Abstract
We characterized two new streptogramin A resistance genes from quinupristin-dalfopristin-resistant Enterococcus faecium JS79, which was selected from 79 E. faecium isolates lacking known genes encoding streptogramin A acetyltransferase. A 5,650-bp fragment of HindIII-digested plasmid DNA from E. faecium JS79 was cloned and sequenced. The fragment contained two open reading frames carrying resistance genes related to streptogramin A, namely, genes for an acetyltransferase and an ATP efflux pump. The first open reading frame comprised 648 bp encoding 216 amino acids with a predicted left-handed parallel β-helix domain structure; this new gene was designated vatG. The second open reading frame consisted of 1,575 bp encoding 525 amino acids with two predicted ATPase binding cassette transporters comprised of Walker A, Walker B, and LSSG motifs; this gene was designated vgaD. vgaD is located 65 bp upstream from vatG, was detected together with vatG in 12 of 179 quinupristin-dalfopristin-resistant E. faecium isolates, and was located on the same plasmid. Also, the 5.6-kb HindIII-digested fragment which was observed in JS79 was detected in nine vgaD- and vatG-containing E. faecium isolates by Southern hybridization. Therefore, it was expected that these two genes were strongly correlated with each other and that they may be composed of a transposon. Importantly, vgaD is the first identified ABC transporter conferring resistance to streptogramin A in E. faecium. Pulsed-field gel electrophoresis patterns and sequence types of vgaD- and vatG-containing E. faecium isolates differed for isolates from humans and nonhumans.
Quinupristin-dalfopristin (Q-D) (70:30), a mixture of streptogramins A and B, has been used to treat patients infected with methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium since the U.S. Food and Drug Admission approved the drug in 1997. Virginiamycin, another streptogramin mixture, had been used as a feed additive for livestock since the late 1970s in the United States, Europe, and Korea and has cross-resistance with Q-D.
Resistance to a synergistic mixture of streptogramins A and B was first identified in clinical S. aureus isolates in France in 1975. The resistance marker vga, conferring resistance to streptogramin A, was characterized in the early 1990s (7), and thereafter many new resistance mechanisms were discovered in staphylococci and E. faecium (3, 4, 6, 8. 25, 33), but resistance to mixtures of streptogramins A and B has been reported to be mediated by streptogramin A alone, irrespective of resistance to streptogramin B (8). Streptogramin A resistance is mediated by vat genes, which encode a streptogramin A acetyltransferase (SAT) that acetylates streptogramin A, and by vga genes, which encode an ATPase binding cassette transporter (ABC transporter).
Multiple alignment of nucleotide sequences of vatA, vatB, and vatD identified four conserved motifs, namely, I, II, III, and IV, and a PCR primer was designed for motifs III and IV (3). Nucleotide sequences of 144 to 147 bp were found from isolates containing vatA, vatB, vatC, vatD, and vatE (3, 6, 33). Most vat genes are located on plasmids and are distributed in staphylococci (vatA, vatB, and vatC) and E. faecium (vatD and vatE) (3, 6, 8, 25, 33), but vatF from Yersinia enterocolitica is located in chromosomal DNA (27). The vga genes, including vgaA, vgaAv, vgaB, and vgaC, encoding the ABC transporter, have been identified in Q-D-resistant staphylococci (4, 7, 14, 22). Recently, both vgaB and vatB were detected in Enterococcus gallinarum (18), but a Q-D resistance mechanism for the ABC transporter has yet to be identified in E. faecium.
E. faecium isolates from healthy humans, poultry, swine, retail meats, clinic patients, and wastewater have been shown to exhibit Q-D resistance and to carry the resistance genes vatD and/or vatE (2, 17, 20, 28, 32). Although the resistance genes encoding SAT have been established in Q-D-resistant E. faecium, some Q-D-resistant isolates that do not carry known resistance genes have been reported (9, 23, 24, 28). Therefore, to identify new determinants conferring resistance to streptogramin A, we selected a Q-D-resistant E. faecium isolate (JS79) lacking known streptogramin A resistance genes and characterized the genes responsible for the resistance.
MATERIALS AND METHODS
Bacterial strains.
Fecal samples from poultry (obtained in 2000) and from healthy humans, poultry, and swine (obtained in 2003) and samples of retail meats (obtained in 2003) were cultivated in 10 ml Enterococcosel broth (BD) for 48 h at 37°C, and one loop of the culture was streaked on Enterococcosel agar containing Q-D (MIC, 2 μg/ml) (Aventis, France) to isolate Q-D-resistant E. faecium. Q-D-resistant isolates were selected after antibiotic susceptibility was determined by disk diffusion and Etest according to Clinical and Laboratory Standards Institute guidelines. A total of 179 Q-D-resistant E. faecium isolates (MICs, ≥4 μg/ml) were used in this experiment (Table 1).
