Abstract
Patterns of HIV-1 protease inhibitor (PI) resistance-associated mutations (RAMs) and effects on PI susceptibility associated with the L76V mutation were studied in a large database. Of 20,501 sequences with ≥1 PI RAM, 3.2% contained L76V; L76V was alone in 0.04%. Common partner mutations included M46I, I54V, V82A, I84V, and L90M. L76V was associated with a 2- to 6-fold decrease in susceptibility to lopinavir, darunavir, amprenavir, and indinavir and a 7- to 8-fold increase in susceptibility to atazanavir and saquinavir.
Combination chemotherapy for treatment of HIV-1 infection is often composed of 2 nucleoside reverse transcriptase inhibitors (RTI) and a nonnucleoside RTI or PR inhibitor (PI) (28). The plasma concentration of most PIs is increased by coadministration of a low dose of ritonavir. Ritonavir-boosted PI-based (PI/r) regimens are very potent and are recommended for use in patients with or without prior antiretroviral treatment (ART) experience (28). In ART-naive individuals, the use of PI/r regimens raises the genetic barrier to resistance by increasing the inhibitory quotient such that multiple mutations are almost always required in order to confer a large enough decrease in susceptibility to allow the virus to replicate in the presence of drug. Remarkably, the majority of viruses present following failure of a PI/r-based, first-line regimen remain sensitive to all PIs (9, 21), even in the absence of the RTI backbone (3, 11, 30). In PI-experienced patients with some resistance-associated mutations (RAMs) already present, switching to a different PI/r with a nonoverlapping pattern of resistance can be as effective as initial therapy (22, 36). In these cases, knowledge of the impact of various resistance mutations on susceptibility and replication capacity can help guide the selection of the most active second- or third-line regimen, especially if options involving other classes of drugs (such as entry or integrase inhibitors) have been exhausted.
As experience with PI treatment increases, diversity of associated patterns of resistance grows, and as novel PIs are brought to market, “new” mutations are often reported among viruses present following treatment failure. The use of inconsistent methods between studies associating PR genotype with clinical response or in vitro susceptibility can result in confusing or even misleading interpretation guidelines. Treatment with lopinavir (1, 11, 12, 23, 26, 27), darunavir (19), or other PIs (5, 7, 29) can lead to selection of the L76V mutation. L76V is associated with reduced response to darunavir (13). Like other mutations in PR (8, 37) and reverse transcriptase (2, 4, 6, 16, 20, 24, 31, 33-35), L76V has also been reported to have the ability to increase susceptibility to some PIs, including saquinavir, atazanavir (5, 23), and tipranavir (15, 32).
(This work was first presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 3 to 6 February 2008, poster 854.)
We used a database containing protease inhibitor susceptibility and sequence information (generated using the PhenoSense and GeneSeq assays at Monogram Biosciences, South San Francisco, CA) to identify isolates containing at least 1 PI RAM, defined as L23I, L24I, D30N, V32A/I, M46I/L/V, I47A/V, G48A/M/V, I50L/V, I54A/L/M/S/T/V, L76V, V82A/C/F/G/L/M/S/T, I84A/C/V, N88S/T, or L90M. Sequences containing mixtures at PI RAM sites were included when calculating mutation frequency estimates but excluded for phenotypic profiling. Data derived from samples tested as part of a clinical trial were excluded from mutation frequency analysis to avoid potential influence of trial selection criteria. Specimens were submitted for routine phenotype/genotype resistance testing between 2000 and 2009; where multiple specimens from the same patient were identified, only one was retained in the analysis.
