Abstract
A mini-Tn5 insertion into a ciprofloxacin (CIP)-resistant mutant of Vibrio cholerae O1 revealed that overexpression of the vca0421 gene, which encodes a hypothetical protein, in the CIP-resistant mutant carrying a mutation in the quinolone resistance-determining region (QRDR) of the gyrA gene causes sensitization to CIP. We propose a new intrinsic mechanism of resistance to fluoroquinolones due to the inherently reduced expression of the vca0421 gene in V. cholerae O1.
Fluoroquinolones, which display potent antibacterial activity against Vibrio cholerae O1 and O139, have been used in the clinical treatment of cholera (13). However, increased therapeutic use of fluoroquinolones has resulted in the appearance of fluoroquinolone-resistant strains of V. cholerae O1 and O139 in clinical isolates from around the world (3, 9, 10).
In addition to V. cholerae O1 and O139, many other bacterial species have developed clinical resistance to fluoroquinolones. The molecular basis of this antibiotic resistance has been studied extensively (2). Most of the acquired resistance can be attributed to mutations in the genes encoding DNA gyrase or topoisomerase IV (Topo IV). Bacterial resistance to fluoroquinolones can also be conferred by increased expression of multidrug efflux pumps or reduced expression of outer membrane proteins, such as porins, resulting in reduced intracellular concentrations of antibiotics (5). DNA gyrase consists of GyrA and GyrB subunits, encoded by the gyrA and gyrB genes, respectively (1, 12). Topo IV is composed of ParC and ParE subunits, encoded by the parC and parE genes, respectively, the amino acid sequences of which are homologous to some degree with those of GyrA and GyrB, respectively (4, 11). The majority of the quinolone resistance mutations have been shown to map to a relatively small region at the N terminus of GyrA, corresponding to the 67th through the 106th amino acid residues in Escherichia coli K-12; this region is called the quinolone resistance-determining region (QRDR) (5, 14). Quinolone resistance mutations in the parC genes were also detected in the region corresponding to the QRDR of ParC (5).
In this study, we attempted to reveal a novel mechanism of V. cholerae O1 resistance to fluoroquinolones. Such data will be key to developing new antibiotics that are effective against fluoroquinolone-resistant V. cholerae O1 strains.
Isolation of ciprofloxacin (CIP)-resistant mutants of 569B was carried out as follows. A single colony of strain 569B was inoculated into 5 ml of Mueller-Hinton broth (MHB; Difco Laboratories, Detroit, MI), and 0.2-ml aliquots of the overnight culture were plated onto a Mueller-Hinton agar (MHA; Difco Laboratories, Detroit, MI) plate containing 0.008 μg of CIP per ml. A resistant colony (the first-step mutant) was selected. The emerging clone was purified on an MHA plate without the antimicrobial agent. The second-step (selective concentration of ciprofloxacin, 0.015 μg/ml) and third-step (selective concentration of ciprofloxacin, 0.25 μg/ml) mutants were obtained in the same manner from the first- and second-step mutants, respectively. A CIP-resistant mutant that grew on the selective medium containing CIP at 0.25 μg/ml was chosen (designated CIP0.25-1), and the MIC of CIP against the CIP0.25-1 strain was determined by the 2-fold agar dilution method recommended by the Japan Society of Chemotherapy (http://www.chemotherapy.or.jp/index.html). The MIC of CIP against the CIP0.25-1 strain was 250-fold higher than that against the wild-type strain (Table 1). As the CIP0.25-1 strain showed a high level of resistance to CIP, we investigated whether point mutations in the QRDRs of the gyrA and parC genes in the CIP-resistant mutant could be detected. Amplification of QRDRs was carried out by using 5′-VC-gyrA (5′-AATGTGCTGGGCAACGACTGG-3′) and 3′-VC-gyrA (5′-GTGCGCGATTTTCGACATACG-3′) primers for the gyrA gene and by using 5′VC-parC (5′-GTCTGAGTTGGGTCTCTCGGC-3′) and 3′-VC-parC (5′-AGAATCTCGGCAAACTTTGACAG-3′) primers for the parC gene, as described previously (2). Sequences of the amplified gyrA and parC QRDRs were determined by using an ABI Prism TM310 genetic analyzer (Applied Biosystems, Foster City, CA). A single point mutation in the QRDR of the gyrA gene (G-to-T substitution at nucleotide position 248; i.e., Ser-to-Ile substitution at deduced amino acid position 83) was detected in the CIP0.25-1 strain compared with the wild-type strain. However, there was no mutation in the QRDR of the parC gene in this strain (Table 2). Because only one point mutation in the QRDR of the gyrA gene was detected in the CIP0.25-1 strain, which exhibits a high level of resistance to CIP, other genetic mechanisms contributing to this high-level resistance were likely to be present.
