Abstract
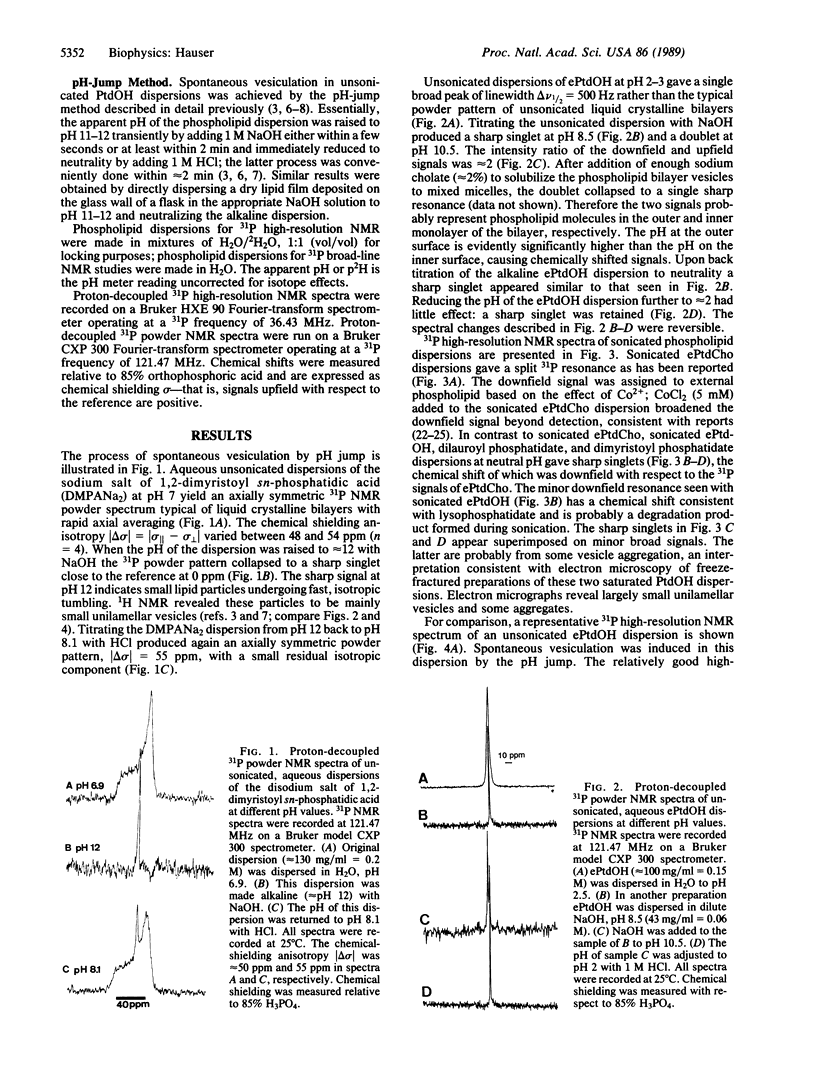
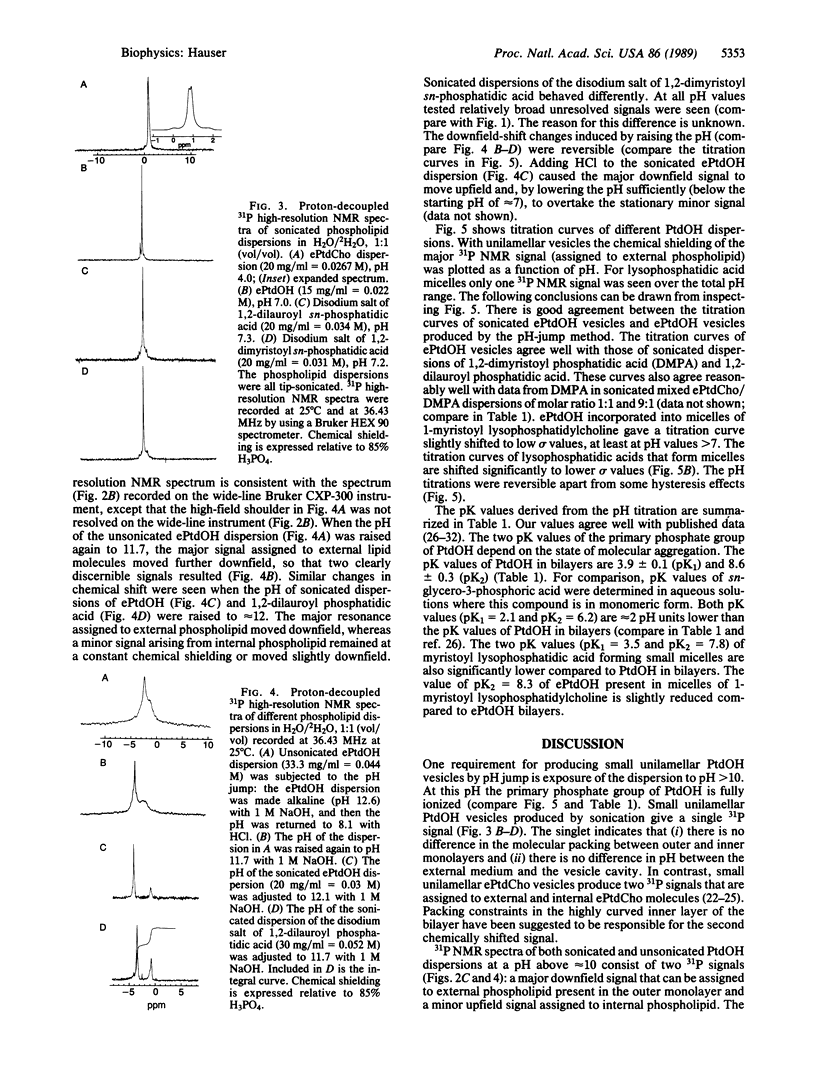
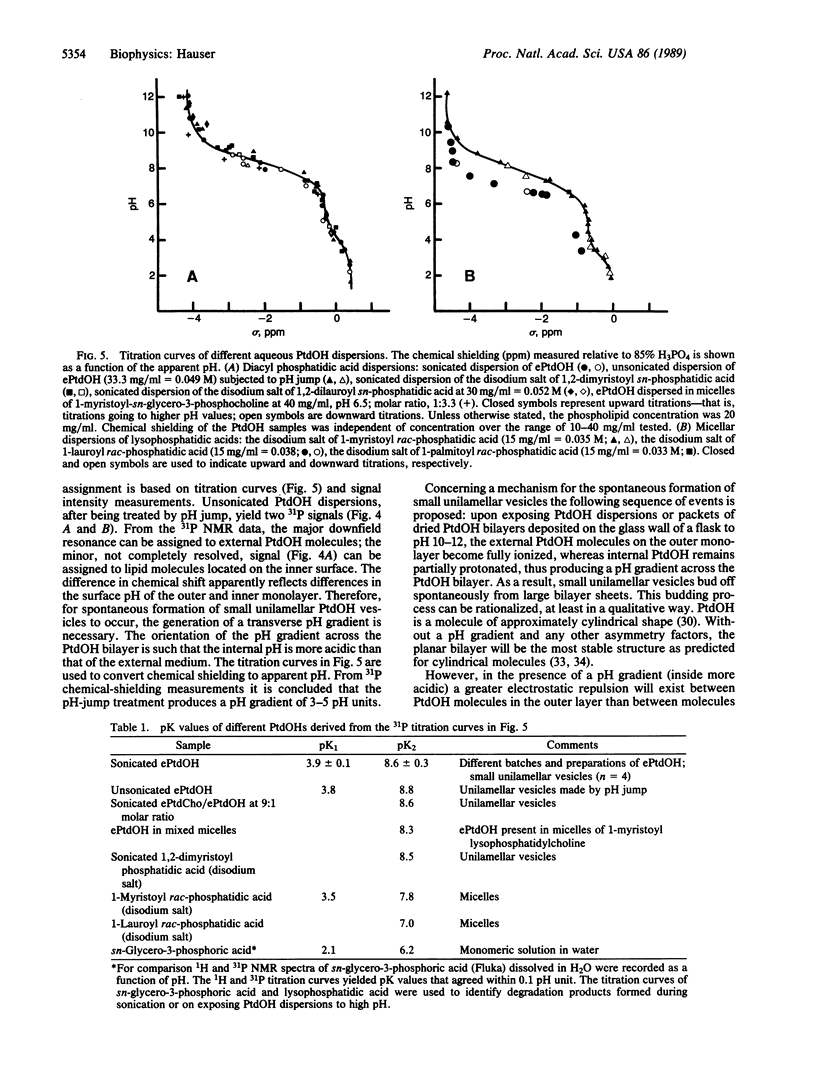
Both naturally occurring and synthetic phosphatidic acid (PtdOH) molecules show the phenomenon of spontaneous vesiculation on jump in pH value. This method involves a transient increase in pH of smectic PtdOH dispersions to values between 10 and 12. Such a pH increase induces spontaneous vesiculation with the formation of small unilamellar vesicles of diameter less than 50 nm as shown by 31P NMR. Both high-resolution and broad-line 31P NMR were used to study the mechanism of this process. When the pH of unsonicated PtdOH dispersions is raised to pH 10-12, lipid molecules on the outer monolayer of the bilayer become fully ionized. The second pK value of PtdOH in bilayers is 8.6 +/- 0.3, determined by 31P NMR. PtdOH molecules on the inner monolayer remain partially protonated. 31P NMR provides unambiguous evidence that the "pH-jump" treatment produces a pH gradient across the PtdOH bilayer. The orientation of the pH gradient is such that the pH in the external medium is 3-5 pH units higher than the internal pH. Associated with the pH gradient is a transverse packing asymmetry: partially protonated PtdOH molecules in the inner layer of the bilayer are more tightly packed than fully ionized molecules present in the outer layer. The pH gradient generated by the pH jump is proposed as the energy source that drives the spontaneous formation of highly curved vesicles.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON M. B., KATZMAN R., WILSON C. E., GREGOR H. P. IONIC PROPERTIES OF AQUEOUS DISPERSIONS OF PHOSPHATIDIC ACID. J Biol Chem. 1964 Dec;239:4066–4072. [PubMed] [Google Scholar]
- Atkinson D., Hauser H., Shipley G. G., Stubbs J. M. Structure and morphology of phosphatidylserine dispersions. Biochim Biophys Acta. 1974 Feb 26;339(1):10–29. doi: 10.1016/0005-2736(74)90329-0. [DOI] [PubMed] [Google Scholar]