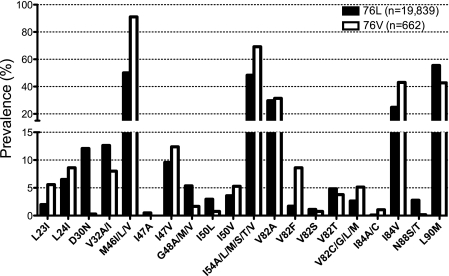
Of 20,501 clinical sequences with at least one PI RAM, 662 (3.2%) contained L76V. No trends in prevalence of L76V were observed over time (not shown). Only 9 samples (0.04%) were found with L76V as the sole PI RAM, while the percentages that also had 1, 2, or 3 or more PI RAMs were 0.16%, 0.51%, and 2.5%, respectively (see Table S1 in the supplemental material). When only one other PI RAM was present, it was most often M46I, -L, or -V (21 of 32 samples). With 2 other PI RAMs, common partner mutations included M46I/L/V and I84V (86 and 43 of 105 samples, respectively), and with 3 or more other PI RAMs, L76V was often seen in combinations that included M46I/L/V, I54A/L/M/V, V82A, I84V, and L90M. The majority (516 of 662, 78%) of L76V-containing sequences also had 3 or more PI RAMs (see Table S1 in the supplemental material). Among all L76V-containing samples, compared to those lacking L76V, combinations with L23I, M46I, V82C/F/G/M, and I84A/C were favored (ratios in prevalence over 2), while D30N, V32A, G48V, I50L, I54A, and N88S/T were disfavored (ratios in prevalence less than 0.5) (Fig. 1). These data are consistent with other recent studies that examined the prevalence of L76V (12, 26).
FIG. 1.
Prevalence of PI RAMs in samples with or without L76V. The percentages of samples containing at least one PI RAM (n = 20,501) that have the indicated mutation in the absence of changes at position 76 (76L) or in combination with L76V (76V) are shown. The vertical axis is split to show differences in prevalence for mutations above (M46I/L/V, I54A/L/M/S/T/V, V82A, and I84V) or below (all others) 15%.
We also examined mutation prevalence in 3 groups of samples defined by the clinically relevant lopinavir susceptibility thresholds: susceptible, fold change in 50% effective concentration (EC50) (FC) of <9-fold; intermediate, FC between 9- and 55-fold; resistant, FC of >55-fold. In general, frequencies of mutation association with L76V were similar to those observed across the entire range of lopinavir susceptibility, although overall the frequency of mutations associated with lopinavir resistance (such as V32I, M46I/L, I47V, G48V, I54V, V82A, I84V, and L90M) increased with decreasing lopinavir susceptibility, as expected (see Table S2 in the supplemental material). In some cases, the relative frequencies of a mutation in the absence or presence of L76V were different in the 3 categories defined by lopinavir susceptibility. For example, I54V prevalence in lopinavir-susceptible samples (FC < 9-fold) was only slightly different from that in resistant samples (FC > 55-fold) but the mutation was less frequent in L76V-containing samples (30%) than in L76V-lacking samples (56%) in the intermediate lopinavir susceptibility group (FC between 9- and 55-fold; Fisher's exact test, P < 0.0001) (see Table S2 in the supplemental material).
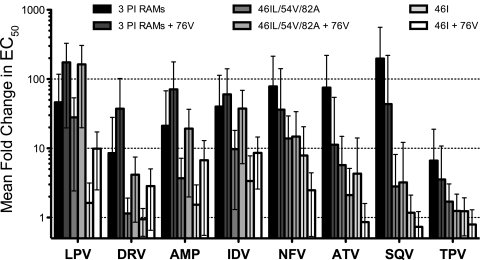
Six of the 9 samples identified with L76V as the only PI RAM did not contain a mixture with wild-type leucine; all but one of these remained susceptible to all PIs (FC < 2-fold). Interestingly, the exceptional sample, with reduced susceptibility to lopinavir (FC = 13.6-fold) and amprenavir (FC = 12.7-fold), also contained changes A431V and I437T in the NC/p1/p6 region of gag; these changes have been reported to occur in combination with L76V and to influence PI susceptibility by others (10, 18, 25, 26). In comparison, 4 of the PI-susceptible samples contained no gag cleavage site mutations (1 sample had no gag data available). Larger groups of samples with certain mutation patterns, with or without L76V, were identified, and the median FCs were compared to assess the impact of L76V on PI susceptibility (Fig. 2; see Table S3 in the supplemental material). Among samples with any 3 PI RAMs, median FCs ranged from ∼2-fold (tipranavir and darunavir) to over 20-fold (atazanavir, nelfinavir, lopinavir, and saquinavir) (Fig. 2); the variation in FC