Abstract
Peramivir, a sialic acid analogue, is a selective inhibitor of neuraminidases produced by influenza A and B viruses. We evaluated the efficacy and safety of a single intravenous dose of peramivir in outpatients with uncomplicated seasonal influenza virus infection. A total of 300 previously healthy adult subjects aged 20 to 64 years with a positive influenza virus rapid antigen test were recruited within 48 h of the onset of influenza symptoms and randomized to three groups: single intravenous infusion of either 300 mg peramivir per kg of body weight, 600 mg peramivir, or matching placebo on study day 1. Influenza symptoms and body temperature were self-assessed for 14 days. Nasal and pharyngeal swabs were collected to determine the viral titer. The primary endpoint was the time to alleviation of symptoms. Of the 300 subjects, 296 were included in the intent-to-treat infected population (300 mg peramivir, n = 99; 600 mg peramivir, n = 97; and placebo, n = 100). Peramivir significantly reduced the time to alleviation of symptoms at both 300 mg (hazard ratio, 0.681) and 600 mg (hazard ratio, 0.666) compared with placebo (adjusted P value, 0.0092 for both comparisons). No serious adverse events were reported. Peramivir was well tolerated, and its adverse-event profile was similar to that of placebo. A single intravenous dose of peramivir is effective and well tolerated in subjects with uncomplicated seasonal influenza virus infection.
Influenza is a highly infectious respiratory tract disease that affects approximately 10% of the world's population annually. The illness is usually self-limiting, with relief of symptoms occurring within 5 to 7 days. Nevertheless, it is an important disease for several reasons, including ease of communicability, morbidity with resultant loss of productivity, severity of complications, and increased risk of death, particularly in high-risk populations. During 19 of the 23 influenza seasons between 1972-1973 and 1994-1995, estimated influenza-associated deaths in the United States ranged from approximately 25 to more than 150 per 100,000 people older than 65, accounting for more than 90% of the deaths attributed to pneumonia and influenza (21). Inevitably, many patients who are elderly or have a chronic pulmonary disease develop life-threatening respiratory failure when they become infected with influenza virus. Furthermore, pandemic H1N1 and avian influenza virus infection have resulted in more deaths than seasonal influenza virus infection among younger patients with no comorbidities (12, 23, 26). Excessive immunological reactions, such as “cytokine storms,” have been suggested to be induced in severe influenza virus pneumonia (18, 19).
Presently, only a few available measures can reduce the impact of influenza: immunoprophylaxis with an inactivated or live attenuated vaccine and chemoprophylaxis or therapy with influenza virus-specific antiviral drugs (i.e., amantadine, rimantadine, oseltamivir, or zanamivir). These antiviral agents have several important limitations: high frequencies of viral resistance (3, 4, 24), limited treatment efficacy, and absence of parenteral formulations for seriously ill patients (14, 20). Thus, alternative antiviral treatments and combination therapies are needed (7).
Peramivir is a neuraminidase inhibitor that represents a potentially promising addition to the armamentarium of drugs for the treatment of influenza. Its advantages include potent antiviral activity against influenza A and B viruses and its parenteral administration (1, 6, 27). Peramivir has strong affinity for influenza virus neuraminidase and a low off rate, suggesting that it could inhibit neuraminidase activity for a prolonged period and allow lower frequency of dosing. Phase 1 studies of intravenous peramivir at doses of up to 800 mg once daily for 6 days in healthy Japanese subjects demonstrated good tolerability (data not shown). We therefore conducted the present study to investigate the efficacy and safety of a single intravenous dose of peramivir for patients with influenza virus infection in the outpatient setting.
MATERIALS AND METHODS
Design.
This study was a randomized, double-blind, placebo-controlled trial conducted at Nagasaki University Hospital, Nagasaki, and 74 other centers in Japan between December 2007 and April 2008. The study protocol was approved by the institutional review board at each center.
Subjects.
Eligible subjects were previously healthy adults aged 20 to 64 years reporting onset of influenza-like illness within the previous 48 h. The time of onset of influenza-like illness was defined as either when the body temperature first rose to ≥1°C above normal or when the subject experienced at least two of the following seven influenza symptoms: headache, aches or pains in muscles or joints, feverishness, fatigue, cough, sore throat, and nasal congestion. At enrollment, a diagnosis of influenza was required based on a positive rapid antigen test for influenza virus, fever of ≥38°C, and the presence of at least two of the seven symptoms listed above at moderate to high severity, based on subjects' self-reporting. Exclusion criteria included respiratory dysfunction or chronic respiratory disorders requiring pharmacotherapy, convulsions or other neurological symptoms, active clinically important chronic illness or known infection with human immunodeficiency virus, renal impairment requiring hemodialysis, suspected bacterial infection, treatment with steroids or other immunosuppressants, use of anti-influenza virus drugs within the past 7 days; and a history of hypersensitivity, allergy, or serious adverse drug reactions to anti-influenza virus drugs or acetaminophen. Women who were pregnant, likely to be pregnant, or breast feeding were also excluded. Prior influenza virus vaccination was not an exclusion criterion. All subjects provided written informed consent.
Study procedures.
Subjects were randomly assigned to receive a single dose of intravenous peramivir (300 mg or 600 mg) or matching placebo (Shionogi & Co., Ltd., Osaka, Japan). Computer-generated randomization was conducted by a central randomization facility with sole access to the code, using a minimization method to balance current smoking behavior at screening and composite symptom scores at screening among the three groups. Each center dispensed the study drug, which was unrecognizable without a drug number, according to the instructions of the randomization center, using assigned randomization numbers. The study drug, a single intravenous infusion of 30- to 60-min duration, was administered by the appropriate personnel at each center. All subjects were dispensed acetaminophen at enrollment and instructed to take it only for symptom relief and to record its use in subject diaries.
