher than that in surgical and/or medical patients. Additionally, considering the intrinsic characteristics of linezolid (a moderately lipophilic drug with mainly nonrenal, nonenzymatic clearance), it is expected that the pathophysiological changes occurring during sepsis should be less relevant than they would be for hydrophilic compounds, like the beta-lactams (29). Indeed, we recognize that measurement of the free moiety rather than of total concentration would have enabled more exhaustive consideration. However, the relatively small extent of protein binding of linezolid (around 30%), which could be even lower in hypoalbuminemic patients, is expected to affect to a limited extent its pharmacokinetics. Besides, in this population of patients, according to pharmacokinetic/pharmacodynamic principles, continuous infusion may be considered a useful tool to improve linezolid activity since it has theoretical advantages over intermittent infusion (2).
In any case, the wide variability observed and the complex management of critically ill patients should induce clinicians to consider TDM an invaluable tool for the management of antibiotic therapy in this setting (22). This could be especially true for patients with severe burn injuries and/or with cystic fibrosis, in whom linezolid clearance was recently shown to be significantly increased (4, 10, 17, 30). Interestingly, in patients with cystic fibrosis, among the various mechanisms that could be responsible for the well-known enhanced clearance of antimicrobial drugs, it has been suggested that the overexpression of P-gp may play a major role (30, 34). Additionally, the overexpression of P-gp was recently cited to explain the significant drop of linezolid concentration during coadministration of rifampin, a potent inducer of P-gp (6, 9). Indeed, although in our database only four patients with rifampin cotreatment underwent TDM, it is noteworthy that very low Cmins of linezolid (median, 1.21 mg/liter; IQ range, 0.68 to 1.73 mg/liter) were observed in these cases.
Obviously, further prospective studies are warranted before any definitive conclusion about the major factors which may affect linezolid pharmacokinetics can be drawn.
We are well aware of the methodological limitations of our study, particularly its retrospective nature; the limited sample size, which is too small to allow us to draw definite conclusions; and the fact that the observational nature of the study obliged us to limit sampling to only two time points. However, the good correlation between Cmin and estimated AUC24 supports the idea that TDM of trough levels might represent a useful predictor of linezolid efficacy and/or toxicity in routine clinical practice. Indeed, the thresholds for Cmin here proposed have not yet been validated and might change with future data that may emerge on this topic.
In conclusion, our study suggests that the standard fixed 600-mg q12h dosage may ensure adequate pharmacodynamic exposure to linezolid in about 60 to 70% of cases but also that, conversely, in the remaining 30 to 40% of cases the application of TDM might be especially worthwhile with the intent of avoiding the risk of treatment failure or of dose-dependent toxicity.
A prospective study focused on assessing the clinical relevance of the correlation between linezolid TDM and efficacy or toxicity is ongoing.
Acknowledgments
No financial support was received for this study.
Federico Pea has been on the speakers' bureau of Pfizer. Mario Furlanut has received grant support from Pfizer. Pierluigi Viale has been a consultant to, has been on the speakers' bureau of, and has received grant support from Pfizer. None of the other authors has a potential conflict of interest to report.
Footnotes
Published ahead of print on 23 August 2010.
REFERENCES
- 1.Abunasser, J., and M. L. Metersky. 2009. A comparison of linezolid with glycopeptides in severe MRSA pneumonia. Expert Rev. Anti Infect. Ther. 7:951-955. [DOI] [PubMed] [Google Scholar]
- 2.Adembri, C., S. Fallani, M. I. Cassetta, S. Arrigucci, A. Ottaviano, P. Pecile, T. Mazzei, R. De Gaudio, and A. Novelli. 2008. Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: intermittent versus continuous infusion. Int. J. Antimicrob. Agents 31:122-129. [DOI] [PubMed] [Google Scholar]