TABLE 1.
Prevalence of Q-D-resistant E. faecium isolates from swine, poultry, chicken meat, and healthy humans in 2000 and 2003
| Source | Isolation yr | No. of samples | No. (%) of Q- Da-resistant isolates | Q-D MIC range (μg/ml) |
|---|---|---|---|---|
| Swine | 2003 | 255 | 13 (5.1) | 8-32 |
| Poultry | 2000 | 396 | 60 (15.2) | 4-32 |
| 2003 | 431 | 82 (19.0) | ||
| Chicken meat | 2003 | 100 | 21 (21) | 4-32 |
| Healthy humans | 2003 | 328 | 3 (0.9) | 4-32 |
| Total | 2000-2003 | 1,510 | 179 (11.9) | 4-32 |
Q-D, quinupristin-dalfopristin.
Determination of genotypes.
The presence or absence of genes conferring resistance to vancomycin (vanA), macrolides (ermB and vgb), or streptogramin A (vatA, vatB, vatC, vatD, and vatE) in the 179 isolates was verified by PCR amplification using primers and PCR conditions as described previously (21, 28). To identify other streptogramin A resistance genes encoding SAT from Q-D-resistant E. faecium isolates lacking known vat genes, the strepto-M and strepto-N primer set was used (28). This primer set was designed based on conserved sequences of vatA, vatB, and vatD (Table 2). Products of 144 to 147 bp were obtained from vat gene-containing isolates with resistance to Q-D.
TABLE 2.
Primer sequences and PCR conditions
| Primer | Sequence | Product size (bp) | PCR conditions |
|---|---|---|---|
| Strepto-M | ATHATGAAYGGIAAYCAYMGIATG | 144-147 | Initial denaturing reaction at 95°C for 5 min; 35 cycles at 95°C for 30 s, 40°C for 2 min, and 72°C for 90 s; final extension reaction at 40°C for 4 min and 72°C for 10 min |
| Strpeto-N | ICCDATCCAIACRTCRTTICC | ||
| vatG1 | GTGGGAAAAGCATACACCT | 200 | Initial denaturing reaction at 94°C for 5 min; 30 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s; final extension reaction at 72°C for 10 min |
| vatG2 | TTGCAGGATTACCACCAAC | ||
| vgaD1 | CAACTGGAGCGAGCTGTTA | 201 | |
| vgaD2 | GACAGCCGGATAATCTTTTG |
To determine whether Q-D-resistant E. faecium isolates carried vatG or vgaD (the two new streptogramin A resistance genes identified in this study), the primer sets were designed based on sequences within vatG and vgaD (Fig. 1 and Table 2), and PCR amplification was performed (Table 2).
FIG. 1.
Schematic diagram depicting a restriction map of a 5.6-kb HindIII fragment of E. faecium JS79 DNA containing two streptogramin A resistance genes and a transposase gene. Bold arrows reflect the orientations of vgaD and vatG and the transposase (IS); black boxes reflect functional domains within the resistance gene translation product. There are two ABC transporter 2 domains, the N-terminal ATP binding domain and the C-terminal ATP binding domain, in the ABC transporter encoded by vgaD and an LβH domain in the streptogramin A acetyltransferase encoded by vatG. Filled arrows indicate primer sequences designed for the vgaD and vatG genes.
Transferability of Q-D and erythromycin resistance.
Seven of 12 vatG-containing E. faecium isolates showed resistance to erythromycin and were used as donors. E. faecium U201, which is susceptible to Q-D and erythromycin and resistant to ampicillin, was used as the recipient. The conjugation was performed using broth mating. One hundred microliters of donor and recipient (1:1) cultured overnight in M17 broth (Oxoid, England) was added in 2 ml fresh M17 broth and incubated at 37°C for 6 h. Transconjugants were selected on brain heart infusion (BHI) agar (BD) containing erythromycin (100 μg/ml) and ampicillin (100 μg/ml) after incubation at 37°C for 24 h.
Isolation of plasmid DNA and Southern hybridization.
Plasmid DNAs were extracted from Q-D-resistant E. faecium isolates using the modified alkaline lysis method of Ehrenfeld and Clewell (10) and then digested using the restriction enzymes BamHI, EcoRI, HindIII, PstI, SalI, and SphI (Takara, Japan). Southern hybridization was then performed using the ECL direct nucleic acid labeling and detection system (Amersham, GE Healthcare Life Science). The PCR products obtained from E. faecium JS79 using the strepto-M and strepto-N primers (144 to 147 bp) to search a new vat gene and using the vatG primer set (200 bp) to identify the vatG gene were used as probes, respectively.