Assessments.
Subjects kept a record of their body temperature, influenza symptoms, and ability to perform their usual activities. Axillary body temperature was measured with a digital thermometer twice daily for 14 days. The presence of the seven influenza symptoms was self-assessed on a 4-point scale (0, absent; 1, mild; 2, moderate; 3, severe) (influenza symptom severity scale [ISS]) and recorded twice daily from day 1 to day 9 and once daily from day 10 to day 14 (16). The ability of subjects to perform their usual activities was also self-assessed on an 11-point visual analogue scale (0, unable to perform usual activity at all, to 10, able to perform all usual activities fully; influenza impact well-being scale [IIWS]) and recorded once daily until day 14. Subject visits were scheduled on days 1 (administration of study drug), 2 (not mandatory), 3, 5, 9, and 14.
A nasal swab from one naris and a single throat swab were collected at baseline and on days 2, 3, 5, and 9. All samples were taken from the same sites throughout the study. These samples were each transported in 2 ml viral transport medium to a central laboratory and were divided for isolation and typing (500 μl) and virus titration (250 μl). Viral titers were calculated as log10 50% tissue culture infective dose (TCID50)/ml of viral transport medium, according to the Spearman-Karber equation. Madin-Darby canine kidney (MDCK) cells were infected in triplicate with 25 μl of a 10-fold dilution series of samples (ranging from undiluted to 107 dilution) in serum-free medium containing 3 μg of trypsin per ml. Virus was adsorbed for 1 h, and then the cells were washed twice to remove unadsorbed virus and residual peramivir. The MDCK cells were then incubated at 37°C in 5% CO2 for 3 days. Following this incubation period, the appearance of cytopathic effect (CPE) on cell monolayers was scored using light microscopy, and the final titer was expressed as TCID50/ml. When no CPE was observed using undiluted viral solution, it was defined as an undetectable level. In the present study, the undetectable level was considered to be 101.1 TCID50/ml. Neuraminidase enzyme inhibitory assays were performed on the isolated virus using a standard fluorometric assay (17). The 50% inhibitory concentration (IC50) was calculated by plotting the percent inhibition of neuraminidase activity versus the inhibitor concentration. The results are reported as the mean ± standard deviation (SD) of three independent experiments.
Blood and urine samples were taken for laboratory tests, which comprised hematological examination (white blood cell count, differential, hemoglobin concentration, hematocrit, red blood cell count, and platelet count), blood biochemistry examination (aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, alkaline phosphatase, creatine phosphokinase, total bilirubin, direct bilirubin, protein total, albumin, blood urea, creatinine, uric acid, glucose, sodium, potassium, chloride, magnesium, calcium, and phosphorus), and urinalysis (bilirubin, protein, glucose, ketone bodies, urobilinogen, occult blood, sediment, β-N-acetyl-d-glucosaminidase, β2 microglobulin, α1 microglobulin, and albumin), were collected at baseline and days 3 and 14. Virological testing and laboratory tests were performed at BML Corporation (Saitama, Japan) by technicians blinded to treatment assignment.
Plasma samples for pharmacokinetic analysis were collected on days 1 (just before completion of infusion), 2, and 3. Peramivir plasma concentrations were determined using a validated liquid chromatography/tandem mass spectrometry (LC/MS-MS) method at Sumika Chemical Analysis Service, Ltd. (Osaka, Japan), after unblinding. Peramivir was extracted from plasma by deproteinization and separated by liquid chromatography with an XBridge C18 column (length, 50 mm; internal diameter, 2.1 mm; Waters Corp., Milford, MA). The column effluent was analyzed using a mass spectrometer (Applied Biosystems/MDS, Concord, Canada; Sciex API4000) equipped with a turbo ion spray in the positive ion detection mode. The lower limit of quantification for peramivir in plasma was 1.00 ng/ml.
The primary efficacy endpoint (time to alleviation of symptoms) was defined as the time from the start of treatment to recovery (i.e., when all seven influenza symptom scores had been at “0” or “1” for at least 21.5 h).
Other efficacy endpoints included a change (from baseline) in composite symptom scores at 24, 36, 48, and 96 h after the start of treatment; the proportion of afebrile subjects (<37°C; axillary); a change in the influenza virus titer from baseline; time to resumption of usual activities; and incidence of influenza-related complications (otitis media, bronchitis, sinusitis, and pneumonia).
In the safety evaluation, adverse events, physical findings, vital signs, and laboratory data were assessed for duration, severity, and causality of the study medication. Severities that were grade 1, grade 2, and grade 3 or higher according to the Division of AIDS table for grading the severity of adult and pediatric adverse events were rated as mild, moderate, and severe, respectively (5).
Statistical analysis.
Efficacy analysis was performed on the intent-to-treat infected (ITTI) population, which included all subjects who had influenza virus infection (confirmed by culture, by PCR, or by a 4-fold elevation in titers of antibody to influenza A or B virus) and were treated.
For the primary endpoint comparison, we used a Cox proportional-hazards model, including the effects of treatment, current smoking behavior, and composite symptom scores at baseline. The efficacy of peramivir was evaluated by comparing the treatment group (consisting of the 300-mg and 600-mg groups) with the placebo group. Only if this analysis showed a statistically significant difference would each peramivir subgroup be compared with the placebo group to determine the recommended dose level. The overall significance level was adjusted by a gate-keeping procedure. In a comparison of each peramivir group with the placebo group, P values were adjusted by the Hochberg method. Subjects whose symptoms failed to improve were censored at the date of their last postbaseline assessment.
