Abstract
HIV-1 RNA level and darunavir concentration in the genital tract were measured in 45 men receiving darunavir-ritonavir mono- or tritherapy. At week 48, a low frequency (3/45) of HIV-1 RNA shedding was observed in patients (1 on monotherapy and 2 on triple therapy), although they had undetectable HIV-1 RNA in plasma. The median darunavir seminal plasma concentration was close to the blood plasma free fraction, demonstrating a good penetration of darunavir into the male genital tract.
Darunavir-ritonavir is an appropriate candidate for protease inhibitor (PI) monotherapy in maintenance therapy due to its high genetic barrier (5), high potency on naive and resistant HIV strains (11, 15), and good pharmacokinetics profile (21). MONOI is an ongoing, 96-week, multicenter, randomized, open-label trial with a primary endpoint at week 48 (W48). This study comprised the first phase, where darunavir at 600/100 mg twice daily (b.i.d.) was introduced for 8 weeks as a component of a triple-drug regimen in replacement of the PI, nonnucleoside reverse transcriptase inhibitors (NNRTI), or third nucleoside RTI (NRTI). Patients whose HIV-1 viral load remained lower than 50 copies/ml 4 weeks after darunavir introduction were randomly assigned 1:1 at day 0 (D0) to continue the triple-drug darunavir-containing regimen (darunavir triple therapy) or to stop the two NRTIs (darunavir monotherapy) (12). Available information on antiretroviral drug penetration into the male genital tract is sparse, and concern remains about the efficacy of PI inhibitor monotherapy in viral sanctuaries such as the male genital tract because of the poor penetration of most PIs into semen and the subsequent risk of persistent viral replication and emergence of resistance (4, 7). The aim of our study was to evaluate the outcomes of HIV-1 shedding in the genital tract in patients receiving darunavir-ritonavir monotherapy versus tritherapy and to determine the darunavir concentrations in blood plasma (BP) (free- and total-protein fractions) and seminal plasma (SP) (total-protein fractions).
(This work was presented at the 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, 16 to 19 February 2010, poster 610.)
From the 225 HIV-1-infected men randomized in the MONOI trial, 47 paired samples of BP and SP were collected at D0 (end of the darunavir tritherapy 8-week run-in period) and W48 after a 3-day period of sexual abstinence. The Cobas TaqMan HIV-1 assay was used to quantify HIV-1 RNA in BP and in SP (at D0 and W48), with limits of quantification of 40 and 200 copies/ml, respectively, as previously described (16, 17). Total and free-fraction BP concentrations and total SP darunavir concentrations were determined at D0 and W48 using the ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method (Acquity UPLC, Acquity TQD) (limit of quantification [LOQ] ∼ 1 ng/ml) as previously described (10). Darunavir plasma protein binding was performed in duplicate using a Centrifree ultrafiltration device. Darunavir concentrations were also determined using high-performance liquid chromatography (HPLC)-fluorimetric detection (LOQ ∼ 5 ng/ml) as previously described for amprenavir to compare with mass spectrometry results (18). Results are presented as medians (interquartile range [IQR], 25% to 75%). Nonparametric tests were used (Spearman's rank test).
Among the 47 patients enrolled in the present study, 23 were randomized in the darunavir-ritonavir monotherapy arm and 24 in the arm receiving darunavir-ritonavir plus 2 NRTIs. HIV-1 RNA at D0 and W48 could be analyzed in paired BP and SP for 45 patients. Overall, 39 patients had concordant undetectable HIV-1 RNA in BP and SP. Six patients had detectable (>200 copies/ml) HIV-1 RNA in SP despite undetectable (<40 copies/ml) HIV-1 RNA in the corresponding BP samples (Table 1): 3 patients at D0 and 3 different patients at W48. At D0 the 3 discordant patients were on darunavir triple therapy, and in two of them, SP HIV-1 RNA became <200 copies/ml at W48. At W48, 3 other patients were discordant, 2 in the tritherapy arm and 1 in the monotherapy arm.
TABLE 1.