PFGE and multilocus ST.
Pulsed-field gel electrophoresis (PFGE) patterns from vatG-containing E. faecium isolates were obtained as described previously (21). The fragments containing total DNA were digested with SmaI (Takara), and electrophoresis conditions were a constant 6 V/cm at 14°C and a pulse time of 1 to 20 s for 24 h using the CHEF-Mapper system (Bio-Rad). Cluster analysis of PFGE patterns was carried out using GelCompar software version 4.0b (Applied Maths, Belgium). The sequence type (ST) and allele number for vatG-containing E. faecium isolates were determined using the multilocus ST site, http://efaecium.mlst.net.
Determination of nucleotide sequences and analysis of the putative amino acids.
A 6-kb HindIII fragment of plasmid DNA from Q-D-resistant E. faecium isolate was sequenced by Macrogen Service Center (Macrogen, Seoul, South Korea) and analyzed using DNAStar software version 5.0 to find open reading frames (ORFs). Clustal W 2.0 (http://www.ebi.ac.uk/Tools/clustalw2/) was used to establish and analyze multiple alignments of the putative amino acid sequences. Conserved Domains from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/) and PROSITE (http://www.ca.expasy.org/prosite/) were used to search for motifs.
Nucleotide sequence accession number.
The sequence data presented here were submitted to GenBank under accession number GQ205627.
RESULTS
Cloning of a resistance gene encoding streptogramin A acetyltransferase.
A total of 179 Q-D-resistant E. faecium isolates (MIC, ≥4 μg/ml) were identified in samples from feces from healthy humans, swine, and poultry in 2000 and 2003 and from chicken meats in 2003. vatD was detected in 26 of the 179 isolates (14.5%), and vatE was detected in 74 of the 179 isolates (41.3%). PCR products were not obtained using primer sets which were specific for known vat genes, including vatA, vatB, vatC, vatD, and vatE, in 79 Q-D-resistant strains (44.1%). In 28 of these 79 Q-D-resistant strains lacking known vat genes as well as in 100 isolates containing vatD and vatE, however, PCR products of 144 to 147 bp were obtained using the strepto-M/strepto-N primer set. The PCR products from the 28 Q-D-resistant isolates were expected to carry a new vat gene sequence encoding SAT. To identify this new determinant conferring resistance to streptogramin A, we selected the highly Q-D-resistant E. faecium strain JS79 (MIC, 32 μg/ml), which was isolated from the stool of a healthy human.
Plasmid DNA isolated from JS79 was digested with BamHI, EcoRI, HindIII, PstI, SalI, and SphI and subjected to 1% agarose gel electrophoresis followed by Southern blot analysis using as a probe the 144- to 147-bp PCR amplification product which was obtained from JS79 using the strepto-M and strepto-N primers. A 6-kb HindIII fragment carrying an acetyltransferase gene was cloned into the HindIII site of pUC18. The nucleotide sequence of the cloned HindIII fragment was determined and consisted of 5,650 bp with 36.6% GC content. The fragment contained three putative ORFs, as determined using DNAStar version 5.0 and the NCBI ORF finder programs. Two ORFs were identified as genes conferring resistance to streptogramin A (encoding SAT and ABC transporter proteins), and the other ORF, containing a transposase insertion sequence, was partially sequenced (Fig. 1).
Characterization of two new ORFs conferring resistance to streptogramin A.