TABLE 1.
Antibacterial activities of fluoroquinolones against V. cholerae 569B, CIP0.25-1, and mTn-321
| Agent | MIC (μg/ml) |
||
|---|---|---|---|
| 569B | CIP0.25-1 | mTn-321 | |
| Ciprofloxacin | 0.004 | 1 | 0.0625 |
| Norfloxacin | 0.0078 | 2 | 0.125 |
| Sparfloxacin | 0.004 | 0.125 | 0.0625 |
| Enoxacin | 0.0313 | 2 | 0.25 |
| Tetracycline | 0.5 | 4 | 0.5 |
TABLE 2.
Mutations detected in the gyrA and parC QRDR sequences of the 569B, CIP0.25-1, and mTn-321 strains
| Strain |
gyrA QRDR sequence |
parC QRDR sequence |
||
|---|---|---|---|---|
| Residue or base change | Residue or amino acid change | Residues or base change | Residues or amino acid change | |
| 569B | G at 248 | Ser at 83 | nt 162 to 411 | aa 54 to 137 |
| CIP0.25-1 | G → T at 248 | Ser → Ile at 83 | No change | No change |
| mTn-321 | G → T at 248 | Ser → Ile at 83 | No change | No change |
To reveal an unknown resistance mechanism in the CIP0.25-1 strain, we carried out a mini-Tn5 insertion to obtain a CIP-susceptible revertant (designated mTn-321). The mini-Tn5 was integrated into the chromosome of the CIP0.25-1 strain as follows. pUTmini-Tn5Sm/Sp was transformed into E. coli SM10λpir and then mobilized from the resulting strain into CIP0.25-1 by conjugation on a membrane filter as described previously (8). The transconjugants were selected on TCBS (thiosulfate-citrate-bile-sucrose) agar (Difco Laboratories) supplemented with spectinomycin (25 μg/ml). To confirm that the transconjugants resulted from the transposition of the mini-Tn5 module, PCR was carried out to detect the 2.1-kb fragment of the mini-Tn5 sequence by using mTn5-I (5′-CGGTGATTGATTGAGCAAGC-3′) and mTn5-O (5′-CTGACTCTTATACACAAGTTCG-3′) primers. A transconjugant (mTn-321), of which the MIC against CIP was 16-fold lower than that of CIP0.25-1 (Table 1), was selected out of the transconjugants which showed possession of the mini-Tn5 module. From mTn-321, a Sau3AI fragment containing the mini-Tn5 module was cloned into pUC119, and the upstream and downstream sequences flanking the mini-Tn5 module were then determined. These sequences were subjected to a BLAST search against the V. cholerae O1 genome released by The Institute for Genomic Research at http://www.tigr.org. The BLAST search revealed that the mini-Tn5 was inserted 110 bp upstream of the vca0421 gene (GenBank accession no. AE003853), which encodes a hypothetical protein (GenBank accession no. AAF96327). Furthermore, no obvious open reading frame (ORF) was detected in the region upstream of the vca0421 gene. MICs of fluoroquinolones and tetracycline (TC) against the wild-type strain, CIP0.25-1, and mTn-321 were determined by the 2-fold agar dilution method (Table 1). CIP0.25-1 showed a high level of resistance to all fluoroquinolones tested. In contrast, the mTn-321 revertant was susceptible to all fluoroquinolones tested.
To examine the relationship between vca0421 gene expression and CIP susceptibility, the vca0421 gene was engineered for overexpression in E. coli JM109. Specifically, the effect of fluoroquinolones and TC on the growth of E. coli JM109 harboring either the ptac85-vca0421 plasmid or vector alone was tested. As shown in Table 3, overexpression of the ptac85-vca0421 plasmid resulted in sensitization of E. coli against fluoroquinolones and tetracycline. Furthermore, overexpression experiments were performed in V. cholerae O1 569B and CIP0.25-1. Semiquantitative reverse transcription-PCR (RT-PCR) did not detect expression of the vca0421 gene under regulation of the tac promoter or the promoter of the cholera toxin gene (ctx) (GenBank accession no. X58785.1) from V. cholerae O1 on the broad-host-range vector pCVD503 (6) in either strain (data not shown). Amplification of a region including the vca0421 gene and its native promoter was also unsuccessful, maybe because of the secondary-structure formation of the promoter region (data not shown).
