Abstract
Antiviral drugs for treating polyomavirus BK (BKV) replication in polyomavirus-associated nephropathy or hemorrhagic cystitis are of considerable clinical interest. Unlike cidofovir, the lipid conjugate 1-O-hexadecyloxypropyl cidofovir (CMX001) is orally available and has not caused detectable nephrotoxicity in rodent models or human studies to date. Primary human renal proximal tubular epithelial cells were infected with BKV-Dunlop, and CMX001 was added 2 h postinfection (hpi). The intracellular and extracellular BKV DNA load was determined by quantitative PCR. Viral gene expression was examined by quantitative reverse transcription-PCR, Western blotting, and immunofluorescence microscopy. We also examined host cell viability, proliferation, metabolic activity, and DNA replication. The titration of CMX001 identified 0.31 μM as the 90% effective concentration (EC90) for reducing the extracellular BKV load at 72 hpi. BKV large T antigen mRNA and protein expression was unaffected at 24 hpi, but the intracellular BKV genome was reduced by 90% at 48 hpi. Late gene expression was reduced by 70 and 90% at 48 and 72 hpi, respectively. Comparisons of CMX001 and cidofovir EC90s from 24 to 96 hpi demonstrated that CMX001 had a more rapid and enduring effect on BKV DNA and infectious progeny at 96 hpi than cidofovir. CMX001 at 0.31 μM had little effect on overall cell metabolism but reduced bromodeoxyuridine incorporation and host cell proliferation by 20 to 30%, while BKV infection increased cell proliferation in both rapidly dividing and near-confluent cultures. We conclude that CMX001 inhibits BKV replication with a longer-lasting effect than cidofovir at 400× lower levels, with fewer side effects on relevant host cells in vitro.
Polyomavirus BK (BKV) infects the majority of humans during childhood and subsequently persists in the renourinary tract, with intermittent periods of asymptomatic shedding into urine (8, 17, 24). Organ-invasive polyomavirus disease is rare and virtually limited to individuals with profound immune dysfunction (17). The prototypic BKV diseases are polyomavirus-associated nephropathy (PyVAN) affecting 1 to 10% of kidney transplant patients and polyomavirus-associated hemorrhagic cystitis affecting 5 to 15% of allogeneic hematopoietic stem cell transplant recipients (13). Unfortunately, antiviral drugs with specific activity against polyomavirus replication are lacking. Polyomaviruses encode only a few proteins and utilize many host proteins for replication, including the cellular DNA polymerase. In the circular double-stranded DNA genome of approximately 5 kb, the viral early genes encoding the regulatory proteins large T antigen (LT-ag) and small T antigen (sT-ag) are separated by the noncoding control region (NCCR) from the late genes encoding the capsid proteins VP1, VP2, and VP3, as well as agnoprotein (agno) (32). Cell culture and biopsy specimen studies indicate that the BKV replication cycle takes approximately 48 to 72 h for completion in renal tubular epithelial cells (1, 21, 33), where early gene expression starts 12 to 24 h postinfection (hpi), followed by the bidirectional replication of the episomal DNA genome at 36 hpi and late gene expression thereafter (5). The replication rate is accelerated for BKV variants derived from kidney transplant patients with rearranged NCCR (11, 22).
The most successful strategy to prevent or treat PyVAN in kidney transplant recipients currently is the reduction of immunosuppression (15, 26), which is followed by increases of BKV-specific cellular immune responses (4, 6). Despite promising long-term results in some studies (12), approximately 10% of patients may experience subsequent acute allograft rejection. Without intervention, progression to premature graft failure and loss is observed in more than 80% of cases (3, 25, 28). For polyomavirus-associated hemorrhagic cystitis, reducing immunosuppression is perceived as a difficult undertaking, since the pathogenesis entails features of an immune reconstitution disease and often coincides with significant graft-versus-host disease requiring immunosuppressive therapy (7, 14). Some potentially active drugs, like cidofovir (CDV), an acyclic nucleotide phosphonate analogue of deoxycytidine monophosphate (dCMP), have been used sporadically for the treatment of BKV replication (19, 20, 31). However, since immunosuppression usually is eased simultaneously with the CDV treatment, it remains unclear whether or not the partly favorable outcomes could be attributed to the antipolyomavirus activity of the drug or the recovery of polyomavirus-specific immunity.
In vitro studies have shown an effect of CDV on BKV replication in human embryonic lung fibroblast cells (WI-38) (9) and in primary human renal proximal tubular epithelial cells (RPTECs) (1). In RPTECs, CDV inhibited BKV replication in a dose-dependent manner with a 90% effective concentration (EC90) of 40 μg/ml (143 μM) (1). The inhibition was mediated at the step of BKV DNA replication but also decreased both host cellular DNA replication and metabolic activity as correlates of nephrotoxicity in vivo. Another caveat of CDV use is that it must be given intravenously, and therefore the patients need to be hospitalized. Recently, a 1-O-hexadecyl-oxypropyl lipid conjugate of CDV (HDP-CDV), denoted CMX001, became available. Unlike CDV, the conjugate seems to be taken up by cells in a manner similar to that of lysophosphatidylcholine followed by the liberation of CDV by phospholipase cleavage and subsequent phosphorylation to the active diphosphate antiviral CDV-PP. Studies of single and repeated dosing in animals and in human volunteers or patients ranging from 0.1 to 4.0 mg/kg of body weight has shown no evidence of significant nephrotoxicity to date (18). In a previous study, CMX001 was reported to inhibit BKV replication in human fetal fibroblasts, but the mechanistic details were not reported (27). Here, we report on the effects of CMX001 on BKV replication in RPTECs, which are the primary target of BKV in PyVAN.
MATERIALS AND METHODS
Cells and virus.
Primary human renal proximal tubule epithelial cells (RPTECs) (Lonza) were propagated as described by the manufacturer. No latent BKV could be detected by PCR of intracellular DNA. All experiments were performed with RPTECs at passage 4, along with BKV-Dunlop supernatants and gradient-purified virus from Vero cells. In addition to being among the most authentic cells for in vitro studies of BKV, RPTECs are about 10 times more permissive for BKV than Vero cells.