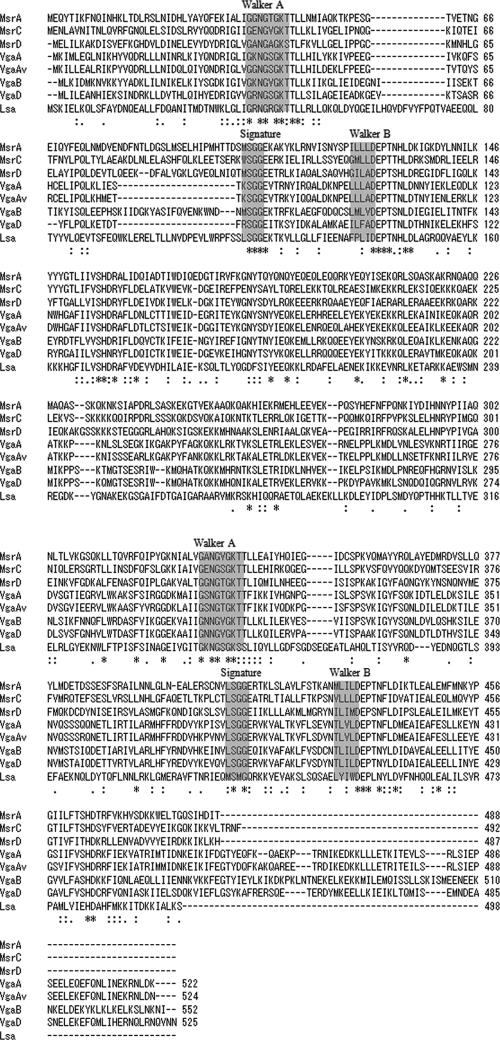
Of the three ORFs identified in the 5,650-bp HindIII fragment of E. faecium JS79 DNA, the first consisted of 1,575 bp (from nucleotide [nt] 1394 to 2968 relative to the 5′ HindIII site) and encoded 525 amino acid residues; the GC content was 36.9%. The ORF contained two ABC transporter type 2 domains (residues 4 to 175 and 271 to 479) as determined via PROSITE and Conserved Domains from the NCBI programs (Fig. 2). Each of these domains contained the characteristic ATP binding cassette comprised of a highly conserved ATP binding cassette (ABC), a typical phosphate binding loop (Walker A motif, P loop), a magnesium binding site (Walker B motif) (29), and a signature conserved motif (LSGG motif, C loop) (Fig. 2). The Walker A motif sequence was A/G-X2-G-X-G-K-S/T, the Walker B motif sequence was hhhhD (h represents hydrophobic residues), and the signature motif sequence was LSGG (Fig. 2). This ORF was designated vgaD. The calculated molecular mass of VgaD was 60.5 kDa, and the calculated isoelectric point was 7.58. In a multiple alignment of amino acid sequences related to ABC transporters, VgaD showed 47% identity with VgaA, 50% with VgaAv, 54% with VgaB, 33% with MsrA, 31% with MsrC, 34% with MsrD, and 22% with Lsa.
FIG. 2.
Multiple-sequence alignment of ABC transporters conferring resistance to streptogramin A/B or macrolides from staphylococci, enterococci, and streptococci. VgaA, accession no. AF186237, S. aureus; VgaAv, M90050, S. aureus; VgaB, U82085, S. aureus; VgaD, a new ABC transporter encoded by vgaD, GQ205627, E. faecium; MsrA, X52085, S. epidermidis; MsrC, AF313494, E. faecium; MsrD, AJ715499, Streptococcus pneumoniae; Lsa, AY225127, E. faecalis.
The second ORF was located 65 bp downstream of the 3′ end of vgaD. This ORF consisted of 648 bp (from nt 3037 to 3684 relative to the 5′ HindIII site) and encoded 216 residues with a calculated molecular mass of 23.9 kDa; the GC content was 36.9%. This ORF contained the common trait of acyl- and acetyltransferases, namely, the LβH domain of hexapeptide repeats containing the transferase signature motif L/I/V-G-X4 (residues 126 to 154 of the peptide sequences) (Fig. 1). This ORF was designated vatG. In a multiple alignment of amino acid sequences related to acetyltransferases, VatG showed 61.7% identity with VatA, 54.7% with VatB, 68.4% with VatC, 63.2% with VatD, 48.6% with VatE, and 55% with VatF.
Distribution of vatG and vgaD in Q-D-resistant E. faecium isolates.
To detect the presence of vatG and vgaD in the 179 Q-D-resistant E. faecium isolates, PCR primer pairs were designed to amplify sequences within vatG and vgaD (Table 2). PCR amplification products of vatG and vgaD were detected in 6.7% (12/179) of the Q-D-resistant isolates from the stool samples of healthy humans, swine, poultry and from chicken meat. All E. faecium isolates containing vatG also harbored vgaD, and Southern hybridization indicated that these genes were located on HindIII fragments of identical size in different plasmids from 10 isolates (Fig. 3).
FIG. 3.
Southern hybridization to identify vatG-containing E. faecium isolates from healthy humans, swine, poultry stool samples, and chicken meat. (A) Agarose gel electrophoresis of HindIII-digested plasmid DNA from E. faecium isolates. (B) Southern hybridization of the samples shown in panel A using a vatG PCR product as probe. M, 100-bp plus ladder. Isolates are as follows: lane 1, A15; lane 2, C19; lane 3, AS115; lane 4, CS098; lane 5, CS103; lane 6, CS106; lane 7, CS121; lane 8, GAV04; lane 9, GAV11 (negative control); lane 10, ES022; lane 11, JS79. ES, isolates from swine feces; A, C, AS, and CS, isolates from poultry feces; JS and KS, isolates from human feces; GAV, isolates from chicken meat.
Relationship of Q-D resistance and erythromycin resistance.