HIV-1 RNA levels and darunavir concentrations in blood and seminal plasma at day 0 and week 48 in discordant patientsa
| Patient no. | Day 0b |
NRTI backbonec | Week 48 |
Arme | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP |
SP |
BP |
SP |
|||||||||
| VLd (copies/ml) | DRV concn (ng/ml) |
VL (copies/ml) | DRV concn (ng/ml) | VL (copies/ml) | DRV concn (ng/ml) |
VL (copies/ml) | DRV concn (ng/ml) | |||||
| Total | Free | Total | Free | |||||||||
| 2 | <40 | 3,384 | 295 | 1,345 | 770 | TDF-FTC | <40 | 6,120 | 604 | <200 | 527 | Mono |
| 3 | <40 | 5,366 | 377 | 345 | 374 | ABC-ddI | <40 | 3,466 | 256 | <200 | 344 | Mono |
| 4 | <40 | 3,310 | 223 | 385 | 144 | AZT-3TC | <40 | 4,730 | 296 | NAf | NA | Triple |
| 1 | <40 | 2,379 | 329 | <200 | 173 | TDF-FTC | <40 | 1,947 | 370 | 270 | 707 | Mono |
| 5 | <40 | 12,404 | 1,262 | <200 | 678 | TDF-FTC | <40 | 2,709 | 276 | 345 | 193 | Triple |
| 6 | <40 | 2,444 | 225 | <200 | 239 | TDF-3TC | <40 | 6,040 | 261 | 475 | 632 | Triple |
Discordant patients had detectable (>200 copies/ml) HIV-1 RNA in seminal plasma despite undetectable (<40 copies/ml) HIV-1 RNA in the corresponding blood plasma sample.
At day 0, all patients had been on a darunavir (DRV) triple drug regimen for the previous 8 weeks (run-in period).
TDF, tenofovir; FTC, emtricitabine; ABC, abacavir; ddI, didanosine; AZT, zidovudine; 3TC, lamivudine.
VL, HIV-1 viral load. Values above the limit of detection are in boldface.
Mono, monotherapy; triple, tritherapy.
NA, not available.
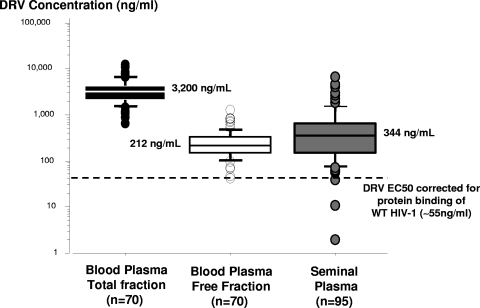
Darunavir concentrations determined using both methods (UPLC-MS/MS and HPLC-fluorimetric detection) were statistically correlated for BP (r2 = 0.935, P < 0.0001) and SP (r2 = 0.961, P < 0.0001). The differences between both methods are in the limits of agreement for both BP and SP samples. Over the linearity range of darunavir concentrations determined by the two methods, only 1/68 and 2/93 pairs of values were outside the mean difference ± 2 standard deviations of the difference intervals for BP and SP, respectively (1). LOQ was consistent with the requirement of the pharmacokinetic analysis in these compartments, and no matrix effect was expected using these methods. Indeed, the darunavir UPLC-MS/MS method, including sample preparation, was developed with a systematic optimization aimed at reducing or eliminating matrix effects as previously described (3). Pharmacokinetics results for BP and in SP are presented in Fig. 1. Total and free BP darunavir concentrations determined 12 h (10.8 to 13.5 h) after the last drug intake were 3,200 ng/ml (2,127 to 4,179 ng/ml; n = 70) and 212 ng/ml (154 to 326 ng/ml; n = 70), respectively. The unbound fraction of drug is considered the only effective fraction available for the diffusion or penetration into tissues. The darunavir plasma free fraction was 7.2% (5.9 to 9.0%) and was consistent with 95% protein binding primarily to plasma alpha 1-acid glycoprotein (21). There was a good correlation between darunavir total and free fractions of plasma proteins (r2 = 0.827; P < 0.0001). The median SP darunavir concentration determined 15.9 h (13.3 to 17.3 h) after the last drug intake was 344 ng/ml (149 to 652 ng/ml; n = 95) and was 6-fold higher than the darunavir 50% effective concentrations (EC50) (corrected for protein binding) for wild-type HIV-1 strains (∼55 ng/ml) (5). Darunavir SP concentrations were below 55 ng/ml for only three patients, and these 3 patients had HIV-1 RNA levels in SP of <200 copies/ml. The SP/BP ratio of darunavir concentrations was 0.086 (0.057 to 0.22).
FIG. 1.
Darunavir (DRV) concentrations in blood plasma (free- and total-protein binding) and in seminal plasma. DRV results from tritherapy and monotherapy were merged for this analysis. n, number of patients; WT, wild type.