for individual samples was high, ranging from susceptible to highly resistant for all PIs except tipranavir and darunavir (see Table S3 in the supplemental material). In the group of samples with any 3 PI RAMs plus L76V, the median FC was increased by 2- to 5-fold for amprenavir, darunavir, indinavir, and lopinavir but was decreased by 7.4-fold for atazanavir and by 7.9-fold for saquinavir. The most common PI RAMs in these 2 groups were M46I, I54V, V82A, I84V, and L90M; M46I was more prevalent in the group that had L76V, while L90M was less prevalent (see Table S3 in the supplemental material). Among samples with M46I or -L, I54V, and V82A as the only PI RAMs, compared to the group with any 3 PI RAMs, lower levels of PI resistance were observed for all PIs except lopinavir. The effect of adding L76V was similar, with median FC increasing by 3- to 6-fold for amprenavir, darunavir, indinavir, and lopinavir, while decreasing by 2.6-fold for atazanavir. The simplest mutation pattern that we examined was M46I alone, which has little effect on median FC (<2-fold for all PIs except nelfinavir); however, in the group with M46I plus L76V, median FC was 7.5-fold for lopinavir, 7-fold for indinavir, 5-fold for amprenavir, and 2-fold for darunavir (increases of 2- to 6-fold compared to M46I alone); the median FCs for atazanavir, tipranavir, and saquinavir were below 1 (decreases of 1.5- to 2-fold). Of the 28 samples with M46I and L76V, 43% had lopinavir FCs over 9-fold (the lower clinical cutoff for ritonavir-boosted lopinavir), compared to only 2% in the absence of L76V (see Table S3 in the supplemental material).
FIG. 2.
PI susceptibility of clinical samples with or without L76V. The mean fold changes in EC50 (±standard deviations) for each PI are shown for 6 groups of samples: any 3 PI RAMs without L76V (n = 4,315); any 3 PI RAMs plus L76V (n = 338); M46I or -L, I54V, and V82A as the only PI RAMs (n = 237), M46I or -L, I54V, and V82A plus L76V (n = 86); M46I only (n = 108); or M46I plus L76V (n = 28). LPV, lopinavir; DRV, darunavir; AMP, amprenavir; IDV, indinavir; NFV, nelfinavir; ATV, atazanavir; SQV, saquinavir; TPV, tipranavir.
In conclusion, the L76V mutation was infrequently observed in this clinical database, with an overall prevalence of 3.2%. As the sole PI RAM, L76V was exceedingly rare (0.04%) among these samples, presumed to be from patients experiencing failure of triple therapy. However, studies of lopinavir-ritonavir monotherapy have reported a higher incidence of L76V at the time of monotherapy failure (11, 26, 27). Phenotypic data indicate that this mutation is not, in isolation, sufficient to confer resistance to lopinavir or other PIs. However, in combination with other PI RAMs, it contributes to a decrease in susceptibility to lopinavir, darunavir, indinavir, and amprenavir and an increase in susceptibility to both atazanavir and saquinavir; the effect on tipranavir susceptibility was modest (∼1.5-fold increase). The magnitudes of several of these effects (2- to 8-fold) strongly suggest that they are likely to be clinically significant, since they could easily cause the susceptibility of the virus to fall well above or below the clinical cutoff for one or more PIs. In comparison to the I47A mutation, previously reported to occur with few other PI RAMs (14), the magnitude of change in phenotypic susceptibility with regard to the L76V mutation on lopinavir is not as dramatic.
Taken together, these results support the inclusion of L76V in the list of mutations contributing to resistance to lopinavir, darunavir, amprenavir, and indinavir (17) and suggest that it should also be considered a suppressive mutation in algorithms for interpretation of resistance to atazanavir and saquinavir.
Supplementary Material
Acknowledgments
We thank Ahkter Molla and Barry Bernstein for their contributions to this work.
Development of the Monogram phenotype-genotype database was supported by a grant from the NIH/NIAID, SBIR-AT R44 AI057068.