In addition, the gene ermB was detected in 9 of the 12 E. faecium isolates containing vatG and vgaD from stool samples from healthy humans and poultry, and the gene vanA was detected in 4 of the 12 isolates from chicken feces in 2001 and from chicken meat in 2003 (Table 3). vgb was not detected in these 12 isolates.
TABLE 3.
Characterization of E. faecium isolates containing vgaD and vatGa
| Isolate(s) | Source | Yr | Resistance gene(s) | MIC (μg/ml) |
MLST profile | ST | ||
|---|---|---|---|---|---|---|---|---|
| Q-D | ERY | VAN | ||||||
| JS79, KS46 | Healthy humans | 2003 | ermB | 32 | 256 | <1 | 1-1-1-1-1-1-1 | 17 |
| ES022 | Swine | 2003 | 4 | 0.1 | <1 | 5-2-22-3-1-1-5 | ND | |
| A15, C19 | Poultry | 2000 | ermB, vanA | 8 | 256 | 256 | 9-2-1-6-1-1-1 | 26 |
| AS115 | Poultry | 2003 | ermB | 8 | 256 | <1 | 9-2-6-32-1-7-1 | ND |
| CS098, CS103, CS106 | Poultry | 2003 | ermB | 8 | 256 | <1 | 9-2-7-6-1-7-1 | 14 |
| CS121 | Poultry | 2003 | ermB | 32 | 256 | <1 | 5-2-6-6-1-1-1 | 12 |
| GAV04, GAV12 | Chicken meat | 2003 | vanA | 4 | <1 | 256 | 5-2-24-14-1-7-1 | 237 |
Abbreviations: Q-D, quinupristin-dalfopristin; ERY, erythromycin; VAN, vancomycin; MLST, multilocus sequence type; ST, sequence type; ND, not determined; ES, isolates from swine feces; A, C, AS, and CS, isolates from poultry feces; JS and KS, isolates from human feces; GAV, isolates from chicken meat.
The transfer frequencies of streptogramin A resistance genes were 10−6 to 10−8 per donor using broth mating conjugation. Although transconjugants were selected on BHI agar containing ampicillin and erythromycin as selection markers, erythromycin resistance of donor isolates was always transferred together with Q-D resistance into the recipient strain, all transconjugants carried ermB and vatG genes on the same plasmid, and MICs of erythromycin (256 μg/ml) and Q-D (8 to 32 μg/ml) in transconjugants were the same as those in donor isolates (data not shown).
Molecular epidemiology of vgaD- and vatG-containing E. faecium isolates.
Among the 12 vgaD- and vatG-containing E. faecium isolates, three isolates (CS98, CS103, and CS106) and two isolates (A15 and C19) from the same farm and two isolates (GAV04 and GAV12) from chickens purchased in the same market were identical based on PFGE and ST analyses, respectively (Table 3). The STs of two isolates from healthy humans, JS79 and KS46, were identical (ST17), and the PFGE patterns and the STs differed in healthy humans and nonhumans (Table 3). Among them, the allele copy numbers of three isolates (AS115, ES022, and KS46) were not determined.
DISCUSSION
In staphylococci and enterococci, Q-D resistance is conferred by the SAT and the ABC transporter molecules. In the early 1990s, Q-D resistance genes were identified using a shotgun cloning method with the Q-D-susceptible strain Escherichia coli DB10 or S. aureus RN4220 as the host, and Q-D-resistant transformants were directly selected on Q-D-containing selection media. Since then, however, new Q-D resistance genes, vatC and vatE, were identified by Southern hybridization using the PCR product of 144 to 147 bp obtained with the primer set of strepto-M and strepto-N as a probe (28), and here we identified a new vat gene, vatG, from Q-D-resistant E. faecium JS79 (which lacks known vat genes) using this primer set (28). Although the seven vat genes from Q-D-resistant staphylococci, E. faecium, and Y. enterocolitica share 45 to 71% identity and the vatF gene is intrinsic, motifs I and II were conserved as shown by multiple-sequence alignment of seven known vat genes. Therefore, new vat genes from Q-D-resistant isolates lacking known vat genes may be identified on an ongoing basis using nucleotide sequences of motifs II and III as queries.