Infection and CMX001 treatment.
Before each experiment, CMX001-060, from here on called CMX001, was freshly dissolved to 1 mg/ml in methanol-water-ammonium hydroxide (50-50-2) and further diluted in RPTEC growth medium. About 50% confluent RPTECs were infected with BKV-Dunlop at a multiplicity of infection (MOI) of 1 for 2 h before removing infectious units by washes and replacing the growth medium with or without CMX001 unless indicated otherwise. Nonconfluent dividing cells were chosen to get high-level BKV replication. The MOI was determined by a modified plaque assay by serial dilution and the seeding of gradient-purified virus or BKV supernatants onto RPTECs. Three days postinfection cells were fixed and stained for agnoprotein or VP1 and the number of infectious virus in the inocula calculated.
Cytotoxicity and cell proliferation assay.
The mitochondrial metabolic activity was monitored by the colorimetric WST-1 assay (Roche), measuring the reduction of the tetrazolium salt WST-1 by mitochondrial dehydrogenases. DNA synthesis was quantified by the colorimetric measurement of bromodeoxyuridine (BrdU) incorporation into DNA using the cell proliferation enzyme-linked immunosorbent assay (ELISA) BrdU kits (Roche). The results were analyzed by the Excel XL fit program for curve fitting to determine the EC90 and the 90% cytotoxic concentration (CC90). The attachment and proliferation of the cells was measured as impedance using E plates and the xCelligence system (Roche). For the RPTECs to attach and proliferate on the E plates, the plates were first coated with fibronectin. The background impedance of the plates then was monitored by the addition of 100 μl medium to each well before the plate was connected to the system and checked in the cell culture incubator for proper electrical contacts. Subsequently, a 100-μl cell suspension containing the indicated cell numbers was seeded. To determine the effect of BKV infection and CMX001 treatment, about 24 h after seeding 150 μl of the medium was replaced with fresh medium with or without purified BKV-Dunlop in the presence or absence of CMX001 (final concentration of 0.31 μM). The cells were grown for 96 h, and impedance was measured every 15 min for the first 6 h and then every 30 min. Impedance was expressed as an arbitrary unit called the cell index.
RNA extraction and cDNA synthesis.
The expression levels of mRNA was quantified by reverse transcription-quantitative PCR (RT-qPCR) as described previously (1). At 24, 48, and 72 hpi, cells were lysed and total RNA extracted using the mirVana PARIS kit (Ambion). RNA samples were treated with DNase turbo (Ambion) to remove residual DNA before the RNA quality was checked by agarose gel electrophoresis, and the RNA concentration was determined using the nanodrop method. cDNA was generated from 250 ng RNA per sample using the high-capacity cDNA kit (Applied Biosystems).
DNA extraction.
To assay extracellular BKV loads, cell culture supernatants were harvested at 24, 48, and 72 hpi and frozen at −70°C until automatic extraction by a robot (GenoM-48; Qiagen). For the determination of intracellular BKV loads, cells were washed, trypsinized, and pelleted at 220 × g for 10 min, resuspended in G2 buffer from the MagAttract DNA mini M48 kit (Qiagen), and frozen at −70°C until extraction with the same robot.
Quantitative PCR for BKV DNA and cellular gene detection.
To quantify intracellular or extracellular BKV DNA load, qPCR with primers and probe targeting the LT-ag gene was used (16). For the normalization of intracellular BKV DNA, each sample was analyzed in parallel by qPCR for the gene for aspartoacylase (ACY) to correct for cellular DNA (1, 29).
Western blotting.
Cells were lysed in cell disruption buffer (mirVana PARIS kit; Ambion) 24, 48, and 72 hpi and stored at −70°C until separation with SDS-polyacrylamide gel electrophoresis (SDS-PAGE), followed by being blotted onto a polyvinyl difluoride (PVDF) membrane. The detection of BKV and cellular proteins was performed with polyclonal rabbit antiserum directed against LT-ag (1:2,000), VP1 (1:10,000), or agno (1:10,000) (1, 32), and a monoclonal mouse antibody directed against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:5,000; Ab8245; Abcam) followed by anti-rabbit and anti-mouse infrared dye-labeled secondary antibodies (IR dye 800; Rockland; and Alexa Fluor 680; Invitrogen), both at 1:5,000, before detection and quantification using the Licor Odyssey and the corresponding commercially available software program. The fluorescent signals are considered to be stable with a greater dynamic range than that of enhanced chemiluminescence. In brief, the protein band signals were measured as integrated intensity (pixel/mm2) using the median lane background method with top and bottom segments with a border width of 1. The expression of viral proteins was normalized against the expression of cellular GAPDH protein on the same blot labeled with a different color, whereby the results in untreated cells at each time point were set as 100%.
Immunofluorescence staining, microscopy, and digital image processing.
Cells were washed in phosphate-buffered saline (PBS), fixed in 100% methanol for 10 min, and blocked with 3% goat serum in PBS for 30 min, both at room temperature, followed by incubation with primary and secondary antibodies for 30 min at 37°C and at room temperature, respectively. Primary antibodies were monoclonal anti-simian virus 40 (SV40) LT-ag (Pab416; 1:100; Chemicon) and polyclonal rabbit antiserum directed against agno or VP1 (both at 1:1,000). The secondary antibodies were anti-mouse conjugated with Alexa Fluor 568 and anti-rabbit conjugated with Alexa Fluor 488 (1:500; Molecular Probes). Nuclei were labeled with DRAQ5 (Biostatus). Images were collected using a Nikon TE2000 microscope equipped and processed with NIS elements basic research software, version 2.2 (Nikon Corporation).
RESULTS
Determination of CMX001 EC90.