- Aurora T. S., Li W., Cummins H. Z., Haines T. H. Preparation and characterization of monodisperse unilamellar phospholipid vesicles with selected diameters of from 300 to 600 nm. Biochim Biophys Acta. 1985 Nov 7;820(2):250–258. doi: 10.1016/0005-2736(85)90118-x. [DOI] [PubMed] [Google Scholar]
- Berden J. A., Barker R. W., Radda G. K. NMR studies on phospholipid bilayers. Some factors affecting lipid distribution. Biochim Biophys Acta. 1975 Jan 28;375(2):186–208. doi: 10.1016/0005-2736(75)90188-1. [DOI] [PubMed] [Google Scholar]
- Berden J. A., Cullis P. R., Hoult D. I., McLaughlin A. C., Radda G. K., Richards R. E. Frequency dependence of 31P NMR linewidths in sonicated phospholipid vesicles: effects of chemical shift anisotropy. FEBS Lett. 1974 Sep 15;46(1):55–58. doi: 10.1016/0014-5793(74)80333-9. [DOI] [PubMed] [Google Scholar]
- Boggs J. M. Intermolecular hydrogen bonding between lipids: influence on organization and function of lipids in membranes. Can J Biochem. 1980 Oct;58(10):755–770. doi: 10.1139/o80-107. [DOI] [PubMed] [Google Scholar]
- Bystrov V. F., Shapiro Y. E., Viktorov A. V., Barsukov L. I., Bergelson L. D. 31P-NMR signals from inner and outer surfaces of phospholipid membranes. FEBS Lett. 1972 Sep 15;25(2):337–338. doi: 10.1016/0014-5793(72)80518-0. [DOI] [PubMed] [Google Scholar]
- Cowley A. C., Fuller N. L., Rand R. P., Parsegian V. A. Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry. 1978 Jul 25;17(15):3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Chemically induced lipid phase separation in model membranes containing charged lipids: a spin label study. Biochim Biophys Acta. 1975 Sep 2;401(3):509–529. doi: 10.1016/0005-2736(75)90249-7. [DOI] [PubMed] [Google Scholar]