ABC transporters are widely distributed in humans as well as in other eukaryotes and prokaryotes. Many ABC transporters contain two hydrophobic membrane-spanning domains and two hydrophilic ATP binding domains. VgaD, however, contains only two distinct ATP binding domains, as do MsrA and VgaB (4, 26). In a multiple sequence alignment of streptogramins A and B and macrolide ABC transporters, the streptogramin A ABC transporters VgaA, VgaB, and VgaD exhibit 44 to 50% identity with each other (not including VgaAv); 31 to 37% identity with MsrA, MsrC, and MsrD; and 22 to 24% identity with Lsa (Fig. 2). We searched for the positions of the N-terminal ATP binding domains (N domains) and C-terminal ATP binding domains (C domains) in the amino acid sequences of MsrA, MsrC, MsrD, VgaA, VgaAv, VgaB, VgaD, and Lsa via PROSITE. In all the proteins except Lsa, the N domain was shorter than the C domain, whereas in Lsa the C domain was the shorter of the two (Fig. 2). Multiple alignments of those sequences excluding Lsa revealed 20 to 80% identity in all of the N domains and the C domains, but the sequence identity between the Msr and Vga C domains (31 to 80%) was, on average, higher than that between the N-domains (33 to 51%). The identity between N domain and C domain in each ABC transporter besides VgaB was 20 to 26% and was lower in different ABC transporters; therefore, ABC transporters such as Msr and Vga may be composed of two domains, the N domain and the C domain, with different ancestries.
Many transposons contain resistance genes with various transposases, which were found in plasmid DNA of staphylococci. In previous reports such findings were observed. For instance, a vgaA variant streptogramin A resistance gene containing a transposon sequence (Tn5406) was previously isolated from S. aureus BM3327, and the structure of Tn5406 is similar to that of Tn554 (13). In this study, such transposon structure was also identified in the DNA plasmid of E. faecium JS79. vgaD was detected in only 12 vatG-containing Q-D-resistant E. faecium isolates and was 65 bp apart from vatG on the same plasmid. However, vgaD was not detected in the remaining Q-D-resistant E. faecium isolates. Nine E. faecium isolates containing vgaD and vatG yielded HindIII fragments identical to that of JS79 (Fig. 3), which included transposase sequences as well as vgaD and vatG (Fig. 1). Although further sequencing for upstream of vgaD and downstream of the insertion sequence is required, such findings in this study suggest that the fragment containing vgaD and vatG carries the structure of the transposon.
Most vat genes isolated from staphylococci and E. faecium are linked with other genes, such as vga-vatA-vgb, vgaB-vatB, and vgbB-vatC in staphylococci (4-6) and ermB-vatD and ermB-vatE in E. faecium (12, 19). It has been reported that transcriptional regulation of vat genes is not controlled by sequences upstream of the start codon (6, 32) but that vat genes are cotranscribed and cotransferred to other strains along with other genes, such as vga, vgaB, vgb, vgbB, or ermB (17, 20, 28). A few researchers have speculated about the linkage between resistance genes in the coselection or persistence of antibiotic resistance. Macrolide resistance is widely distributed in many isolates, especially in vancomycin-resistant E. faecium. The ermB gene is closely linked together with vanA on the same plasmid, and cotransfer of vanA and ermB to other strains and a high frequency of vancomycin-resistant enterococci (VRE) result from the link with macrolide resistance (1, 11). Linkage with ermB and vatE using PCR and transference of the Q-D resistance gene to other strains were also reported for Q-D-resistant E. faecium isolates (30, 31). We found that both ermB and vatG were transferred using broth mating conjugation, and ermB and vatG were detected using PCR. Therefore, it was expected that vatG was more closely linked to ermB.
Many Q-D-resistant E. faecium isolates that do not contain any known streptogramin A resistance genes have been identified (14, 16, 23, 24, 32). In one study, no known streptogramin A resistance genes were detected in 29% of the Q-D-resistant isolates (MIC, 4 to 16 μg/ml) (28), and a clinical study from Korea reported that 10% of Q-D-resistant E. faecium isolates did not carry streptogramin A resistance genes (MIC, 4 μg/ml). Although vat-vgb and vgaB-vatB were isolated from E. faecium and E. gallinarum, respectively (14, 18), vat genes from E. faecium have not been detected in staphylococci (14), and vat genes from staphylococci are rarely found in enterococci (15). This suggests that genes encoding SAT are specific according to genus.
It has been reported that clonal complex 17 (CC17), including ST17, is widely disseminated in E. faecium isolates from hospitals throughout the world and carries mostly virulence marker esp genes. E. faecium JS79 was isolated from a healthy human and had ST17, but JS79 is susceptible to ampicillin and does not carry the esp gene (data not shown). The STs of two isolates, AS115 and ES022, were not determined, and we expect to find that the ST(s) of these two isolates is new.
Acknowledgments
This work was supported by a research grant from the Korea Food and Drug Administration of the Republic of Korea in 2006 (06042-ARM-127) and by the Korea National Institute of Health in 2007 (2007-N00299-00).
Footnotes
Published ahead of print on 16 August 2010.