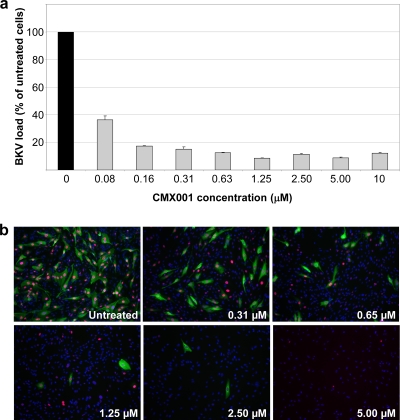
To investigate the effect of CMX001 on BKV progeny, increasing concentrations of CMX001 were added 2 hpi, and supernatants were harvested at 72 hpi. We observed that CMX001 reduced the extracellular BKV load in a concentration-dependent manner (Fig. 1a). Recent results from the mathematical modeling of BKV replication in kidney transplant recipients suggest that a more-than 80% reduction of renal BKV replication must be maintained for up to 10 weeks to observe the clearing of plasma and urine viral loads (10). We therefore focused on the concentration of CMX001 that reduced the extracellular BKV load by 90%. The CMX001 concentration of 0.31 μM consistently provided this level of inhibition (Fig. 1a) and was used for further characterization. Immunofluorescence staining of BKV-infected RPTECs 72 hpi demonstrated a concentration-dependent decrease in the number and intensity of LT-ag- and agno-expressing cells (Fig. 1b). At concentrations as high as 10 μM CMX001, no BKV-infected cell was observed, but the total number of cells also was reduced (also see below). We concluded that CMX001 reduced the expression of early and late BKV proteins and the production of extracellular progeny but also seemed to have a concentration-dependent effect on the proliferation of RPTECs.
FIG. 1.
Effect of increasing concentrations of CMX001 on BKV load and expression of BKV proteins. (a) RPTEC supernatants were harvested 72 hpi, i.e., 70 h after the start of treatment with indicated CMX001 concentrations, and BKV load was measured by qPCR. The DNA load in untreated cells (1.19 × 109 genome equivalent [Geq]/ml) was set as 100%. Determinations were in triplicate. The mean values are shown, and the error bars represent standard deviations. (b) Indirect immunofluorescence of BKV-infected RPTECs either untreated or treated with the indicated CMX001 concentrations. The cells were methanol fixed 72 hpi and stained using as primary antibodies polyclonal rabbit anti-agno serum (green) for the visualization of the late agno and the SV40 LT-ag monoclonal Pab416 for the visualization of early LT-ag (red). Cell nuclei (blue) were stained with Drac 5.The pictures are taken with the 10× objective.
CMX001 and BKV early gene expression.
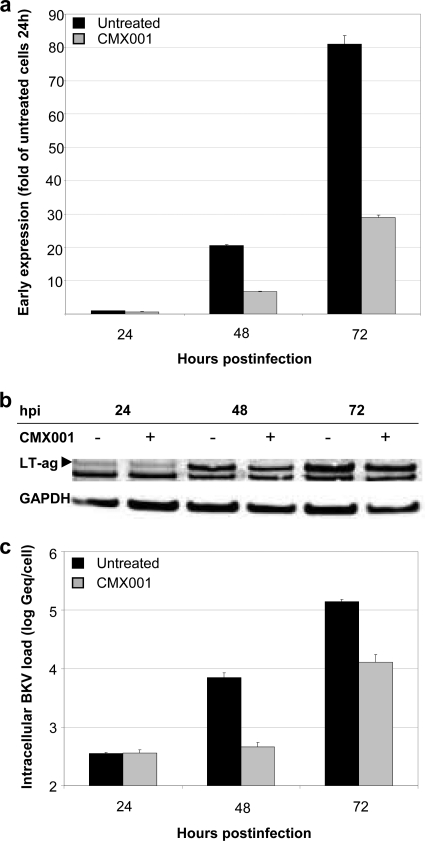
To study the effect of CMX001 at 0.31 μM on BKV early gene expression, we compared LT-ag mRNA levels in treated and untreated RPTECS at 24, 48, and 72 hpi by RT-qPCR. The results were normalized to the housekeeping gene huHPRT and are presented as the changes relative to the levels of the untreated sample at 24 hpi. We found no difference in early gene expression at 24 hpi, but reductions of 33 and 64% were seen at 48 and 72 hpi, respectively (Fig. 2a). Analyzing LT-ag expression by Western blotting and normalization to the constitutively expressed enzyme GAPDH revealed a corresponding result showing little difference at 24 hpi but a 20 to 30% reduction at later time points (Fig. 2b). The band seen below LT-ag is a cellular protein of unknown origin cross-reacting with the polyclonal rabbit antiserum used. We concluded that CMX001 did not inhibit BKV early protein expression early in the viral life cycle, but it did so later at 48 and 72 hpi.
FIG. 2.
Influence of CMX001 at 0.31 μM on BKV-Dunlop early expression and DNA replication in RPTECs. (a) Early mRNA expression. RNA was extracted from CMX001-treated and untreated BKV-infected RPTECs at the indicated time points. LT-ag mRNA expression was measured by RT-qPCR and normalized to huHPRT transcripts. Results are presented as changes in the LT-ag mRNA level, with the level in the untreated sample at 24 hpi arbitrarily set to 1. Determinations were in triplicate. The mean values are shown, and the error bars represent standard deviations. (b) Early protein expression. Cell extracts from CMX001-treated (+) and untreated (−) BKV-infected RPTECs were harvested 24, 48, and 72 hpi, and Western blotting was performed with polyclonal rabbit anti-LT-ag serum and with a monoclonal antibody directed against the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The anti-LT-ag serum also recognizes a cellular protein of unknown origin. (c) BKV DNA replication. CMX001-treated and untreated BKV-infected RPTECs were harvested at the indicated time points and DNA extracted. Intracellular BKV DNA load was measured by qPCR and normalized for cellular DNA using the aspartoacyclase (ACY) qPCR. Data are presented as log Geq/cell. Determinations were in triplicate. The mean values are shown, and the error bars represent standard deviations.
CMX001 and BKV genome replication.