- Gulik-Krzywicki T., Rivas E., Luzzati V. Structure et polymorphisme des lipides: étude par diffraction des rayons X du systéme formé de lipides de mitochondries de coeur de boeuf et d'eau. J Mol Biol. 1967 Jul 28;27(2):303–322. doi: 10.1016/0022-2836(67)90022-8. [DOI] [PubMed] [Google Scholar]
- Haines T. H. Anionic lipid headgroups as a proton-conducting pathway along the surface of membranes: a hypothesis. Proc Natl Acad Sci U S A. 1983 Jan;80(1):160–164. doi: 10.1073/pnas.80.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H. O. The effect of ultrasonic irradiation on the chemical structure of egg lecithin. Biochem Biophys Res Commun. 1971 Nov;45(4):1049–1055. doi: 10.1016/0006-291x(71)90443-8. [DOI] [PubMed] [Google Scholar]
- Hauser H., Gains N., Eibl H. J., Müller M., Wehrli E. Spontaneous vesiculation of aqueous lipid dispersions. Biochemistry. 1986 Apr 22;25(8):2126–2134. doi: 10.1021/bi00356a042. [DOI] [PubMed] [Google Scholar]
- Hauser H., Gains N., Müller M. Vesiculation of unsonicated phospholipid dispersions containing phosphatidic acid by pH adjustment: physicochemical properties of the resulting unilamellar vesicles. Biochemistry. 1983 Sep 27;22(20):4775–4781. doi: 10.1021/bi00289a025. [DOI] [PubMed] [Google Scholar]
- Hauser H., Gains N. Spontaneous vesiculation of phospholipids: a simple and quick method of forming unilamellar vesicles. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1683–1687. doi: 10.1073/pnas.79.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Irons L. The effect of ultrasonication on the chemical and physical properties of aqueous egg yolk lecithin dispersions. Hoppe Seylers Z Physiol Chem. 1972 Oct;353(10):1579–1590. doi: 10.1515/bchm2.1972.353.2.1579. [DOI] [PubMed] [Google Scholar]
- Hauser H. Spontaneous vesiculation of uncharged phospholipid dispersions consisting of lecithin and lysolecithin. Chem Phys Lipids. 1987 May;43(4):283–299. doi: 10.1016/0009-3084(87)90024-7. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Jähnig F., Harlos K., Vogel H., Eibl H. Electrostatic interactions at charged lipid membranes. Electrostatically induced tilt. Biochemistry. 1979 Apr 17;18(8):1459–1468. doi: 10.1021/bi00575a012. [DOI] [PubMed] [Google Scholar]