Footnotes
Published ahead of print on 30 August 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Baras, A., K. Champenois, P. Choisy, Y. Yazdanpanah, and L. Bocket. 2009. Factors associated with the L76V protease mutation in HIV-1-infected patients with virological failure of lopinavir/ritonavir. Antivir. Ther. 14(Suppl. 1):A114. [Google Scholar]
- 2.Bazmi, H. Z., J. L. Hammond, S. C. Cavalcanti, C. K. Chu, R. F. Schinazi, and J. W. Mellors. 2000. In vitro selection of mutations in the human immunodeficiency virus type 1 reverse transcriptase that decrease susceptibility to (-)-β-d-dioxolane-guanosine and suppress resistance to 3′-azido-3′-deoxythymidine. Antimicrob. Agents Chemother. 44:1783-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bierman, W. F., M. A. van Agtmael, M. Nijhuis, S. A. Danner, and C. A. Boucher. 2009. HIV monotherapy with ritonavir-boosted protease inhibitors: a systematic review. AIDS 23:279-291. [DOI] [PubMed] [Google Scholar]
- 4.Boucher, C. A. B., N. Cammack, P. Schipper, R. Schuurman, P. Rouse, M. A. Wainberg, and J. M. Cameron. 1993. High-level resistance to (−) enantiomeric 2′-deoxy-3′-thiacytidine in vitro is due to one amino acid substitution in the catalytic site of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 37:2231-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun, P., H. Walter, D. Hoffmann, M. Däumer, R. Ehret, K. Korn, B. Thiele, T. Berg, M. Stürmer, F. Wiesmann, and R. Kaiser. 2007. Clinically relevant resensitization of protease inhibitors (PIs) saquinavir and atazanavir (ATV) by L76V mutation in multidrug-resistant HIV-1-infected patients. Antivir. Ther. 12:S142. [Google Scholar]
- 6.Byrnes, V. W., E. A. Emini, W. A. Schleif, J. H. Condra, C. L. Schneider, W. J. Long, J. A. Wolfgang, D. J. Graham, L. Gotlib, A. J. Schlabach, et al. 1994. Susceptibilities of human immunodeficiency virus type 1 enzyme and viral variants expressing multiple resistance-engendering amino acid substitutions to reserve transcriptase inhibitors. Antimicrob. Agents Chemother. 38:1404-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charpentier, C., F. Talla, A. Joko, A. Si-Mohamed, and L. Bélec. 2009. Prevalence of HIV-1 drug resistance in HIV-1-infected patients treated in Douala, Cameroon. Antivir. Ther. 14(Suppl. 1):A191. [Google Scholar]
- 8.Colonno, R., R. Rose, C. McLaren, A. Thiry, N. Parkin, and J. Friborg. 2004. Identification of I50L as the signature atazanavir (ATV)-resistance mutation in treatment-naive HIV-1-infected patients receiving ATV-containing regimens. J. Infect. Dis. 189:1802-1810. [DOI] [PubMed] [Google Scholar]
- 9.Cuzin, L., C. Allavena, P. Morlat, and P. Dellamonica. 2008. Boosted protease inhibitor-based or nonnucleoside reverse transcriptase-based HAART: is there a best choice for antiretroviral-naive HIV-1 infected patients? AIDS Rev. 10:205-211. [PubMed] [Google Scholar]
- 10.Dam, E., R. Quercia, B. Glass, D. Descamps, O. Launay, X. Duval, H. G. Krausslich, A. J. Hance, and F. Clavel. 2009. Gag mutations strongly contribute to HIV-1 resistance to protease inhibitors in highly drug-experienced patients besides compensating for fitness loss. PLoS Pathog. 5:e1000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaugerre, C., P. Flandre, M. L. Chaix, J. Ghosn, F. Raffi, P. Dellamonica, H. Jaeger, D. Shurmann, I. Cohen-Codar, P. N. Van, M. Norton, A. M. Taburet, J. F. Delfraissy, and C. Rouzioux. 2009. Protease inhibitor resistance analysis in the MONARK trial comparing first-line lopinavir-ritonavir monotherapy to lopinavir-ritonavir plus zidovudine and lamivudine triple therapy. Antimicrob. Agents Chemother. 53:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Mendoza, C., C. Garrido, A. Corral, N. Zahonero, and V. Soriano. 2008. Prevalence and impact of HIV-1 protease mutation L76V on lopinavir resistance. AIDS 22:311-313. [DOI] [PubMed] [Google Scholar]