Since BKV episome replication is known to occur at around 36 hpi (1, 21, 33), we investigated whether the BKV genome replication was affected by CMX001. The intracellular BKV load at 24, 48, and 72 hpi was measured by qPCR and normalized to the cell number using the aspartoacylase (ACY) gene as a cellular reference gene (30). Compared to results with untreated RPTECs, CMX001 at 0.31 μM reduced the intracellular BKV load by 94% at 48 hpi and 91% at 72 hpi (Fig. 2c). Thus, we could identify a significant inhibitory effect of CMX001 on intracellular BKV genome replication. This step is known to require LT-ag function, which increases viral late gene expression by two synergistic mechanisms, namely, by increasing the DNA templates and thereby the gene dose per cell for late gene transcription and by activating transcription from the late promoter (5).
CMX001 and BKV late gene expression.
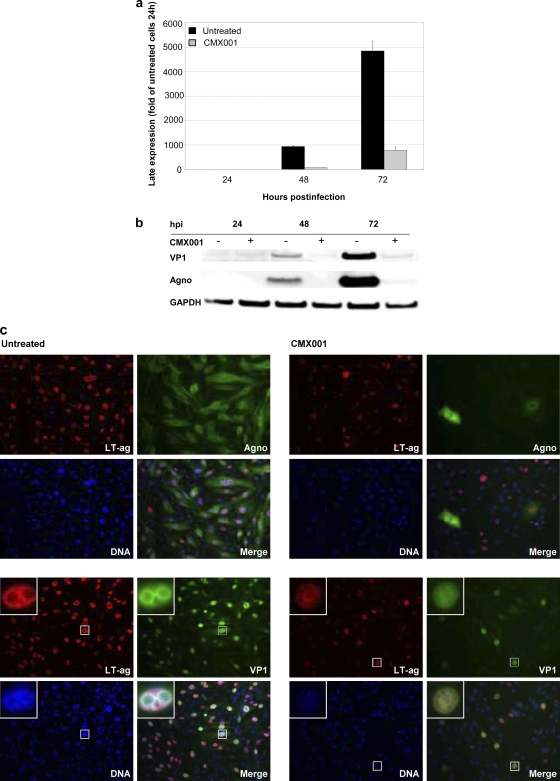
To study the effect of CMX001 on BKV late gene expression, we compared VP1 and agno mRNA levels in CMX001-treated and untreated RPTECS at 24, 48, and 72 hpi by RT-qPCR. Late mRNA levels were normalized to the housekeeping gene huHPRT and are presented as the changes relative to the untreated sample at 24 hpi. A 93 and 82% reduction was found at 48 and 72 hpi, respectively (Fig. 3a). By Western blotting, we found a decrease of VP1 of 85 and 96%, respectively, while agno was reduced by 97 and 96% (Fig. 3b).
FIG. 3.
Influence of CMX001 at 0.31 μM on BKV-Dunlop late expression in RPTECs. (a) Late mRNA expression. RNA was extracted from CMX001-treated and untreated BKV-infected RPTECS at the indicated time points. VP1 mRNA expression was measured by RT-qPCR and normalized to huHPRT transcripts. Results are presented as changes in the VP1 mRNA level, with the level in the untreated sample at 24 hpi arbitrarily set to 1. Determinations were in triplicate. The mean values are shown, and the error bars represent standard deviations. (b) Late protein expression. Cell extracts from CMX001-treated (+) and untreated (−) BKV-infected RPTECs were harvested 24, 48, and 72 hpi, and Western blotting was performed with polyclonal rabbit anti-agno and anti-VP1 serum and with the monoclonal antibody anti-GAPDH. (c) Early and late protein expression. Indirect immunofluorescence of BKV-infected RPTECs either untreated or treated with CMX001. The cells were methanol fixed 72 hpi and stained using the primary antibodies polyclonal rabbit anti-agno serum or anti-VP1 serum (both green) for the visualization of the late agno and VP1 protein, respectively, in combination with the SV40 LT-ag monoclonal Pab416 for the visualization of early LT-ag (red). Cell nuclei (blue) were stained with Drac 5. The pictures are taken with the 20× objective. Inserts show selected BKV-infected cells.
To investigate the effects of CMX001 on BKV gene expression at the single-cell level, we performed immunofluorescence for the early LT-ag and the late agno and VP1 at 72 hpi. We found that the number and intensity of nuclear LT-ag signals clearly was reduced, but the decrease was even more pronounced for the late agno and VP1 expression (Fig. 3c). When immunofluorescence staining was performed at 48 hpi, LT-ag, agno, and VP1 expression was even more affected, indicating a weak increase in BKV protein expression in the treated cells from 48 to 72 hpi (data not shown). Of note, the nuclear VP1 staining in CMX001-treated cells was weak and dispersed and devoid of the strong VP1-stained inclusions characteristic of untreated BKV-infected cells. Immunofluorescence also revealed some refractory cells in the CMX001-treated culture expressing agno at levels comparable to those of untreated cells even with CMX001 concentrations up to 2.5 μM (Fig. 1b). We also noted a decrease in nuclear DNA fluorescence with increasing CMX001 concentrations, suggesting a decrease in total nuclear DNA consisting of BKV and host DNA, which was further addressed by BrdU incorporation (see below). We concluded that CMX001 significantly reduces late protein expression but also inhibits early protein expression at later time points after BKV genome replication had occurred.
Kinetics of CMX001 inhibition.