The proportion of subjects reporting postbaseline normal temperature (<37.0°C) and the proportion of subjects who had positive viral titers after baseline in each peramivir group and the placebo group were compared by the Mantel-Haenszel test stratified for current smoking behavior and composite symptom scores at baseline. Fisher's exact test was used to compare the incidences of the above-mentioned influenza-related complications. The times to resumption of usual activities were compared between each peramivir group and the placebo group using a stratified log rank test.
A safety analysis was performed on the safety population, which included all subjects who took at least one dose of study medication. Intergroup comparison of the incidences of adverse events was performed using Fisher's exact test.
Sample size calculations assumed a two-sided significance level of 0.05 to be distributed equally among groups (300 mg peramivir, 600 mg peramivir, and placebo). Using the Lakatos method (11), a per-group sample size of 67 was estimated to have at least 80% power to detect a difference in the median time to alleviation of symptoms of 87 h in the treatment group and 137 h in the placebo group. However, the sample size was increased to 300 subjects to increase the precision of the efficacy evaluation and to accumulate safety information.
All analyses were performed using SAS version 8.2 (or higher) software (SAS Institute, Cary, NC). Statistics were reported to one decimal place beyond the number of decimal places present as the original endpoint.
RESULTS
Subjects.
Figure 1 shows the trial profiles. A total of 300 subjects were randomly assigned to one of the treatment groups. Of these, one subject did not have laboratory-confirmed influenza virus infection, two subjects withdrew after randomization but before treatment, and one subject did not have any symptom assessment data after randomization. Therefore, 296 subjects (300 mg peramivir, n = 99, and 600 mg peramivir, n = 97; placebo, n = 100) were included in the ITTI population. Subject characteristics were well distributed across treatment groups (Table 1). The predominant influenza virus strain was the A/H1 subtype. Considering that the study was conducted before the emergence of the 2009 pandemic H1N1 virus, the predominant influenza virus strain in our study could be the seasonal A (H1N1) Russian strain. At baseline, the median IC50s for peramivir by virus subtype were 1.11 to 2.81 nmol/liter (Table 2). IC50s were significantly higher for the A/H3 subtype than for the A/H1 subtype in an additional analysis using the Wilcoxon rank sum test (data not shown). Sequence data were obtained for three viruses showing IC50s beyond the mean IC50 plus 3 SD at baseline. In one of three viruses, the H275Y mutation was detected in the H1N1 virus tested.
FIG. 1.
Study profiles.
TABLE 1.
Demographic data (intent-to-treat infected population)
| Parameter | Value |
||
|---|---|---|---|
| Peramivir |
Placebo (n = 100) | ||
| 300 mg (n = 99) | 600 mg (n = 97) | ||
| Male sex [n (%)] | 46 (46.5) | 53 (54.6) | 51 (51.0) |
| Age (mean ± SD [yr]) | 34.2 ± 9.8 | 33.9 ± 10.4 | 34.4 ± 9.6 |
| Weight (mean ± SD [kg]) | 61.15 ± 12.69 | 63.12 ± 15.18 | 61.85 ± 13.11 |
| Current smoker [n (%)] | 34 (34.3) | 32 (33.0) | 34 (34.0) |
| Symptom duration before study [n (%)] | |||
| 0-24 h | 59 (59.6) | 51 (52.6) | 48 (48.0) |
| 24-48 h | 40 (40.4) | 46 (47.4) | 52 (52.0) |
| Composite symptom score at baseline (mean ± SD) | 11.5 ± 2.8 | 11.8 ± 2.5 | 12.0 ± 2.7 |
| Body temp at baseline (mean ± SD) [°C] | 38.44 ± 0.43 | 38.64 ± 0.53 | 38.50 ± 0.46 |
| Influenza virus subtype [n (%)] | |||
| A/H1 | 74 (74.7) | 69 (71.1) | 72 (72.0) |
| A/H3 | 21 (21.2) | 25 (25.8) | 24 (24.0) |
| A/- | 2 (2.0) | 2 (2.1) | 4 (4.0) |
| B | 2 (2.0) | 1 (1.0) | 0 (0.0) |
TABLE 2.
IC50 for peramivir at baseline
| Influenza virus subtype | n | IC50 (nmol/liter) |
||
|---|---|---|---|---|
| Minimum | Median | Maximum | ||
| A/H1 | 158 | 0.56 | 1.15 | 9.43 |
| A/H3 | 69 | 1.06 | 1.36 | 3.51 |
| A/- | 6 | 0.56 | 1.11 | 1.72 |
| B | 3 | 2.62 | 2.81 | 3.00 |
Clinical efficacy.
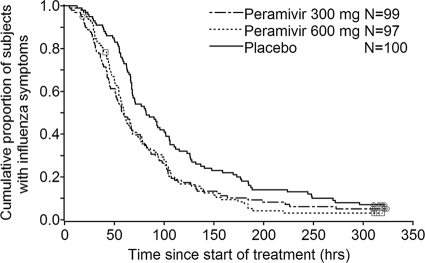
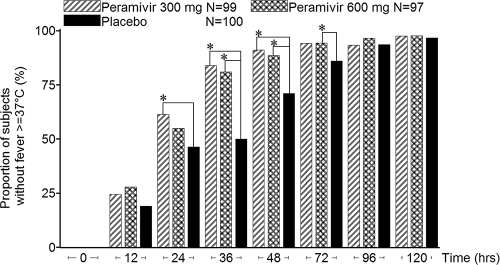
Peramivir significantly reduced the time to alleviation of symptoms compared with placebo. The hazard ratio of the treatment to the placebo for the time to alleviation of symptoms was 0.681 (adjusted P value, 0.0092) in the 300-mg group and 0.666 (adjusted P value, 0.0092) in the 600-mg group, and this effect occurred regardless of the influenza virus subtype or duration of symptoms before the study (Table 3). The efficacy of peramivir was apparent as early as 24 h after the start of treatment (Fig. 2). The proportion of afebrile (temperature < 37.0°C) subjects was increased by treatment, and a reduction in fever was evident within 24 h of therapy (Fig. 3). In addition, peramivir recipients reported shorter times to resumption of their usual activities (43.6 h and 41.7 h earlier in the 300-mg and 600-mg groups, respectively; 300 mg, median duration, 125.6 h [95% confidence interval [CI], 103.8 to 148.5], P = 0.0367; 600 mg, 127.4 h [95% CI, 122.1 to 153.1], P = 0.0152; and placebo, 169.1 h [95% CI, 142.0 to 180.0]).