- 13.de Meyer, S., T. Vangeneugden, B. van Baelen, E. de Paepe, H. van Marck, G. Picchio, E. Lefebvre, and M. P. de Bethune. 2008. Resistance profile of darunavir: combined 24-week results from the POWER trials. AIDS Res. Hum. Retroviruses 24:379-388. [DOI] [PubMed] [Google Scholar]
- 14.Friend, J., N. Parkin, T. Liegler, J. N. Martin, and S. G. Deeks. 2004. Isolated lopinavir resistance after virological rebound of a ritonavir/lopinavir-based regimen. AIDS 18:1965-1966. [DOI] [PubMed] [Google Scholar]
- 15.Hall, D. B., J. D. Baxter, J. M. Schapiro, M. Boucher, T. Clemens, and J. Scherer. 2008. Mutations 24I, 50L/V, 54L, and 76V, selected by other protease inhibitors, predict durable response to tipranavir in treatment experienced patients when two or more are present. Antivir. Ther. 13(Suppl. 3):A136. [Google Scholar]
- 16.Huang, W., A. Gamarnik, K. Limoli, C. J. Petropoulos, and J. M. Whitcomb. 2003. Amino acid substitutions at position 190 of human immunodeficiency virus type 1 reverse transcriptase increase susceptibility to delavirdine and impair virus replication. J. Virol. 77:1512-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, V. A., F. Brun-Vezinet, B. Clotet, H. F. Gunthard, D. R. Kuritzkes, D. Pillay, J. M. Schapiro, and D. D. Richman. 2009. Update of the drug resistance mutations in HIV-1: December 2009. Top. HIV Med. 17:138-145. [PubMed] [Google Scholar]
- 18.Knops, E., I. Kemper, E. Schulter, H. Pfister, R. Kaiser, and J. Verheyen. 2010. The evolution of protease mutation 76V is associated with protease mutation 46I and gag mutation 431V. AIDS 24:779-781. [DOI] [PubMed] [Google Scholar]
- 19.Lambert-Niclot, S., P. Flandre, A. Canestri, G. Peytavin, C. Blanc, R. Agher, C. Soulie, M. Wirden, C. Katlama, V. Calvez, and A. G. Marcelin. 2008. Factors associated with the selection of mutations conferring resistance to protease inhibitors (PIs) in PI-experienced patients displaying treatment failure on darunavir. Antimicrob. Agents Chemother. 52:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larder, B. A. 1992. 3′-Azido-3′-deoxythymidine resistance suppressed by a mutation conferring human immunodeficiency virus type 1 resistance to nonnucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 36:2664-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llibre, J. M. 2009. First-line boosted protease inhibitor-based regimens in treatment-naive HIV-1-infected patients—making a good thing better. AIDS Rev. 11:215-222. [PubMed] [Google Scholar]
- 22.Martinez-Cajas, J. L., and M. A. Wainberg. 2008. Antiretroviral therapy: optimal sequencing of therapy to avoid resistance. Drugs 68:43-72. [DOI] [PubMed] [Google Scholar]
- 23.Mueller, S. M., M. Daeumer, R. Kaiser, H. Walter, R. Colonno, and K. Korn. 2004. Susceptibility to saquinavir and atazanavir in highly protease inhibitor (PI) resistant HIV-1 is caused by lopinavir-induced drug resistance mutation L76V. Antivir. Ther. 9:S44. [Google Scholar]
- 24.Nijhuis, M., R. Schuurman, D. de Jong, R. van Leeuwen, J. Lange, S. Danner, W. Keulen, T. de Groot, and C. A. Boucher. 1997. Lamivudine-resistant human immunodeficiency virus type 1 variants (184V) require multiple amino acid changes to become co-resistant to zidovudine in vivo. J. Infect. Dis. 176:398-405. [DOI] [PubMed] [Google Scholar]