- Li W., Haines T. H. Uniform preparations of large unilamellar vesicles containing anionic lipids. Biochemistry. 1986 Nov 18;25(23):7477–7483. doi: 10.1021/bi00371a033. [DOI] [PubMed] [Google Scholar]
- Mantelli S., Speiser P., Hauser H. Phase behaviour of a diglyceride prodrug: spontaneous formation of unilamellar vesicles. Chem Phys Lipids. 1985 Aug 15;37(4):329–343. doi: 10.1016/0009-3084(85)90087-8. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D. Surface properties of acidic phospholipids: interaction of monolayers and hydrated liquid crystals with uni- and bi-valent metal ions. Biochim Biophys Acta. 1968 Sep 17;163(2):240–254. doi: 10.1016/0005-2736(68)90103-x. [DOI] [PubMed] [Google Scholar]
- Tenchov B. G., Yanev T. K., Tihova M. G., Koynova R. D. A probability concept about size distributions of sonicated lipid vesicles. Biochim Biophys Acta. 1985 Jun 11;816(1):122–130. doi: 10.1016/0005-2736(85)90400-6. [DOI] [PubMed] [Google Scholar]
- Träuble H., Eibl H. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proc Natl Acad Sci U S A. 1974 Jan;71(1):214–219. doi: 10.1073/pnas.71.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Cullis P. R., Radda G. K. Differential scanning calorimetry and 31P NMR studies on sonicated and unsonicated phosphatidylcholine liposomes. Biochim Biophys Acta. 1975 Sep 16;406(1):6–20. doi: 10.1016/0005-2736(75)90038-3. [DOI] [PubMed] [Google Scholar]
- van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L., de Gier J. Comparative studies on the effects of pH and Ca2+ on bilayers of various negatively charged phospholipids and their mixtures with phosphatidylcholine. Biochim Biophys Acta. 1978 Sep 11;512(1):84–96. doi: 10.1016/0005-2736(78)90219-5. [DOI] [PubMed] [Google Scholar]