TABLE 3.
Time to alleviation of symptoms (intent-to-treat infected population)
| Population | Parameter | Value |
||
|---|---|---|---|---|
| Peramivir |
Placebo | |||
| 300 mg | 600 mg | |||
| Overall | n | 99 | 97 | 100 |
| Median (h) (95% CI) | 59.1 (50.9-72.4) | 59.9 (54.4-68.1) | 81.8 (68.0-101.5) | |
| Hazard ratioa (95% CI) | 0.681 (0.511-0.909) | 0.666 (0.499-0.890) | ||
| Adjusted P valueb | 0.0092 | 0.0092 | ||
| Influenza virus subtype | ||||
| A/H1 | n | 74 | 69 | 72 |
| Median (h) | 52.5 | 62.6 | 81.4 | |
| Hazard ratio | 0.779 | 0.899 | ||
| Adjusted P value | 0.1458 | 0.5384 | ||
| A/H3 | n | 21 | 25 | 24 |
| Median (h) | 76.1 | 50.5 | 81.0 | |
| Hazard ratio | 0.542 | 0.326 | ||
| Adjusted P value | 0.0556 | 0.0008 | ||
| Symptom duration before study | ||||
| 0-24 h | n | 59 | 51 | 48 |
| Median (h) | 57.2 | 56.1 | 86.7 | |
| Hazard ratio | 0.653 | 0.663 | ||
| Adjusted P value | 0.0516 | 0.0516 | ||
| 24-48 h | n | 40 | 46 | 52 |
| Median (h) | 69.1 | 64.7 | 70.8 | |
| Hazard ratio | 0.708 | 0.694 | ||
| Adjusted P value | 0.1118 | 0.1118 | ||
Hazard ratios compared to the placebo group were estimated using Cox proportional-hazards modeling, adjusted for current smoking behavior and composite symptom scores at baseline.
P values for comparisons between peramivir and placebo were adjusted using the Hochberg method for multiple comparisons.
FIG. 2.
Kaplan-Meier curve of time to alleviation of symptoms (intent-to-treat infected population). □, ○, and ⋄, censored subjects who withdrew before resolution of symptoms.
FIG. 3.
Proportions of subjects reporting normal temperature (intent-to-treat infected population). *, P < 0.05 between peramivir and placebo as determined by the Mantel-Haenszel test stratified for current smoking behavior and composite symptom scores at baseline.
Physician-diagnosed secondary complications (pneumonia, bronchitis, sinusitis, and otitis media) occurred in three recipients of 300 mg peramivir (3 cases of bronchitis; 3.0%), one recipient of 600 mg peramivir (1 case of otitis media; 1.0%), and three placebo recipients (3 cases of bronchitis; 3.0%).
Virology.
The effect of peramivir on virus shedding was evaluated for observed data on the subset of subjects with a baseline sample positive for influenza virus. The influenza virus titer (log10 TCID50/ml) and the proportion of virus-positive subjects over time are shown in Fig. 4 A and B, respectively. At baseline, the viral titers were similar for all three groups; however, on day 3, the proportions of virus-positive subjects were significantly decreased in the 300-mg and 600-mg groups (300 mg, 35/95, 36.8%, P = 0.0485; 600 mg, 24/93, 25.8%, P = 0.0003; placebo, 50/97, 51.5%). Virus was not detected in most subjects on day 9 (300 mg, 0/95, 0.0%; 600 mg, 1/91, 1.1%; placebo, 0/96, 0.0%).
FIG. 4.
(A) Means and SD of influenza virus titers (log10 TCID50/ml) over time (intent-to-treat infected population). Analysis was performed for observed data on the subset of subjects who were positive for influenza virus at baseline. Virus titers under the lower limit of quantification (1.1 log10 TCID50/ml) were set equal to 1.1. (B) Proportions of subjects showing positive viral titers (intent-to-treat infected population). Analysis was performed for observed data on the subset of subjects who were positive for influenza virus at baseline. The positive virus titer was at least 1.1 log10 TCID50/ml. *, P < 0.05 between peramivir and placebo as determined by the Mantel-Haenszel test, stratified by current smoking behavior and composite symptom scores at baseline.
Tolerability.
Peramivir was generally well tolerated. Of 298 treated subjects (safety population), one subject receiving placebo withdrew from the study because of an adverse event, and no serious adverse events were reported.