- 25.Nijhuis, M., N. M. van Maarseveen, S. Lastere, P. Schipper, E. Coakley, B. Glass, M. Rovenska, D. de Jong, C. Chappey, I. W. Goedegebuure, G. Heilek-Snyder, D. Dulude, N. Cammack, L. Brakier-Gingras, J. Konvalinka, N. Parkin, H. G. Krausslich, F. Brun-Vezinet, and C. A. Boucher. 2007. A novel substrate-based HIV-1 protease inhibitor drug resistance mechanism. PLoS Med. 4:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nijhuis, M., A. M. Wensing, W. F. Bierman, D. de Jong, R. Kagan, A. Fun, C. A. Jaspers, K. A. Schurink, M. A. van Agtmael, and C. A. Boucher. 2009. Failure of treatment with first-line lopinavir boosted with ritonavir can be explained by novel resistance pathways with protease mutation 76V. J. Infect. Dis. 200:698-709. [DOI] [PubMed] [Google Scholar]
- 27.Norton, M., C. Delaugere, G. Batot, J. F. Delfraissy, and C. Rouzioux. 2006. Drug resistance outcomes in a trial comparing lopinavir/ritonavir monotherapy to LPV/r + zidovudine/lamivudine (MONARK trial). Antivir. Ther. 11:S84. [Google Scholar]
- 28.Panel on Antiretroviral Guidelines for Adults and Adolescents. 2009. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, p. 1-161. Department of Health and Human Services, Washington, DC.
- 29.Poveda, E., C. de Mendoza, L. Martin-Carbonero, A. Corral, V. Briz, J. Gonzalez-Lahoz, and V. Soriano. 2007. Prevalence of darunavir resistance mutations in HIV-1-infected patients failing other protease inhibitors. J. Antimicrob. Chemother. 60:885-888. [DOI] [PubMed] [Google Scholar]
- 30.Sahali, S., M. L. Chaix, J. F. Delfraissy, and J. Ghosn. 2008. Ritonavir-boosted protease inhibitor monotherapy for the treatment of HIV-1 infection. AIDS Rev. 10:4-14. [PubMed] [Google Scholar]
- 31.Schmit, J. C., J. Martinez-Picado, L. Ruiz, C. Tural, K. Van Laethem, C. Cabrera, A. Ibanez, T. Puig, M. Witvrouw, J. Desmyter, E. De Clercq, B. Clotet, and A. M. Vandamme. 1998. Evolution of HIV drug resistance in zidovudine/zalcitabine- and zidovudine/didanosine-experienced patients receiving lamivudine-containing combination therapy. Antivir. Ther. 3:81-88. [Google Scholar]
- 32.Tartaglia, A., A. Saracino, L. Monno, C. Tinelli, and G. Angarano. 2009. Both a protective and a deleterious role for the L76V mutation. Antimicrob. Agents Chemother. 53:1724-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tisdale, M., S. D. Kemp, N. R. Parry, and B. A. Larder. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. U. S. A. 90:5653-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wainberg, M. A., M. D. Miller, Y. Quan, H. Salomon, A. S. Mulato, P. D. Lamy, N. A. Margot, K. E. Anton, and J. M. Cherrington. 1999. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir. Ther. 4:87-94. [DOI] [PubMed] [Google Scholar]
- 35.Whitcomb, J. M., N. T. Parkin, C. Chappey, N. S. Hellmann, and C. J. Petropoulos. 2003. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J. Infect. Dis. 188:992-1000. [DOI] [PubMed] [Google Scholar]
- 36.Wilson, L. E., and J. E. Gallant. 2009. HIV/AIDS: the management of treatment-experienced HIV-infected patients: new drugs and drug combinations. Clin. Infect. Dis. 48:214-221. [DOI] [PubMed] [Google Scholar]
- 37.Ziermann, R., K. Limoli, K. Das, E. Arnold, C. J. Petropoulos, and N. T. Parkin. 2000. A mutation in human immunodeficiency virus type 1 protease, N88S, that causes in vitro hypersensitivity to amprenavir. J. Virol. 74:4414-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.