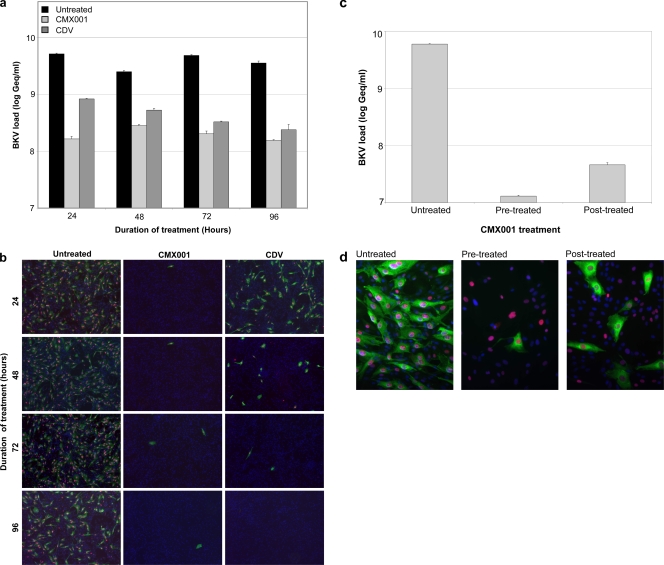
To examine the kinetics of CMX001 inhibition on BKV replication, RPTECs were treated after the 2-h infection for 22, 46, 70, or 94 h, and BKV loads were determined in the supernatants at 96 hpi. At the indicated times, the supernatant was harvested, the cells were washed once, and new complete medium was added. At 96 hpi, BKV loads were measured in the supernatants. As shown, CMX001 treatment at 0.31 μM for 22 h was enough to reduce the BKV load at 96 hpi by approximately 90% (Fig. 4a). Longer exposure times had only a marginal effect. Under these conditions, CDV treatment at 143 μM (40 μg/ml) (1) for at least 46 h was needed to reduce the extracellular BKV load to this level. To investigate whether or not the observed reductions in BKV load corresponded to a reduced number of infectious units, the supernatant harvested at the different time points were 10-fold diluted and seeded on new RPTECs. Three days postinfection, cells were fixed and immunofluorescence staining with antibodies against LT-ag and agno was performed. The results demonstrated that little infectious virus was detectable after treating cells with CMX001 for only 22 h, while CDV treatment for 70 h was needed to obtain a similar result (Fig. 4b). Next, we examined if CMX001 pretreatment affected BKV replication. RPTECs were treated for 24 h and washed before and after 2 h of BKV inoculation and also at 24 hpi. We found that pretreatment reduced the extracellular viral load at 72 hpi by more than 99% (Fig. 4c). Treatment with CMX001 after the 2-h virus inoculation for 24 h also reduced BKV loads at 72 hpi, but not as effectively as pretreatment. These observations were confirmed by immunofluorescence staining, revealing only a few agno-expressing cells in the pretreated wells compared to treatment at 24 hpi or to untreated wells (Fig. 4d). We concluded that CMX001 has a more rapid and enduring inhibitory effect than CDV and can prevent the completion of the BKV life cycle even if administered for only 24 h before or after BKV infection.
FIG. 4.
Kinetics of CMX001 at 0.31 μM treatment of BKV-infected RPTECs. (a) Extracellular BKV load. Cells were infected for 2 h, and CMX001 was added for 22, 46, 70, or 94 h, respectively. At the indicated times, supernatant was removed, cells were washed, and new medium added. At 96 hpi all supernatants were harvested and qPCR was performed. Data are presented as BKV load in log Geq/ml. Determinations were in triplicate. The mean values are shown, and the error bars represent standard deviations. (b) Infection and indirect immunofluorescence. The supernatant collected 96 hpi from the cells described above where diluted 1:10 and seeded on new RPTEC cells. At 72 hpi cells were methanol fixed, and immunofluorescence staining with polyclonal rabbit anti-agno serum (green) and the SV40 LT-ag monoclonal Pab416 was performed (red). Cell nuclei (blue) were stained with Drac 5. The pictures were taken with the 10× objective. (c) Extracellular BKV load. CMX001 was added 24 h before infection was indicated. Before infection, all cells were washed once with complete medium. BKV was added for 2 h and then removed, and cells were washed once with medium. Where indicated, previously untreated cells now were treated with CMX001 for 24 h. After 24 h, all supernatants were removed and cells were washed once more before complete medium was given for 48 h. Supernatants were harvested 72 hpi, and BKV loads were measured by qPCR. Determinations were in triplicate. The mean values are shown, and the error bars represent standard deviations. (d) Indirect immunofluorescence of BKV-infected RPTECs treated as outlined above. The cells were methanol fixed and stained using the primary antibodies polyclonal rabbit anti-agno serum (green) for the visualization of the viral late gene protein agno and the SV40 LT-ag monoclonal Pab416 for the visualization of viral early protein LT-ag (red). Cell nuclei (blue) were stained with Drac 5. The pictures were taken with a 20× objective.
Effects of CMX001 on RPTECs.
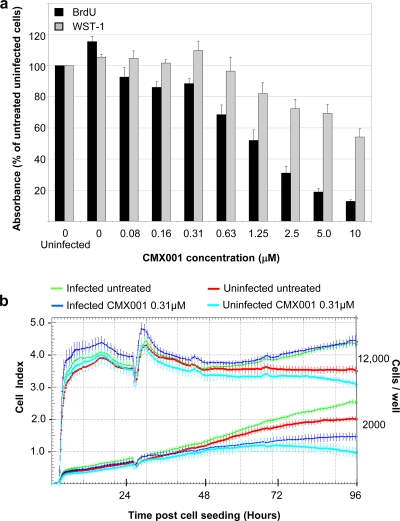
Phase-contrast microscopy did not reveal any crude signs of impaired host cell viability during the 3-day exposure to CMX001 at 0.31 μM, while a certain reduction was seen at concentrations around 10 μM. To use more-sensitive assays, we investigated host cell DNA replication and metabolic activity using BrdU incorporation and WST-1 assays in uninfected and infected RPTECs. We found that CMX001 reduced both DNA replication and the metabolic activity of infected RPTECs in a concentration-dependent manner (Fig. 5a). Of note, CMX001 at 0.31 μM, the EC90 of BKV replication, induced a 25% reduction in BrdU incorporation but no significantly altered metabolic activity. As shown in Fig. 5, at a CMX001 concentration of 10 μM we observed a 50% reduction in WST-1 activity, but BrdU incorporation was reduced by approximately 90%.
FIG. 5.
Influence of CMX001 on DNA replication, metabolic activity, cell adhesion, and the proliferation of uninfected and BKV-infected RPTECs. (a) Cellular DNA replication was examined with a cell proliferation enzyme-linked immunosorbent assay (ELISA) monitoring BrdU incorporation, and metabolic activity was examined with cell proliferation reagent WST-1 to measure WST-1 cleavage. Medium with indicated CMX001 concentrations was added 2 hpi, and absorbance was measured 72 hpi. Absorbance for untreated uninfected cells was set as 100%. Determinations were based on measurements of quintuplicates (5 wells). The mean values are shown, and the error bars represent standard deviations. (b) For a dynamic monitoring of cell adhesion and the proliferation of RPTECs, the XCELLigence system was used. RPTECs at a density of 2,000 and 12,000 cells/well were seeded on E plates. Twenty-seven hours after seeding, 150 μl of the medium in each well (a total of 200 μl) was replaced with fresh medium with or without purified BKV-Dunlop (MOI, 5) and with or without CMX001 (total concentration of 0.31 μM), and the cells were left until 96 h after cell seeding.