The incidence of all adverse events for peramivir was comparable to that for placebo (300 mg peramivir, P = 0.4986; 600 mg peramivir, P = 1.0000) (Table 4). Adverse events were generally mild to moderate. The most common adverse events in terms of clinical symptoms were gastrointestinal. In the 300-mg, 600-mg, and placebo groups, diarrhea occurred in 14.1%, 15.2%, and 17.0% and nausea in 3.0%, 6.1%, and 1.0%, respectively. Severe adverse events occurred with two subjects (2.0%; two subjects with electrocardiogram QT interval prolonged) in the 300-mg group, three subjects (3.0%; one subject each with electrocardiogram QT interval prolonged, blood glucose increased, and blood creatinine increased) in the 600-mg group, and five subjects (5.0%; three subjects with electrocardiogram QT interval prolonged and one subject each with blood pressure increased and blood glucose increased) in the placebo group. Two of these events (one subject each with electrocardiogram QT interval prolonged in the 300-mg group and blood creatinine increased in the 600-mg group) were considered by investigators to be related to study medications. Both of these events were resolved without treatment. The other severe adverse events were attributed to influenza virus infection or its complications.
TABLE 4.
Summary of adverse events (safety population)
| Parameter | Value |
||
|---|---|---|---|
| Peramivir |
Placebo (n = 100) | ||
| 300 mg (n = 99) | 600 mg (n = 99) | ||
| No. of events | 252 | 252 | 257 |
| No. (%) of patients with ≥1 event | 87 (87.9) | 90 (90.9) | 91 (91.0) |
| 95% CI (%) | 79.8, 93.6 | 83.4, 95.8 | 83.6, 95.8 |
| Pa | 0.4986 | 1.0000 | |
| Adverse events (≥6% in either group) [n (%) of patients] | |||
| Monocyte % increased | 20 (20.2) | 18 (18.2) | 31 (31.0) |
| Blood glucose increased | 18 (18.2) | 17 (17.2) | 18 (18.0) |
| Diarrhea | 14 (14.1) | 15 (15.2) | 17 (17.0) |
| Lymphocyte % increased | 14 (14.1) | 14 (14.1) | 5 (5.0) |
| Proteinuria present | 9 (9.1) | 11 (11.1) | 18 (18.0) |
| White blood cells urine positive | 8 (8.1) | 9 (9.1) | 8 (8.0) |
| β2 Microglobulin in urine increased | 14 (14.1) | 8 (8.1) | 11 (11.0) |
| White blood cell count decreased | 9 (9.1) | 7 (7.1) | 4 (4.0) |
| Blood bilirubin increased | 7 (7.1) | 8 (8.1) | 7 (7.0) |
| Alanine aminotransferase increased | 4 (4.0) | 7 (7.1) | 8 (8.0) |
| Aspartate aminotransferase increased | 1 (1.0) | 7 (7.1) | 6 (6.0) |
| α1 Microglobulin increased | 6 (6.1) | 6 (6.1) | 6 (6.0) |
| Nausea | 3 (3.0) | 6 (6.1) | 1 (1.0) |
| Blood lactate dehydrogenase increased | 2 (2.0) | 6 (6.1) | 4 (4.0) |
| β-N-Acetyl-d-glucosaminidase | 9 (9.1) | 5 (5.1) | 5 (5.0) |
| Urine albumine present | 5 (5.1) | 5 (5.1) | 6 (6.0) |
| Protein total decreased | 3 (3.0) | 4 (4.0) | 6 (6.0) |
| Lymphocyte morphology abnormal | 11 (11.1) | 4 (4.0) | 6 (6.0) |
| Nasopharyngitis | 0 (0.0) | 4 (4.0) | 6 (6.0) |
| Blood phosphate increased | 6 (6.0) | 3 (3.0) | 4 (4.0) |
The P value was calculated by intergroup comparison between peramivir and placebo groups using Fisher's exact test.
Drug concentration.
The mean dosages based on weight were 5.0 mg/kg (range, 3.0 to 7.2 mg/kg) and 10.0 mg/kg (range, 5.5 to 15.3 mg/kg) in the 300- and 600-mg groups, respectively. The median duration of infusion was 0.63 h (range, 0.43 to 0.97 h). The median plasma concentrations at the end of infusion were 18,100 ng/ml (range, 1,780 to 31,000 ng/ml; n = 98) in the 300-mg group and 36,300 ng/ml (range, 9,200 to 72,400 ng/ml; n = 98) in the 600-mg group. The values 18 to 24 h after the end of infusion were 14.8 ng/ml (range, 6.71 to 28.4 ng/ml; n = 34) and 32.8 ng/ml (range, 14.4 to 71.9 ng/ml; n = 25), and those 36 to 48 h after the end of infusion were 5.01 ng/ml (range, 0 to 11.1 ng/ml; n = 85) and 10.7 ng/ml (range, 5.49 to 22.9 ng.ml; n = 89) in the 300- and 600-mg groups, respectively.
DISCUSSION
In this study, intravenous administration of peramivir in the ambulatory setting at a single dose of 300 mg or 600 mg was associated with significant clinical and antiviral effects in healthy adults with seasonal influenza virus infection and was generally well tolerated. The improvement of symptoms was apparent within 24 h of peramivir administration, which corresponded to the acute phase of illness, when influenza symptoms are commonly most troublesome. These decreases in the severity and duration of illness were accompanied by significant improvements in the time to resumption of usual activities.
Peramivir showed a high neuraminidase-inhibitory activity (median IC50 range by virus subtype, 1.11 to 2.81 nmol/liter; range, 0.38 to 0.92 ng/ml) at baseline. After intravenous administration of peramivir, median plasma concentrations were nearly 2 orders of magnitude higher than those achieved with standard doses of oral oseltamivir, and a potent and significant antiviral effect was seen with the peramivir groups compared to the placebo group (P = 0.0485 and 0.0003 in the 300- and 600-mg groups, respectively) in the number of subjects shedding virus on day 3. The strong affinity for influenza virus neuraminidase and low off rate of peramivir may have led to favorable efficacy with a single administration.