REFERENCES
- 1.Aarestrup, F. M. 2000. Characterization of glycopeptides-resistant Enterococcus faecium (GRE) from broilers and pigs in Denmark: genetic evidence that persistence of GRE in pig herds is associated with coselection by resistance to macrolides. J. Clin. Microbiol. 38:2774-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarestrup, F. M., Y. Agerso, P. Gerner-Smidt, M. Madsen, and L. B. Jensen. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37:127-137. [DOI] [PubMed] [Google Scholar]
- 3.Allignet, J., and N. El Solh. 1995. Diversity among the gram-positive acetyltransferases inactivating streptogramin A and structurally related compounds and characterization of a new staphylococcal determinant, vatB. Antimicrob. Agents Chemother. 39:2027-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allignet, J., and N. El Solh. 1997. Characterization of a new staphylococcal gene, vgaB, encoding a putative ABC transporter conferring resistance to streptogramin A and related compounds. Gene 202:133-138. [DOI] [PubMed] [Google Scholar]
- 5.Allignet, J., and N. El Solh. 1999. Comparative analysis of staphylococcal plasmids carrying three streptogramin-resistance genes:vat-vgb-vga. Plasmid 42:134-138. [DOI] [PubMed] [Google Scholar]
- 6.Allignet, J., N. Liassine, and N. El Sohl. 1998. Characterization of a staphylococcal plasmid related to pUB110 and carrying two novel genes, vatC and vgbB, encoding resistance to streptogramins A and B and similar antibiotics. Antimicrob. Agents Chemother. 42:1794-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allignet, J., V. Loncle, and N. El Sohl. 1992. Sequence of a staphylococcal plasmid gene, vga, encoding a putative ATP-binding protein involved in resistance to virginiamycin A-like antibiotics. Gene 117:45-51. [DOI] [PubMed] [Google Scholar]
- 8.Allignet, J., V. Loncle, C. Simenel, M. Delepierre, and N. El Solh. 1993. Sequence of a staphylococcal gene, vat, encoding an acetyltransferase inactivating the A-type compounds of virginiamycin-like antibiotics. Gene 130:91-98. [DOI] [PubMed] [Google Scholar]
- 9.Donabedian, S. M., M. B. Perri, D. Vager, E. Hershberger, P. Malani, S. Simjee, J. Chow, E. N. Vergis, R. R. Muder, K. Gay, F. J. Angulo, P. Bartlett, and M. J. Zervos. 2006. Quinupristin-dalfopristin resistance in Enterococcus faecium isolates from humans, farm animals, and grocery store meat in the United States. J. Clin. Microbiol. 44:3361-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrenfeld, E. E., and D. B. Cleweell. 1987. Transfer functions of the Streptococcus faecalis plasmid pAD1 organization of plasmid DNA encoding response to sex pheromone. J. Bacteriol. 169:3473-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Migura, L., E. Liebana, and L. B. Jensen. 2007. Transposon characterization of vancomycin-resistant Enterococcus faecium (VREF) and dissemination of resistance associated with transferable plasmids. J. Antimicrob. Chemother. 60:263-268. [DOI] [PubMed] [Google Scholar]
- 12.Hammerum, A. M., S. E. Flannagan, D. B. Clewell, and L. B. Jensen. 2001. Indication of transposition of a mobile DNA element containing the vatD and ermB genes in Enterococcus faecium. Antimicrob. Agents Chemother. 45:3223-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haroche, J., J. Allignet, and N. El Solh. 2002. Tn5406, a new staphylococcal transposon conferring resistance to streptogramin A and related compounds, including dalfopristin. Antimicrob. Agents Chemother. 46:2337-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haroche, J., J. Allignet, C. Buchrieser, and N. El Solh. 2000. Characterization of a variant of vgaA conferring resistance to streptogramin A and related compounds. Antimicrob. Agents Chemother. 44:2271-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haroche, J., J. Allignet, S. Aubert, A. E. Van Den Bogaard, and N. El Solh. 2000. satG, conferring resistance to streptogramin A, is widely distributed in Enterococcus faecium strains but not in staphylococci. Antimicrob. Agents Chemother. 44:190-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes, J. R., D. D. Wagner, L. L. English, L. E. Carr, and S. W. Joseph. 2005. Distribution of streptogramin resistance determinants among Enterococcus faecium from a poultry production environment of the U.S.A. J. Antimicrob. Chemother. 55:123-126. [DOI] [PubMed] [Google Scholar]