- 3.Alffenaar, J. W., J. G. Kosterink, R. V. Altena, T. S. van der Werf, D. R. Uges, and J. H. Proost. 2010. Limited sampling strategies for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Ther. Drug Monit. 32:97-101. [DOI] [PubMed] [Google Scholar]
- 4.Bosso, J. A., P. A. Flume, and S. L. Gray. 2004. Linezolid pharmacokinetics in adult patients with cystic fibrosis. Antimicrob. Agents Chemother. 48:281-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 6.Egle, H., R. Trittler, K. Kummerer, and S. W. Lemmen. 2005. Linezolid and rifampin: drug interaction contrary to expectations? Clin. Pharmacol. Ther. 77:451-453. [DOI] [PubMed] [Google Scholar]
- 7.Fenner, K. S., M. D. Troutman, S. Kempshall, J. A. Cook, J. A. Ware, D. A. Smith, and C. A. Lee. 2009. Drug-drug interactions mediated through P-glycoprotein: clinical relevance and in vitro-in vivo correlation using digoxin as a probe drug. Clin. Pharmacol. Ther. 85:173-181. [DOI] [PubMed] [Google Scholar]
- 8.Garrabou, G., A. Soriano, S. Lopez, J. P. Guallar, M. Giralt, F. Villarroya, J. A. Martinez, J. Casademont, F. Cardellach, J. Mensa, and O. Miro. 2007. Reversible inhibition of mitochondrial protein synthesis during linezolid-related hyperlactatemia. Antimicrob. Agents Chemother. 51:962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebhart, B. C., B. C. Barker, and B. A. Markewitz. 2007. Decreased serum linezolid levels in a critically ill patient receiving concomitant linezolid and rifampin. Pharmacotherapy 27:476-479. [DOI] [PubMed] [Google Scholar]
- 10.Hallam, M. J., J. M. Allen, S. E. James, P. M. Donaldson, J. G. Davies, G. W. Hanlon, and B. S. Dheansa. 2010. Potential subtherapeutic linezolid and meropenem antibiotic concentrations in a patient with severe burns and sepsis. J. Burn Care Res. 31:207-209. [DOI] [PubMed] [Google Scholar]
- 11.Hanley, M. J., D. R. Abernethy, and D. J. Greenblatt. 2010. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 49:71-87. [DOI] [PubMed] [Google Scholar]
- 12.Islinger, F., P. Dehghanyar, R. Sauermann, C. Burger, C. Kloft, M. Muller, and C. Joukhadar. 2006. The effect of food on plasma and tissue concentrations of linezolid after multiple doses. Int. J. Antimicrob. Agents 27:108-112. [DOI] [PubMed] [Google Scholar]
- 13.Jang, H. C., S. H. Kim, K. H. Kim, C. J. Kim, S. Lee, K. H. Song, J. H. Jeon, W. B. Park, H. B. Kim, S. W. Park, N. J. Kim, E. C. Kim, M. D. Oh, and K. W. Choe. 2009. Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin. Infect. Dis. 49:395-401. [DOI] [PubMed] [Google Scholar]
- 14.Jones, R. N., T. R. Fritsche, H. S. Sader, and J. E. Ross. 2007. Zyvox annual appraisal of potency and spectrum program results for 2006: an activity and spectrum analysis of linezolid using clinical isolates from 16 countries. Diagn. Microbiol. Infect. Dis. 59:199-209. [DOI] [PubMed] [Google Scholar]
- 15.Katoh, M., M. Nakajima, H. Yamazaki, and T. Yokoi. 2001. Inhibitory effects of CYP3A4 substrates and their metabolites on P-glycoprotein-mediated transport. Eur. J. Pharm. Sci. 12:505-513. [DOI] [PubMed] [Google Scholar]
- 16.Lacarelle, B., P. Pisano, T. Gauthier, P. H. Villard, F. Guder, J. Catalin, and A. Durand. 1994. Abbott PKS system: a new version for applied pharmacokinetics including Bayesian estimation. Int. J. Biomed. Comput. 36:127-130. [DOI] [PubMed] [Google Scholar]
- 17.Lovering, A. M., R. Le Floch, L. Hovsepian, J. Stephanazzi, P. Bret, G. Birraux, and C. Vinsonneau. 2009. Pharmacokinetic evaluation of linezolid in patients with major thermal injuries. J. Antimicrob. Chemother. 63:553-559. [DOI] [PubMed] [Google Scholar]
- 18.Myrianthefs, P., S. L. Markantonis, K. Vlachos, M. Anagnostaki, E. Boutzouka, D. Panidis, and G. Baltopoulos. 2006. Serum and cerebrospinal fluid concentrations of linezolid in neurosurgical patients. Antimicrob. Agents Chemother. 50:3971-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pai, M. P., and D. T. Bearden. 2007. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 27:1081-1091. [DOI] [PubMed] [Google Scholar]
- 20.Pauli-Magnus, C., S. Rekersbrink, U. Klotz, and M. F. Fromm. 2001. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedebergs Arch. Pharmacol. 364:551-557. [DOI] [PubMed] [Google Scholar]