This is the first study demonstrating darunavir penetration into the seminal fluid and concomitantly the virological outcomes in BP and SP compartments. The median darunavir SP concentration is close to the BP free fraction and approximately 6-fold higher than the darunavir EC50 corrected for protein binding of wild-type HIV-1 (∼55 ng/ml) (5), demonstrating good penetration of darunavir into the male genital tract and suggesting passive diffusion of the free fraction from BP to SP. The percentage of patients (6.4%) who had detectable HIV-1 RNA in semen although they had concomitantly undetectable HIV-1 RNA in blood despite adequate concentrations in the two compartments was consistent with the value of 5% reported in a previous study (13) and reflected intermittent HIV-1 excretion (2, 8). For a small number of patients, no relationship between the treatment arm (darunavir monotherapy versus triple therapy) or the NRTI backbone and intermittent HIV-1 excretion was evidenced. This suggests that the use of darunavir-ritonavir monotherapy does not increase intermittent HIV-1 excretion, probably because darunavir has good penetration into the male genital tract. Two previous studies comparing lopinavir-ritonavir monotherapy and triple therapy, one in initiation (6) and the other in maintenance (9), also showed no difference in the proportion of patients with detectable HIV-1 RNA in semen between arms.
The capacity of a drug to enter the male genital tract depends on a number of factors: molecular size, lipophilicity, degree of ionization, plasma protein binding, and whether or not the drug is a substrate for efflux transporters (20). The PIs have in general a number of unfavorable characteristics, which results in more-difficult passive diffusion (4, 19). Like most PIs, darunavir is highly bound to plasma proteins (95%), with a small fraction (5%) of unbound drug available to penetrate the male genital tract. The median BP darunavir concentration of 3,200 ng/ml determined in this study was close to that obtained from TMC114-C213 and TMC114-C202 studies of 3,539 ng/ml (14). Only one study showed that darunavir has good penetration into SP of HIV-1-infected men receiving a once-daily regimen, with total concentrations determined 12 h after the last drug intake approximating 11% of the BP concentrations (19). However, in that study no distinction between darunavir total and free fractions was made. Moreover, no data are available on protein binding in SP. All darunavir SP concentrations were above the published protein-corrected darunavir EC50, and 1/3 of all darunavir SP concentrations exceeded the protein-corrected EC50 required to inhibit PI-resistant HIV.
In conclusion, darunavir concentrations in the seminal fluid due to its use either alone or in combination with NRTIs did not correlate with detection of HIV in the seminal fluid.
Acknowledgments
This study was supported by ANRS (Agence Nationale de Recherches sur le SIDA et les hépatites virales). The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007-2013), under the project Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN), grant agreement 223131.
Footnotes
Published ahead of print on 16 August 2010.
REFERENCES
- 1.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. [PubMed] [Google Scholar]
- 2.Bujan, L., M. Daudin, T. Matsuda, L. Righi, L. Thauvin, L. Berges, J. Izopet, A. Berrebi, P. Massip, and C. Pasquier. 2004. Factors of intermittent HIV-1 excretion in semen and efficiency of sperm processing in obtaining spermatozoa without HIV-1 genomes. AIDS 18:757-766. [DOI] [PubMed] [Google Scholar]
- 3.Chambers, E., D. M. Wagrowski-Diehl, Z. Lu, and J. R. Mazzeo. 2007. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 852:22-34. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, M. S., C. Gay, A. D. Kashuba, S. Blower, and L. Paxton. 2007. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann. Intern. Med. 146:591-601. [DOI] [PubMed] [Google Scholar]
- 5.De Meyer, S., H. Azijn, D. Surleraux, D. Jochmans, A. Tahri, R. Pauwels, P. Wigerinck, and M. P. de Bethune. 2005. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob. Agents Chemother. 49:2314-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosn, J., M. L. Chaix, G. Peytavin, J. L. Bresson, J. Galimand, P. M. Girard, F. Raffi, I. Cohen-Codar, J. F. Delfraissy, and C. Rouzioux. 2008. Absence of HIV-1 shedding in male genital tract after 1 year of first-line lopinavir/ritonavir alone or in combination with zidovudine/lamivudine. J. Antimicrob. Chemother. 61:1344-1347. [DOI] [PubMed] [Google Scholar]
- 7.Ghosn, J., M. L. Chaix, G. Peytavin, E. Rey, J. L. Bresson, C. Goujard, C. Katlama, J. P. Viard, J. M. Treluyer, and C. Rouzioux. 2004. Penetration of enfuvirtide, tenofovir, efavirenz, and protease inhibitors in the genital tract of HIV-1-infected men. AIDS 18:1958-1961. [DOI] [PubMed] [Google Scholar]
- 8.Gupta, P., C. Leroux, B. K. Patterson, L. Kingsley, C. Rinaldo, M. Ding, Y. Chen, K. Kulka, W. Buchanan, B. McKeon, and R. Montelaro. 2000. Human immunodeficiency virus type 1 shedding pattern in semen correlates with the compartmentalization of viral quasispecies between blood and semen. J. Infect. Dis. 182:79-87. [DOI] [PubMed] [Google Scholar]
- 9.Gutmann, C., M. Opravil, C. Fux, H. J. Furrer, L. A. Decosterd, M. Cavassini, S. Yerly, B. Hirschel, P. Vernazza, and the Swiss HIV Cohort Study. 2009. Abstr. Deutsch Österreichisch Schweizerische AIDS Kongr. (SÖDAK), St Gallen, Switzerland, 24 to 27 June 2009, abstr. P421.