The benefit demonstrated by neuraminidase inhibitor therapy in the outpatient setting is assumed to also occur in seriously ill subjects requiring hospital care (8, 13, 15, 22). Current antiviral treatments, such as oseltamivir and zanamivir, are administered either orally or by inhalation. These routes may not provide rapid, reliable drug delivery in seriously ill patients. For example, failure of zanamivir therapy to treat pneumonia in a bone marrow transplant recipient has been reported, even though the infecting virus (influenza A [H1N1] virus) was sensitive to the agent (14). In addition, the oral bioavailability of oseltamivir, especially when given by nonstandard means (e.g., via nasogastric tube) is uncertain, although a recent report on three subjects found adequate absorption under such circumstances (20). Parenteral administration can circumvent these limitations by guaranteeing rapid delivery and high levels in blood, increasing the likelihood of drug delivery to infection sites, particularly the lungs of patients with pneumonia and the extrapulmonary tissues of those with influenza A (H5N1 or pandemic H1N1) virus infection.
A potential limitation of the present study is that the protocol specifically excluded individuals from high-risk populations, and use of antiviral agents such as peramivir in severe cases of influenza virus infection should be considered. Based mainly on the results of the present study, the U.S. Food and Drug Administration issued an emergency use authorization for peramivir exclusively for severe pandemic H1N1 (2), and this authorized regimen is 600 mg/day for 5 to 10 days. Some investigators have reported that influenza virus infection in immunocompromised or severely ill patients is likely to require longer duration of antiviral therapy than that in uncomplicated patients (9, 25), and such patients might need multiple administrations of peramivir. In an influenza A (H5N1) virus infection model, mice received multiple oral doses of oseltamivir over 5 consecutive days or peramivir (via either a single intramuscular injection or 5 intramuscular injections over 5 consecutive days), starting at 1 h after virus inoculation, and all these groups were more likely to survive than were control mice injected with saline (27). However, the multiple-dose peramivir regimen was the only one that prevented paralysis by day 15, suggesting that multidose regimens of peramivir may provide greater benefit. In practice, treatment duration will be selected on the basis of the expected need for a longer duration in hospitalized patients and is consistent with the design of ongoing phase 3 trials in hospitalized patients.
The extent to which the higher serum drug levels achieved in the present study may provide greater antiviral efficacy and reduce the frequency of resistance emergence remains to be determined in clinical trials. Future studies will be needed to clarify whether such high plasma neuraminidase inhibition levels provide greater clinical benefits for high-risk or hospitalized subjects with influenza virus infection.
In conclusion, we found that a single 300- or 600-mg intravenous dose of peramivir in the outpatient setting was efficacious for the treatment of uncomplicated influenza virus infection in adult subjects. The efficacy, tolerability, and ease of administration of peramivir in healthy adults with uncomplicated influenza virus infection support continued investigation of this agent in high-risk populations and severe cases.
Acknowledgments
This study was funded by Shionogi & Co., Ltd. (Osaka, Japan).
We thank Tohru Ohe of Okayama University, Okayama, Japan, for his help with the evaluation of electrocardiograms obtained in the study; the subjects for their participation; and all those who acted as coworkers and monitors in the participating centers for their dedicated work.
S-021812 Clinical Study Group. Writing Committee: S. Kohno, H. Kida, M. Mizuguchi, J. Shimada, and Masafumi Seki (Nagasaki University, Nagasaki, Japan), who take responsibility for the content and accuracy of the paper. Investigator Group (Japan): Yoshitaka Sugawara, Obihiro Respiratory and Medical Hospital, Hokkaido; Hironi Makita, Makita Hospital, Hokkaido; Masahiro Notani, Sapporo Ryokuai Hospital, Hokkaido; Hiroshi Hachinohe, Aoba Medical Clinic, Hokkaido; Toshimichi Itoh, Misono Itoh Internal Clinic, Hokkaido; Rie Tanzawa, Kohyou Clinic, Hokkaido; Shin Tsutahara, Sapporo Naika Clinic, Hokkaido; Yasushi Amada, Amada Internal Clinic, Fukushima; Mayumi Eiro, Social Insurance Nihonmatsu Hospital, Fukushima; Motohiko