To investigate the influence of CMX001 at 0.31 μM on the proliferation of uninfected and BKV-infected RPTECs in real time, we measured the impedance in arbitrary cell index units using the xCelligence system. Cells were at two different densities: one that permitted exponential growth up to 72 h (2,000 cells/well, bottom) and one at subconfluency entering confluence within the first 24 h after seeding (12,000 cells/well, top). One day postseeding the medium was replaced, and four conditions were examined: (i) uninfected and untreated, (ii) uninfected but CMX001 treated, (iii) BKV infected but untreated, or (iv) BKV infected and CMX001 treated. The cell index was measured in 30-min intervals up to 96 h. The data showed that BKV infection increased cell proliferation in exponentially growing and in subconfluent cell cultures (Fig. 5b). In exponentially growing cells, CMX001 reduced the rate of RPTEC proliferation by approximately 25% in uninfected cells and by approximately 35% in BKV-infected cells at 48 h postexposure (72 h after seeding). In subconfluent cells, CMX001 had only a minimal inhibitory effect on infected and uninfected cells alike. We concluded that CMX001 at an EC90 of 0.31 μM had a certain inhibitory effect on RPTEC proliferation that was inversely proportional to cell density but did not appear to be toxic at this concentration.
To determine the selectivity index, we modeled the concentration-dependent effect of CMX001 on the increase in supernatant BKV loads as well as on the BrdU incorporation. The results were fitted to an exponential decay function (see Fig. S1a in the supplemental material), where the EC90 was between 0.16 μM (SD, 0.02) and 0.31 μM (SD, 0.04). Similarly, the effect of CMX001 on BrdU incorporation was fitted (see Fig. S2 in the supplemental material), indicating a 90% cytotoxic concentration (CC90) of 8.86 μM (SD, 1.6). The results suggested that the corresponding selectivity index (SI) for CMX001 SI90 (CC90/EC90) could be estimated as being between 28 and 55.
DISCUSSION
Antiviral drugs with higher efficacy and specificity are needed to improve current outcomes of BKV-mediated nephropathy after kidney transplantation and hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation (31). In this study, we characterized the lipid conjugate 1-O-hexadecyloxypropyl cidofovir (CMX001) regarding the inhibition of BKV replication in human primary proximal tubular epithelial cells. Our results demonstrate that CMX001 at 0.31 μM given after infection was sufficient to reduce the extracellular progeny BKV load by 90% at 72 hpi. Our detailed investigation of the BKV life cycle indicated that CMX001 inhibition occurred at the level of BKV genome replication following the initial phase of early gene expression at 24 hpi. Compared to levels for untreated controls, the increase of intracellular BKV genomes was inhibited between 24 and 48 hpi. Moreover, the burst of late gene expression at 48 and 72 hpi was significantly reduced. In untreated cells, late gene expression results from the synergy of the LT-ag-mediated activation of late gene expression and the increased copy number of replicated viral genomes. Interestingly, CMX001 treatment led to the same alteration of nuclear VP1 architecture as that previously described for BKV-infected RPTECs exposed to leflunomide (2). Since both CMX001 and leflunomide impair efficient BKV genome replication, the data suggest that this altered replication architecture is not specific to leflunomide but partly results from slowed-down replication, possibly as a result of low nuclear genome copy numbers and hence reduced VP1 expression. CMX001 was active at about 400-times-lower concentrations than the EC90 for CDV in the same test system (CDV EC90 at 40 μg/ml, 143 μM; CMX001 EC90, 0.31 μM). The inhibitory activity of CMX001 was more immediate and enduring than that of CDV, requiring an exposure time of only 1 day, compared to 2 to 3 days for an EC90 of BKV progeny loads at 96 hpi, and it could be conferred by a pretreatment of 24 h prior to infection. This difference in inhibitory kinetics also was apparent in infectious units when seeding diluted supernatants onto new RPTECs and is likely to result from higher intracellular levels of active antiviral following the more-rapid uptake of the lipid conjugate (23). When CMX001 was added at 24 h before infection, a more-than 99% inhibition in extracellular BKV DNA was found. Taken together, the data indicate a significantly enhanced BKV-inhibitory potency of CMX001 compared to that of the parent compound, CDV.