TABLE 3.
Antibacterial activities of fluoroquinolones against E. coli JM109(ptac85) and JM109(ptac85-vca0421)
| Agent | MIC (μg/ml) |
|
|---|---|---|
| JM109(ptac85) | JM109(ptac85-vca0421) | |
| Ciprofloxacin | 0.5 | 0.25 |
| Norfloxacin | 1 | 1 |
| Sparfloxacin | 0.25 | 0.125 |
| Enoxacin | 4 | 2 |
| Chloramphenicol | 16 | 16 |
| Tetracycline | 16 | 8 |
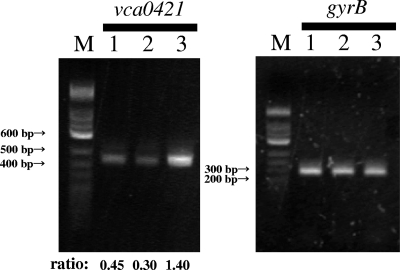
Then, expression levels of the vca0421 gene in the three V. cholerae O1 strains (wild-type strain, CIP-resistant mutant, and mTn-321 revertant) were compared by semiquantitative RT-PCR as described previously (7). For RT-PCR, bacterial cells were cultured in LB (Difco Laboratories) at 25°C overnight. Total RNAs from the three strains were isolated with Isogen (Nippongene, Tokyo, Japan) by using the method recommended by the manufacturer. The RNAs were purified again after treatment with RNase-free DNase I (Takara Bio, Otsu, Japan), and the amount and purity of the RNAs were determined by measuring the A260/A280 ratio. A total of 100 ng of the RNA was used to amplify the vca0421 and gyrB transcripts by RT-PCR. The gyrB transcript level was used as the internal control for RT-PCR as described previously (7). RT-PCR was performed using a SuperScript one-step RT-PCR with Platinum Taq system (Invitrogen, Carlsbad, CA) and primers for vca0421 (5′-VC-vca0421 [5′-GAGAGGATCCATGAAAAAATTGTTAATGGTACTG-3′] and 3′-VC-vca0421 [5′-AGAGGTCGACTTACATAACGCACTCTTTCG-3′]) and gyrB (5′-VC-gyrB [5′-ATGTCGAACAATTACGATTCATC-3′] and 3′-VC-gyrB [5′-CAGTACAGTCATGATGACTTCTG-3′]). The RT-PCR was performed with the following cycle profile: 35 cycles of vca0421 gene annealing at 48°C and 35 cycles of gyrB annealing at 50°C. The RT-PCR products (446 bp for vca0421 and 294 bp for gyrB) were subjected to agarose gel electrophoresis (2% gel) and visualized by staining with ethidium bromide. Semiquantitative RT-PCR was carried out as described previously (7), with slight modifications. The relative expression level of the vca0421 transcript was measured by normalizing the PCR product of the vca0421 gene to that of the gyrB gene. After the densitometric intensities of the RT-PCR products were quantified with NIH Image software, the relative expression level of the vca0421 transcript was calculated as the ratio of the final RT-PCR product of the vca0421 gene to that of the gyrB gene. The amount of contaminating chromosomal DNA in each sample was determined in control reactions without reverse transcriptase. Consequently, it was found that the relative expression level of the vca0421 gene in the mTn-321 revertant was 4.7-fold higher than that in the CIP-resistant mutant (Fig. 1). However, in the mTn-321 revertant, there was no change in the single point mutation in the QRDR of the gyrA gene that was detected in the CIP0.25-1 strain (Table 2). In addition, we performed sequence analysis of the 250-bp upstream regions of the vca0421 ORF in the wild-type and CIP0.25-1 strains and found that there was no difference between the two sequences.
FIG. 1.