- 10.Jung, B. H., N. L. Rezk, A. S. Bridges, A. H. Corbett, and A. D. Kashuba. 2007. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 21:1095-1104. [DOI] [PubMed] [Google Scholar]
- 11.Katlama, C., R. Esposito, J. M. Gatell, J. C. Goffard, B. Grinsztejn, A. Pozniak, J. Rockstroh, A. Stoehr, N. Vetter, P. Yeni, W. Parys, and T. Vangeneugden. 2007. Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS 21:395-402. [DOI] [PubMed] [Google Scholar]
- 12.Katlama, C., M. A. Valantin, M. Algarte-Genin, C. Duvivier, S. Lambert-Niclot, P. M. Girard, J. M. Molina, B. Hosten, S. Pakianather, G. Peytavin, A. G. Marcelin, and P. Flandre. 2009. Abstr. 5th IAS Conf. HIV Pathol. Treat. Prevent., Cape Town, South Africa, 19 to 22 June, abstr. WELBB102.
- 13.Marcelin, A. G., R. Tubiana, S. Lambert-Niclot, G. Lefebvre, S. Dominguez, M. Bonmarchand, D. Vauthier-Brouzes, F. Marguet, N. Mousset-Simeon, G. Peytavin, and C. Poirot. 2008. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. AIDS 22:1677-1679. [DOI] [PubMed] [Google Scholar]
- 14.Molina, J. M., and A. Hill. 2007. Darunavir (TMC114): a new HIV-1 protease inhibitor. Expert Opin. Pharmacother. 8:1951-1964. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz, R., E. Dejesus, H. Khanlou, E. Voronin, J. van Lunzen, J. Andrade-Villanueva, J. Fourie, S. De Meyer, M. De Pauw, E. Lefebvre, T. Vangeneugden, and S. Spinosa-Guzman. 2008. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS 22:1389-1397. [DOI] [PubMed] [Google Scholar]
- 16.Pasquier, C., D. Anderson, C. Andreutti-Zaugg, R. Baume-Berkenbosch, F. Damond, A. Devaux, Y. Englert, J. Galimand, C. Gilling-Smith, O. Guist'hau, L. Hollander, M. Leruez-Ville, B. Lesage, A. Maillard, A. G. Marcelin, M. P. Schmitt, A. Semprini, M. Vourliotis, C. Xu, and L. Bujan. 2006. Multicenter quality control of the detection of HIV-1 genome in semen before medically assisted procreation. J. Med. Virol. 78:877-882. [DOI] [PubMed] [Google Scholar]
- 17.Pasquier, C., K. Saune, S. Raymond, N. Moinard, M. Daudin, L. Bujan, and J. Izopet. 2009. Determining seminal plasma human immunodeficiency virus type 1 load in the context of efficient highly active antiretroviral therapy. J. Clin. Microbiol. 47:2883-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadler, B. M., C. D. Hanson, G. E. Chittick, W. T. Symonds, and N. S. Roskell. 1999. Safety and pharmacokinetics of amprenavir (141W94), a human immunodeficiency virus (HIV) type 1 protease inhibitor, following oral administration of single doses to HIV-infected adults. Antimicrob. Agents Chemother. 43:1686-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor, S., A. Jayasuriya, N. Dufty, G. Gilleran, A. Berry, D. Back, and E. Smit. 2010. Abstr. 17th Conf. Retrovir. Opport. Infect., San Fransisco, CA, 16 to 19 February 2010, abstr. 611.
- 20.Taylor, S., and A. S. Pereira. 2001. Antiretroviral drug concentrations in semen of HIV-1 infected men. Sex. Transm. Infect. 77:4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermeir, M., S. Lachau-Durand, G. Mannens, F. Cuyckens, B. van Hoof, and A. Raoof. 2009. Absorption, metabolism, and excretion of darunavir, a new protease inhibitor, administered alone and with low-dose ritonavir in healthy subjects. Drug Metab. Dispos. 37:809-820. [DOI] [PubMed] [Google Scholar]