Randhawa and colleagues reported the increased potency of lipid derivatives of CDV on BKV replication in lung embryonic fibroblasts using the BKV Gardner strain (27). We have used the BKV-Dunlop strain, which is characterized by a rearranged NCCR and rapid replication kinetics similar to those of highly pathogenic variants found in kidney transplant patients (11, 22). Given the mechanism of CMX001 inhibition at the level of genome replication, it is no surprise that less well-replicating nonrearranged BKV also are inhibited, as is the polyomavirus JC (our unpublished data). Our previous work on CDV indicated that the inhibitory activity of CDV was closely linked to inhibitory effects of the host cells: CDV EC90 reduced the proliferation of RPTEC by 30 to 40% according to BrdU incorporation, while the overall metabolic activity was reduced by 20 to 30% (1). Given the increased potency of CMX001 on BKV replication, it was of considerable interest to monitor effects on the host cells. Our results indicated that the CMX001 EC90 had only a little effect on the overall metabolic activity of BKV-infected RPTECs and reduced the overall proliferative activity by up to 25%. As reported previously (1), BKV infection by itself increases the metabolic activity of RPTECs over that of uninfected cells and also increases the proliferative activity as measured by BrdU incorporation. Comparing RPTEC proliferation in a novel real-time proliferation assay, the stimulating effect of BKV infection on cell proliferation was clearly demonstrated on exponentially growing cultures as well as on cell cultures reaching confluence. In accordance with the BrdU results, this assay showed that CMX001 BKV EC90 reduced the proliferation rate of exponentially growing RPTECs by approximately 25%. This effect was less apparent at higher cell densities, suggesting that the specificity of CMX001 on BKV infection increased when confluent cells were infected. It can be envisaged that this feature also contributes to an overall reduced nephrotoxicity, especially when treating focal diseases such as PyVAN. There was no discernible effect of CMX001 at 0.31 μM on early gene expression at 24 hpi, but we consistently observed an approximately 25% reduction in LT-ag expression at 48 and 72 hpi, i.e., at a time point after viral genome replication. Since LT-ag is known to activate the proliferative state of the host cell and the recruitment of building blocks for virus replication, it is possible that the CMX001-mediated inhibition of viral genome replication reduced LT-ag expression at 48 hpi due to a limited gene dosage effect and contributed to the slight decrease in the overall proliferative activity of CMX001 at 0.31 μM. Higher concentrations of CMX001 of up to 10 μM were needed for a 90% inhibition of cell proliferation (see below). CMX001 pretreatment for 24 h also was effective to reduce BKV progeny loads, while more than 48 h were required for CDV. Although the reasons for the more-pronounced effect of CMX001 than that of CDV is not clear, we suspect that more-rapid uptake and/or equilibration with intracellular nucleotide pools may be involved, which might contribute to an effective antiviral state without excessive toxicity.
CMX001 was found to inhibit BKV replication in human embryonic lung fibroblasts cells (WI-38), with a more-than 800-fold-increased EC50 of 0.13 μM, whereas 115.1 μM was observed for CDV (27). These results were obtained by determining the intracellular BKV levels of cells harvested 7 days after infection and normalizing values to the host cell load using a housekeeping gene for the CC50, and an SI50 of 113 was indicated. Our results aimed at determining the EC90 parameters for the 72 h BKV life cycle in RPTECs based on a detailed infection model of polyomavirus-associated nephropathy in kidney transplants, where these parameters have indicated that an EC90 is needed for the contraction of the virus pool and hence the clearance of viremia and viruria, by 3 and 10 weeks, respectively (10). Since BrdU incorporation at a CMX001 concentration of 10 μM was reduced to approximately 10% of what was found in untreated infected cells as the CC90, we could estimate the SI90 as being 32.3. This SI90 must be regarded as very favorable for preclinical and clinical studies and extend earlier observations to a clinically relevant host cell model of primary human renal proximal tubular epithelial cells.
Taken together, we conclude that CMX001-like CDV inhibits BKV replication in primary human RPTECs downstream of initial LT-ag expression at the level of viral genome replication. Although polyomavirus replication is dependent on host cell DNA polymerase function, the specificity of CMX001 for BKV replication may result from an enhanced susceptibility of infected cells through their LT-ag-mediated activation and preferential recruitment of the host cell replication machinery to the site of viral genome replication. The lipid modification causes a more-rapid and enduring antiviral effect of CMX001 at an approximately 400-fold-lower concentration than that for CDV and an estimated SI90 of 28 to 55. Taken together with the oral bioavailability and the lack of nephrotoxicity from CMX001 observed to date in preclinical and clinical studies (18), our results suggest that the antiviral potential of CMX001 should be further explored in clinical studies of BKV-mediated diseases.
Supplementary Material
Acknowledgments
We thank Bettina Aasnæs at the Department of Microbiology and Infection Control at the University Hospital of North Norway for excellent technical assistance and Severine Louvel, InPheno AG Basel, Switzerland, for helping with fitting the EC90 and CC90 results.
This work was supported by an institutional grant of the University of Basel to H.H.H., by Extrafunds from the Norwegian Foundation for Health and Rehabilitation to E.B., and by an unrestricted research grant from Chimerix Inc. to H.H.H.
The authors declare that there is no conflict of interest regarding the role of the sponsors for this research.
Footnotes
Published ahead of print on 16 August 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Bernhoff, E., T. J. Gutteberg, K. Sandvik, H. H. Hirsch, and C. H. Rinaldo. 2008. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am. J. Transplant. 8:1413-1422. [DOI] [PubMed] [Google Scholar]
- 2.Bernhoff, E., G. D. Tylden, L. J. Kjerpeseth, T. J. Gutteberg, H. H. Hirsch, and C. H. Rinaldo. 2010. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J. Virol. 84:2150-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binet, I., V. Nickeleit, H. H. Hirsch, O. Prince, P. Dalquen, F. Gudat, M. J. Mihatsch, and G. Thiel. 1999. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation 67:918-922. [DOI] [PubMed] [Google Scholar]