RT-PCR analysis of the vca0421 transcript levels. Lane M, 100-bp DNA ladder marker; lane 1, wild-type strain; lane 2, CIP-resistant mutant CIP0.25-1; lane 3, mTn-321. The gyrB gene was used as an internal control. The relative expression level of the vca0421 transcript was calculated as the ratio of the RT-PCR product of the vca0421 gene to that of the gyrB gene (values shown at bottom).
Taken together, our results suggest that overexpression of the vca0421 gene in a CIP-resistant mutant carrying a mutation in the QRDR of the gyrA gene causes sensitization to CIP. However, the precise mechanism underlying this observed sensitization remains unclear. In conclusion, we propose a new intrinsic mechanism of V. cholerae O1 resistance to fluoroquinolones due to the inherently reduced expression of the vca0421 gene.
Footnotes
Published ahead of print on 30 August 2010.
REFERENCES
- 1.Adachi, T., M. Mizuuchi, E. Robinson, E. Appella, M. O'Dea, M. Gellert, and K. Mizuuchi. 1987. DNA sequence of the E. coli gyrB gene: application of a new sequencing strategy. Nucleic Acids Res. 15:771-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baranwal, S., K. Dey, T. Ramamurthy, G. B. Nair, and M. Kundu. 2002. Role of active efflux in association with target gene mutations in fluoroquinolone resistance in clinical isolates of Vibrio cholerae. Antimicrob. Agents Chemother. 46:2676-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg, P., S. Sinha, R. Chakraborty, S. K. Bhattacharya, G. B. Nair, T. Ramamurthy, and Y. Takeda. 2001. Emergence of fluoroquinolone-resistant strains of Vibrio cholerae O1 biotype El Tor among hospitalized patients with cholera in Calcutta, India. Antimicrob. Agents Chemother. 45:1605-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato, J., Y. Nishimura, R. Imamura, H. Niki, S. Hiraga, and H. Suzuki. 1990. New topoisomerase essential for chromosome segregation in E. coli. Cell 63:393-404. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura, S. 1997. Mechanisms of quinolone resistance. J. Infect. Chemother. 3:128-138. [Google Scholar]
- 6.Nishibuchi, M., and J. B. Kaper. 1985. Nucleotide sequence of the thermostable direct hemolysin gene of Vibrio parahaemolyticus. J. Bacteriol. 162:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okuda, J., F. Murayama, E. Yamanoi, E. Iwamoto, S. Matsuoka, M. Nishibuchi, and T. Nakai. 2007. Base changes in the fliC gene of Edwardsiella tarda: possible effects on flagellation and motility. Dis. Aquat. Organ. 76:113-121. [DOI] [PubMed] [Google Scholar]
- 8.Okuda, J., and M. Nishibuchi. 1998. Manifestation of the Kanagawa phenomenon, the virulence-associated phenotype, of Vibrio parahaemolyticus depends on a particular single base change in the promoter of the thermostable direct haemolysin gene. Mol. Microbiol. 30:499-511. [DOI] [PubMed] [Google Scholar]
- 9.Okuda, J., T. Ramamurthy, and S. Yamasaki. 2007. Antibacterial activity of ciprofloxacin against clinical strains of Vibrio cholerae O139 recently isolated from India. Yakugaku Zasshi 127:903-904. [DOI] [PubMed] [Google Scholar]
- 10.Okuda, J., T. Ramamurthy, and S. Yamasaki. 2007. The potent antibacterial activity of sitafloxacin against fluoroquinolone-resistant clinical isolates of Vibrio cholerae O1. Microbiol. Immunol. 51:467-469. [DOI] [PubMed] [Google Scholar]
- 11.Peng, H., and K. V. Marians. 1993. Escherichia coli topoisomerase IV. Purification, characterization, subunit structure, and subunit interactions. J. Biol. Chem. 268:24481-24490. [PubMed] [Google Scholar]
- 12.Swanberg, S., and J. Wang. 1987. Cloning and sequencing of the Escherichia coli gyrA gene coding for the A subunit of DNA gyrase. J. Mol. Biol. 197:729-736. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto, T., G. B. Nair, M. J. Albert, C. C. Parodi, and Y. Takeda. 1995. Survey of in vitro susceptibilities of Vibrio cholerae O1 and O139 to antimicrobial agents. Antimicrob. Agents Chemother. 39:241-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida, H., M. Bogaki, M. Nakamura, and S. Nakamura. 1990. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob. Agents Chemother. 34:1271-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]