Aida, Aida Hospital, Fukushima; Takeshi Nawa, Hitachi General Hospital, Ibaraki; Naka Araki, Iryohojin Keiyukai Moriya Keiyu Hospital, Ibaraki; Takao Ishizuka, Tomioka General Hospital, Gunma; Hitoshi Sakai, Sakai Clinic, Chiba; Shin Totokawa, Gyotoku Flower Street Clinic, Chiba; Akihiko Ohwada, Ohwada Internal and Respiratory Clinic, Chiba; Kiyoshi Niwa, Niwa Family Clinic, Tokyo; Mitumi Ikeda, Nishio Hospital, Tokyo; Akinori Yamashita, Yamashita Clinic, Tokyo; Masaru Oritsu, Japan Red Cross Medical Center, Tokyo; Keiko Kono, Kono Medical Clinic, Tokyo; Sumio Aizawa, Nakano Egota Hospital, Tokyo; Masaaki Tomonari, Tomonari Daini Clinic, Tokyo; Tsuyoshi Yamato, Kouwa Clinic, Tokyo; Hiroyoshi Kanemitsu, Musashikoganei Clinic, Tokyo; Nobuyuki Saitoh, J-Tower Clinic, Tokyo; Noriya Hori, Sakura Clinic, Tokyo; Yoshihiko Maezawa, Maezawa Clinic, Tokyo; Hatsumi Hamada, Futaba ENT Clinic, Tokyo; Takahiro Yokoyama, Yotsuya Internal Clinic, Tokyo; Shigenao Kan, K Clinic Sanno, Tokyo; Tadashi Fujikawa, Fujikawa Clinic, Tokyo; Takashi Ishikawa, Ishikawa Nippori Clinic, Tokyo; Kiyomitsu Miyachi, Kuroda Medical Clinic, Tokyo; Takahiro Katoh, Medical Plaza Edogawa, Tokyo; Hiroaki Shirai, Iryohojin Koganeibashi Sakura Clinic, Tokyo; Kiyomitsu Miyachi, Keigu Clinic, Kanagawa; Kouichi Miyashita, Fukui General Hospital, Fukui; Michiko Indou, Komatsu Hospital, Aichi; Masaharu Kitada, Iryohojin Kowakai Kitada-iin, Osaka; Motokazu Kato, Kishiwada City Hospital, Osaka; Eikichi Cho, Cho Clinic, Osaka; Hideyuki Sato, Keisaikai Medical Mall, Osaka; Michio Yagi, Osaka Pharmacology Clinical Research Hospital, Osaka; Hiroshi Tsujioka, Iryouhoujin Isseikai Ohara Hospital, Hyogo; Masato Baden, Iryouhoujin Kaiseikai Takarazuka Hospital, Hyogo; Masahiro Shibagaki, Iryouhoujinn Shoutoukai Irie Hospital, Hyogo; Masanobu Funamoto, Funamoto Clinic, Hyogo; Satoko Wada, Wada Clinic, Hyogo; Tomohiko Sugimoto, Sugimoto Clinic, Hyogo; Soichiro Hozawa, Hiroshima Allergy and Respiratory Clinic, Hiroshima; Fumiko Saeki, Kouri Hifuka Naika Geka, Ehime; Osamu Kubota, Kubota Naika Junkankika Kokyukika, Ehime; Teiichi Nishio, Medical Corporation Ichijukai Nishio Hospital, Fukuoka; Masaharu Kinoshita, Nagata Hospital, Fukuoka; Nobuo Yuino, Shin Yukuhashi Hospital, Fukuoka; Toru Rikimaru, Fukuokaken Saiseikai Futsukaichi Hospital, Fukuoka; Ken Inoue, Seishinkai Inoue Hospital, Fukuoka; Kouichi Fukuda, Medical Co. Chiyukai Fukuoka Wajiro Hospital, Fukuoka; Toru Umezu, Iryouhoujin Houmankai Umezu Medical Clinic, Fukuoka; Yousuke Miyagawa, Koga Hospital 21, Fukuoka; Shigeru Fujii, Medical Co. Chiyukai Fukuoka Shin Mizumaki Hospital, Fukuoka; Nobukuni Yoshida, Momochihama Clinic Twins Momochi Zaitakushinryosyo, Fukuoka; Munemitu Yamamoto, Hakataeki-higashi Clinic, Fukuoka; Shinichi Osaki, Osaki Internal Medicine, Respiratory Clinic, Fukuoka; Shigeki Hatama, Hatama Internal Medicine Clinic, Fukuoka; Junji Shibata, Medical Corporation Shibata-Clinic, Fukuoka; Masafumi Seki, Nagasaki University Hospital, Nagasaki; Yuichi Inoue, Isahaya Health Insurance General Hospital, Nagasaki; Toyomitsu Sawai, Sasebo General Hospital, Nagasaki; Yasuhito Higashiyama, Hokusho Central Hospital, Nagasaki; Kiyoyasu Fukushima, Japanese Red Cross Nagasaki Genbaku Isahaya Hospital, Nagasaki; Issei Tokimatsu, Oita University Hospital, Oita; Toshie Onodera, Mie Memorial Hospital, Oita; Takashige Miyazaki, Miyazaki Clinic, Oita.
Footnotes
Published ahead of print on 16 August 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Bantia, S., C. S. Arnold, C. D. Parker, R. Upshaw, and P. Chand. 2006. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antiviral Res. 69:39-45. [DOI] [PubMed] [Google Scholar]
- 2.Birnkrant, D., and E. Cox. 2009. The emergency use authorization of peramivir for treatment of 2009 H1N1 influenza. N. Engl. J. Med. 361:2204-2207. [DOI] [PubMed] [Google Scholar]
- 3.Bright, R. A., M. J. Medina, X. Xu, G. Perez-Oronoz, T. R. Wallis, X. M. Davis, L. Povinelli, N. Cox, and A. I. Klimov. 2005. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 366:1175-1181. [DOI] [PubMed] [Google Scholar]
- 4.Deyde, V. M., X. Xu, R. A. Bright, M. Shaw, C. B. Smith, Y. Zhang, Y. Shu, L. V. Gubareva, N. J. Cox, and A. I. Klimov. 2007. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 196:249-257. [DOI] [PubMed] [Google Scholar]
- 5.Division of Acquired Immunodeficiency Syndrome, NIAID, NIH. December 2004, posting date. Division of AIDS table for grading the severity of adult and pediatric adverse events. http://www3.niaid.nih.gov/research/resources/DAIDSClinRsrch/PDF/Safety/DAIDSAEGradingTable.pdf.