- 21.Pea, F., L. Scudeller, M. Lugano, U. Baccarani, F. Pavan, M. Tavio, M. Furlanut, G. D. Rocca, F. Bresadola, and P. Viale. 2006. Hyperlactacidemia potentially due to linezolid overexposure in a liver transplant recipient. Clin. Infect. Dis. 42:434-435. [DOI] [PubMed] [Google Scholar]
- 22.Pea, F., and P. Viale. 2009. Bench-to-bedside review: appropriate antibiotic therapy in severe sepsis and septic shock—does the dose matter? Crit. Care 13:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pea, F., and P. Viale. 2009. Could de-escalation of antibiotic therapy be feasible even for documented methicillin-resistant Staphylococcus aureus ventilator-associated pneumonia? J. Trauma 67:893-894. [DOI] [PubMed] [Google Scholar]
- 24.Pea, F., and P. Viale. 2007. Pharmacodynamics of antibiotics to treat multidrug-resistant Gram-positive hospital infections. Expert Rev. Anti Infect. Ther. 5:255-270. [DOI] [PubMed] [Google Scholar]
- 25.Pea, F., P. Viale, M. Lugano, F. Pavan, L. Scudeller, G. Della Rocca, and M. Furlanut. 2004. Linezolid disposition after standard dosages in critically ill patients undergoing continuous venovenous hemofiltration: a report of 2 cases. Am. J. Kidney Dis. 44:1097-1102. [DOI] [PubMed] [Google Scholar]
- 26.Peng, G. W., R. P. Stryd, S. Murata, M. Igarashi, K. Chiba, H. Aoyama, M. Aoyama, T. Zenki, and N. Ozawa. 1999. Determination of linezolid in plasma by reversed-phase high-performance liquid chromatography. J. Pharm. Biomed. Anal. 20:65-73. [DOI] [PubMed] [Google Scholar]
- 27.Peppard, W. J., C. J. Johnston, and A. M. Urmanski. 2008. Pharmacologic options for CNS infections caused by resistant Gram-positive organisms. Expert Rev. Anti Infect. Ther. 6:83-99. [DOI] [PubMed] [Google Scholar]
- 28.Rayner, C. R., A. Forrest, A. K. Meagher, M. C. Birmingham, and J. J. Schentag. 2003. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin. Pharmacokinet. 42:1411-1423. [DOI] [PubMed] [Google Scholar]
- 29.Roberts, J. A., and J. Lipman. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37:840-851. [DOI] [PubMed] [Google Scholar]
- 30.Santos, R. P., C. B. Prestidge, M. E. Brown, B. Urbancyzk, D. K. Murphey, C. M. Salvatore, H. S. Jafri, G. H. McCracken, Jr., N. Ahmad, P. J. Sanchez, and J. D. Siegel. 2009. Pharmacokinetics and pharmacodynamics of linezolid in children with cystic fibrosis. Pediatr. Pulmonol. 44:148-154. [DOI] [PubMed] [Google Scholar]
- 31.Slatter, J. G., D. J. Stalker, K. L. Feenstra, I. R. Welshman, J. B. Bruss, J. P. Sams, M. G. Johnson, P. E. Sanders, M. J. Hauer, P. E. Fagerness, R. P. Stryd, G. W. Peng, and E. M. Shobe. 2001. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C]linezolid to healthy human subjects. Drug Metab. Dispos. 29:1136-1145. [PubMed] [Google Scholar]
- 32.Stalker, D. J., and G. L. Jungbluth. 2003. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin. Pharmacokinet. 42:1129-1140. [DOI] [PubMed] [Google Scholar]
- 33.Stein, G. E., and E. M. Wells. 2010. The importance of tissue penetration in achieving successful antimicrobial treatment of nosocomial pneumonia and complicated skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus: vancomycin and linezolid. Curr. Med. Res. Opin. 26:571-588. [DOI] [PubMed] [Google Scholar]
- 34.Susanto, M., and L. Z. Benet. 2002. Can the enhanced renal clearance of antibiotics in cystic fibrosis patients be explained by P-glycoprotein transport? Pharm. Res. 19:457-462. [DOI] [PubMed] [Google Scholar]
- 35.Swoboda, S., M. C. Ober, C. Lichtenstern, S. Saleh, V. Schwenger, H. G. Sonntag, W. E. Haefeli, G. Hempel, T. Hoppe-Tichy, and M. A. Weigand. 2010. Pharmacokinetics of linezolid in septic patients with and without extended dialysis. Eur. J. Clin. Pharmacol. 66:291-298. [DOI] [PubMed] [Google Scholar]
- 36.Thallinger, C., C. Buerger, N. Plock, S. Kljucar, S. Wuenscher, R. Sauermann, C. Kloft, and C. Joukhadar. 2008. Effect of severity of sepsis on tissue concentrations of linezolid. J. Antimicrob. Chemother. 61:173-176. [DOI] [PubMed] [Google Scholar]
- 37.Vinh, D. C., and E. Rubinstein. 2009. Linezolid: a review of safety and tolerability. J. Infect. 59(Suppl. 1):S59-S74. [DOI] [PubMed] [Google Scholar]