- 4.Binggeli, S., A. Egli, S. Schaub, I. Binet, M. Mayr, J. Steiger, and H. H. Hirsch. 2007. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am. J. Transplant. 7:1131-1139. [DOI] [PubMed] [Google Scholar]
- 5.Cole, C. N. 1996. Polyomavirinae: the viruses and their replication, p. 917-945. In D. M. K. Bernard, N. Fields, and Peter M. Howley (ed.), Fundamental virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 6.Comoli, P., A. Azzi, R. Maccario, S. Basso, G. Botti, G. Basile, I. Fontana, M. Labirio, A. Cometa, F. Poli, F. Perfumo, F. Locatelli, and F. Ginevri. 2004. Polyomavirus BK-specific immunity after kidney transplantation. Transplantation 78:1229-1232. [DOI] [PubMed] [Google Scholar]
- 7.Dropulic, L. K., and R. J. Jones. 2008. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 41:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egli, A., L. Infanti, A. Dumoulin, A. Buser, J. Samaridis, C. Stebler, R. Gosert, and H. H. Hirsch. 2009. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 199:837-846. [DOI] [PubMed] [Google Scholar]
- 9.Farasati, N. A., R. Shapiro, A. Vats, and P. Randhawa. 2005. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation 79:116-118. [DOI] [PubMed] [Google Scholar]
- 10.Funk, G. A., R. Gosert, P. Comoli, F. Ginevri, and H. H. Hirsch. 2008. Polyomavirus BK replication dynamics in vivo and in silico to predict cytopathology and viral clearance in kidney transplants. Am. J. Transplant. 8:2368-2377. [DOI] [PubMed] [Google Scholar]
- 11.Gosert, R., C. H. Rinaldo, G. A. Funk, A. Egli, E. Ramos, C. B. Drachenberg, and H. H. Hirsch. 2008. Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J. Exp. Med. 205:841-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardinger, K. L., M. J. Koch, D. J. Bohl, G. A. Storch, and D. C. Brennan. 2010. BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am. J. Transplant. 10:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch, H. H. 2005. BK virus: opportunity makes a pathogen. Clin. Infect. Dis. 41:354-360. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch, H. H. 2010. Polyoma and papilloma virus infections after hematopoietic stem cell or solid organ transplantation, p. 465-482. In P. Bowden, P. Ljungman, and D. R. Snydman (ed.), Transplant infections, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 15.Hirsch, H. H., D. C. Brennan, C. B. Drachenberg, F. Ginevri, J. Gordon, A. P. Limaye, M. J. Mihatsch, V. Nickeleit, E. Ramos, P. Randhawa, R. Shapiro, J. Steiger, M. Suthanthiran, and J. Trofe. 2005. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79:1277-1286. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch, H. H., M. Mohaupt, and T. Klimkait. 2001. Prospective monitoring of BK virus load after discontinuing sirolimus treatment in a renal transplant patient with BK virus nephropathy. J. Infect. Dis. 184:1494-1496. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch, H. H., and J. Steiger. 2003. Polyomavirus BK. Lancet Infect. Dis. 3:611-623. [DOI] [PubMed] [Google Scholar]
- 18.Hostetler, K. Y. 2009. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 82:A84-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuypers, D. R., B. Bammens, K. Claes, P. Evenepoel, E. Lerut, and Y. Vanrenterghem. 2009. A single-centre study of adjuvant cidofovir therapy for BK virus interstitial nephritis (BKVIN) in renal allograft recipients. J. Antimicrob. Chemother. 63:417-419. [DOI] [PubMed] [Google Scholar]
- 20.Kuypers, D. R., A. K. Vandooren, E. Lerut, P. Evenepoel, K. Claes, R. Snoeck, L. Naesens, and Y. Vanrenterghem. 2005. Adjuvant low-dose cidofovir therapy for BK polyomavirus interstitial nephritis in renal transplant recipients. Am. J. Transplant. 5:1997-2004. [DOI] [PubMed] [Google Scholar]
- 21.Low, J., H. D. Humes, M. Szczypka, and M. Imperiale. 2004. BKV and SV40 infection of human kidney tubular epithelial cells in vitro. Virology 323:182-188. [DOI] [PubMed] [Google Scholar]
- 22.Olsen, G. H., H. H. Hirsch, and C. H. Rinaldo. 2009. Functional analysis of polyomavirus BK non-coding control region quasispecies from kidney transplant recipients. J. Med. Virol. 81:1959-1967. [DOI] [PubMed] [Google Scholar]
- 23.Painter, G. R., and K. Y. Hostetler. 2004. Design and development of oral drugs for the prophylaxis and treatment of smallpox infection. Trends Biotechnol. 22:423-427. [DOI] [PubMed] [Google Scholar]
- 24.Polo, C., J. L. Perez, A. Mielnichuck, C. G. Fedele, J. Niubo, and A. Tenorio. 2004. Prevalence and patterns of polyomavirus urinary excretion in immunocompetent adults and children. Clin. Microbiol. Infect. 10:640-644. [DOI] [PubMed] [Google Scholar]
- 25.Ramos, E., C. B. Drachenberg, J. C. Papadimitriou, O. Hamze, J. C. Fink, D. K. Klassen, R. C. Drachenberg, A. Wiland, R. Wali, C. B. Cangro, E. Schweitzer, S. T. Bartlett, and M. R. Weir. 2002. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J. Am. Soc Nephrol. 13:2145-2151. [DOI] [PubMed] [Google Scholar]
- 26.Ramos, E., C. B. Drachenberg, R. Wali, and H. H. Hirsch. 2009. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation 87:621-630. [DOI] [PubMed] [Google Scholar]
- 27.Randhawa, P., N. A. Farasati, R. Shapiro, and K. Y. Hostetler. 2006. Ether lipid ester derivatives of cidofovir inhibit polyomavirus BK replication in vitro. Antimicrob. Agents Chemother. 50:1564-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randhawa, P. S., S. Finkelstein, V. Scantlebury, R. Shapiro, C. Vivas, M. Jordan, M. M. Picken, and A. J. Demetris. 1999. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation 67:103-109. [DOI] [PubMed] [Google Scholar]
- 29.Randhawa, P. S., K. Khaleel-Ur-Rehman, P. A. Swalsky, A. Vats, V. Scantlebury, R. Shapiro, and S. Finkelstein. 2002. DNA sequencing of viral capsid protein VP-1 region in patients with BK virus interstitial nephritis. Transplantation 73:1090-1094. [DOI] [PubMed] [Google Scholar]
- 30.Randhawa, P. S., A. Vats, D. Zygmunt, P. Swalsky, V. Scantlebury, R. Shapiro, and S. Finkelstein. 2002. Quantitation of viral DNA in renal allograft tissue from patients with BK virus nephropathy. Transplantation 74:485-488. [DOI] [PubMed] [Google Scholar]
- 31.Rinaldo, C. H., and H. H. Hirsch. 2007. Antivirals for the treatment of polyomavirus BK replication. Expert Rev. Anti Infect. Ther. 5:105-115. [DOI] [PubMed] [Google Scholar]
- 32.Rinaldo, C. H., T. Traavik, and A. Hey. 1998. The agnogene of the human polyomavirus BK is expressed. J. Virol. 72:6233-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seemayer, C. A., N. H. Seemayer, U. Durmuller, F. Gudat, S. Schaub, H. H. Hirsch, and M. J. Mihatsch. 2008. BK virus large T and VP-1 expression in infected human renal allografts. Nephrol. Dial Transplant. 23:3752-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.