- 6.Gubareva, L. V., R. G. Webster, and F. G. Hayden. 2001. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob. Agents Chemother. 45:3403-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayden, F. G. 2009. Developing new antiviral agents for influenza treatment: what does the future hold? Clin. Infect. Dis. 48:S3-S13. [DOI] [PubMed] [Google Scholar]
- 8.Hayden, F. G., A. D. Osterhaus, J. J. Treanor, D. M. Fleming, F. Y. Aoki, K. G. Nicholson, A. M. Bohnen, H. M. Hirst, O. Keene, and K. Wightman, for the GG167 Influenza Study Group. 1997. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. N. Engl. J. Med. 337:874-880. [DOI] [PubMed] [Google Scholar]
- 9.Kidd, I. M., E. Nastouli, R. Shulman, P. R. Grant, D. C. J. Howell, and M. Singer. 2009. H1N1 pneumonitis treated with intravenous zanamivir. Lancet 374:1036. [DOI] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Lakatos, E. 1988. Sample sizes based on the log-rank statistic in complex clinical trials. Biometrics 44:229-241. [PubMed] [Google Scholar]
- 12.Louie, J. K., M Acosta, K. Winter, C. Jean, S. Gevali, R. Schechter, D. Vugia, K Harriman, B. Matyas, C. A. Glaser, M. C. Samuel, J. Rosenberg, J. Talarico, and D. Hatch, for the California Pandemic (H1N1) Working Group. 2009. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 302:1896-1902. [DOI] [PubMed] [Google Scholar]
- 13.McGeer, A., K. A. Green, A. Plevneshi, A. Shigayeva, N. Siddiqi, J. Raboud, D. E. Low, and the Toronto Invasive Bacterial Diseases Network. 2007. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin. Infect. Dis. 45:1568-1575. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros, R., M. A. Rameix-Welti, V. Lorin, P. Ribaud, J. C. Manuguerra, G. Socie, C. Scieux, N. Naffakh, and S. van der Werf. 2007. Failure of zanamivir therapy for pneumonia in a bone-marrow transplant recipient infected by a zanamivir-sensitive influenza A (H1N1) virus. Antivir. Ther. 12:571-576. [PubMed] [Google Scholar]
- 15.Nicholson, K. G., F. Y. Aoki, A. D. M. E Osterhaus, S. Trottier, O. Carewicz, C. H. Mercier, A. Rode, and P. Ward, for the Neuraminidase Inhibitor Flu Investigator Group. 2000. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 355:1845-1850. [DOI] [PubMed] [Google Scholar]
- 16.Osborne, R. H., G. Hawthorne, M. Panicolaou, and Y. Wegmueller. 2000. Measurement of rapid changes in health outcomes in people with influenza symptoms. J. Outcome Res. 4:15-30. [Google Scholar]
- 17.Potier, M., L. Mameli, M. Belislem, L. Dallaire, and S. B. Melanxon. 1979. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyla-D-N-acetylneuraminate) substrate. Anal. Biochem. 94:287-296. [DOI] [PubMed] [Google Scholar]
- 18.Seki, M., S. Kohno, M. W. Newstead, X. Zeng, U. Bhan, N. W. Lukacs, S. L. Kunkel, and T. J. Standiford. 2010. Critical role of IL-1 receptor-associated kinase-M in regulating chemokine-dependent deleterious inflammation in murine influenza pneumonia. J. Immunol. 184:1410-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki, M., K. Yanagihara, Y. Higashiyama, Y. Fukuda, Y. Kaneko, H. Ohno, Y. Miyazaki, Y. Hirakata, K. Tomono, J. Kadota, T. Tashiro, and S. Kohno. 2004. Immunokinetics in severe pneumonia due to influenza virus and bacteria coinfection in mice. Eur. Respir. J. 24:143-149. [DOI] [PubMed] [Google Scholar]
- 20.Taylor, W. R., B. N. Thinh, G. T. Anh, P. Horby, H. Wertheim, N. Lindegardh, M. D. de jong, K. Stepniewska, T. T. Hanh, N. D. Hien, N. M. Bien, N. Q. Chau, A. Fox, N. M. Ngoc, M. Crusat, J. J. Farrar, N. J. White, N. H. Ha, T. T. Lien, N. V. Trung, N. Day, and N. G. Binh. 2008. Oseltamivir is adequately absorbed following nasogastric administration to adult patients with severe H5N1 influenza. PLoS One 3410:e3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson, W. W., D. K. Shay, E. Weitraub, L. Brammer, N. Cox, L. J. Anderson, and K. Fukuda. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179-186. [DOI] [PubMed] [Google Scholar]
- 22.Treanor, J. J., F. G. Hayden, P. S. Vrooman, R. Barbarash, R. Bettis, D. Riff, S. Singh, N. Kinnersley, P. Ward, and R. G. Mills, for the U.S. Oral Neuraminidase Study Group. 2000. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treatment of acute influenza: a randomized controlled trial. JAMA 283:1016-1024. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. 2007. Update: WHO-confirmed human cases of avian influenza A (H5N1) infection, 25 November 2003-24 November 2006. Wkly. Epidemiol. Rec. 82:41-48. [Google Scholar]
- 24.World Health Organization. March 2008, posting date. Influenza A(H1N1) virus resistance to oseltamivir—2008/2009 influenza season, northern hemisphere. http://www.who.int/csr/disease/influenza/H1N1webupdate20090318%20ed_ns.pdf.
- 25.World Health Organization. November 2009, posting date. Clinical management of human infection with pandemic (H1N1) 2009: revised guidance. http://www.who.int/csr/resources/publications/swineflu/clinical_management/en/index.html.
- 26.Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus. 2008. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358:261-273. [DOI] [PubMed] [Google Scholar]
- 27.Yun, N. E., N. S. Linde, M. A., Zacks, I. G. Barr, A. C. Hurt, J. N. Smith, N. Dziuba, M. R. Holbrook, L. Zhang, J. M. Kilpatrick, C. S. Arnold, and S. Paessler. 2008. Injectable peramivir mitigates disease and promotes survival in ferrets and mice infected with the highly virulent influenza virus, A/Vietnam/1203/04 (H5N1). Virology 374:198-209. [DOI] [PMC free article] [PubMed] [Google Scholar]