- 17.Jackson, C. R., P. Fedorka-Cray, J. B. Barrett, L. M. Hiott, and T. A. Woodley. 2007. Prevalence of streptogramin resistance in enterococci from animals: identification of vatD from animal sources in the U.S.A. Int. J. Antimicrob. Agents 30:60-66. [DOI] [PubMed] [Google Scholar]
- 18.Jackson, C. R., P. J. Fedorka-Cray, L. M. Hiott, and T. A. Woodley. 2008. First report of vatB and vgaB from Enterococcus gallinarum in the U.S.A. Int. J. Antimicrob. Agents 31:175-187. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, L. B., A. M. Hammerum, and F. M. Aarestup. 2000. Linkage of vatE and ermB in streptogramin-resistant Enterococcus faecium isolates from Europe. Antimicrob. Agents Chemother. 44:2231-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen, L. B., A. M. Hammerum, F. Bager, and F. M. Aerestrup. 2002. Streptogramin resistance among Enterococcus faecium isolated from production animals in Denmark in 1997. Microb. Drug Resist. 8:369-374. [DOI] [PubMed] [Google Scholar]
- 21.Jung, Y.-H., Y. S. Lee, J. O. Ahn, H. R. Lee, J. K. Lee, J. I. Yoo, H.-S. Kwak, and B. S. Kim. 2006. Prevalence and genetic relatedness of vancomycin-resistant enterococci isolated from livestock and humans after ban of avoparcin in Korea. Infect. Chemother. 38:7-14. [Google Scholar]
- 22.Kadlec, K., and S. Schwarz. 2009. Novel ABC transporter gene, vagC, located on a multiresistance plasmid from a porcine methicillin-resistant Staphylococcus aureus ST398 strain. Antimicrob. Agents Chemother. 53:3589-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott, P. F., P. Cullen, S. K. Hubert, S. D. McDermott, M. Bartholomew, S. Simjee, and D. D. Wagner. 2005. Changes in antimicrobial susceptibility of native Enterococcus faecium in chickens fed virginiamycin. Appl. Environ. Microbiol. 71:4986-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh, W. S., K. S. Ko, J.-H. Song, M. Y. Lee, S. Perk, K. R. Peck, N. Y. Lee, C.-K. Kim, H. Lee, S.-W. Kim, J.-S. Yeom, H. K. Ki, and G.-J. Woo. 2005. High rate of resistance to quinupristin/dalfopristin in Enterococcus faecium clinical isolates from Korea. Antimicrob. Agents Chemother. 49:5176-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rende-Fournier, R., R. Leclercq, M. Galimand, J. Duval, and P. Courvalin. 1993. Identification of the satA gene encoding a streptogramin A acetyltransferase in Enterococcus faecium BM4145. Antimicrob. Agents Chemother. 37:2119-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross, J. I., E. A. Eady, J. H. Cove, W. J. Cunliffe, S. Baumberg, and J. C. Wootton. 1990. Inducible erythromycin resistance in staphylococci is encoded by a member of the ATP-binding transport super-gene family. Mol. Microbiol. 4:1207-1214. [DOI] [PubMed] [Google Scholar]
- 27.Seoane, A., and J. M. García Lobo. 2000. Identification of a streptogramin A acetyltransferase gene in the chromosome of Yersinia enterocolitica. Antimicrob. Agents Chemother. 44:905-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soltani, M., D. Beighton, J. Philpott-Howard, and N. Woodford. 2000. Mechanisms of resistance to quinupristin-dalfopristin among isolates of Enterococcus faecium from animals, raw meat, and hospital patients in Western Europe. Antimicrob. Agents Chemother. 44:433-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases, and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werner, G., B. Hildebrandt, I. Klare, and W. Witte. 2000. Linkage of determinants for streptogramin A, macrolide-lincosamide-streptogramin B, and chloramphenicol resistance on a conjugative plasmid in Enterococcus faecium and dissemination of this cluster among streptogramin-resistant enterococci. Int. J. Med. Microbiol. 290:543-548. [DOI] [PubMed] [Google Scholar]
- 31.Werner, G., I. Klare, and W. Witte. 2002. Molecular analysis of streptogramin resistance in enterococci. Int. J. Med. Microbiol. 292:81-94. [DOI] [PubMed] [Google Scholar]
- 32.Werner, G., I. Klare, H. Heier, K.-H. Hinz, G. Böhme, M. Wendt, and W. Witte. 2000. Quinupristin/dalfopristin enterococci of the satA (vatD) and satG (vatE) genotypes from different ecological origins in Germany. Microb. Drug Resist. 6:37-47. [DOI] [PubMed] [Google Scholar]
- 33.Werner, W., and W. Witte. 1999. Characterization of a new enterococcal gene, satG, encoding a putative acetyltransferase conferring resistance to streptogramin A compounds. Antimicrob. Agents Chemother. 43:1813-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]