Abstract
We describe a genetic system for producing specific gene knockouts in Cyanothece sp. strain PCC 7822 using a single-stranded DNA technique (B. Zorin, P. Hegemann, and I. Sizova, Eukaryot. Cell 4:1264-1272, 2005). The first fully segregated mutant was a ΔnifK mutant, and it was unable to grow on medium lacking combined nitrogen and produced virtually no hydrogen.
Cyanobacteria are important model organisms for the study of many biological processes, including photosynthesis, N2 fixation, and gene regulation of metabolism. This has led to the development of genetic systems in key organisms such as Synechococcus elongatus (10, 11, 16), Synechocystis sp. strain PCC 6803 (30, 31, 33), and Anabaena sp. strain PCC 7120 (6, 7, 8, 9, 12, 13, 25, 34, 35, 36) and in some other bacteria (14).
We wish to develop a genetic system for a strain within the genus Cyanothece (19), large (3- to 8-μm) unicellular, diazotrophic cyanobacteria. When cells are grown in the absence of combined nitrogen under 12-h light-dark conditions, Cyanothece sp. strain ATCC 51142 has an extreme temporal regulation that coordinates photosynthesis during the daytime and N2 fixation at night (3, 4, 19-22). This is a very valuable system for the study of photosynthesis, N2 fixation, cellular morphology, and gene regulation (24, 27, 28, 32). Because of these properties, six Cyanothece genomes have been sequenced, starting with Cyanothece sp. strain ATCC 51142 (32). Subsequently, five other Cyanothece strains have been sequenced through the Department of Energy (DOE) Joint Genome Initiative (http://www.jgi.doe.gov/), including Cyanothece sp. strain PCC 7822 (29).
Cyanothece sp. strain ATCC 51142 was grown on ASP2, and PCC 7424, PCC 7425, PCC 7822, PCC 8801, and PCC 8802 were grown on BG11 as previously described (19). Cyanothece strains were grown until late log phase (∼7 days), and cells were electroporated essentially as described previously (26), with the pulse controller set to 200 Ω. The cells were transformed with 1 μl DNA (5 pg to 0.5 μg) for 0.4 s and plated on either ASP2 plates (0.5% Phytagel) for Cyanothece sp. strain ATCC 51142 or BG11 plates (1.5% agar) for all of the other strains. The next day, after about 20 h of growth in continuous dim light (10 microeinsteins m−2 s−1), spectinomycin (Sp; 10 μg/ml), streptomycin (1 μg/ml), or kanamycin (Km; 25 μg/ml) was underlaid under the agar.
Cyanothece genes were PCR amplified using hybrid primers with an upstream 21-nucleotide sequence matching either the upstream region of the EcoRI site or the downstream region of the HindIII site on pUC19 (Table 1). pUC19 was double digested by EcoRI and HindIII. Linearized pUC19 and PCR products of Cyanothece genes were transformed into XL-1 Blue (18).
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant genotype, description, or sequence | Source or reference |
|---|---|---|
| E. coli strains | ||
| KC8 | Kmr RecA+lacΔ | Clontech |
| XL-1 Blue | Tcr nalidixic acid resistant | Agilent Technologies |
| Cyanothece sp. strains | ||
| ATCC 51142 | Isolated from intertidal area near Port Aransas, TX | Laboratory collection |
| PCC 7424 | Isolated from rice field soil, Senegal, 1972 | Laboratory collection |
| PCC 7425 | Isolated from rice field soil, Senegal, 1972 | Laboratory collection |
| PCC 7822 | Isolated from rice field soil at Central Rice Research Institute, Cuttack, Orissa, India | Laboratory collection |
| PCC 8801 | Isolated from rice field soil (during spring), Ping-Tong District, southern Taiwan, as Synechococcus sp. strain RF-1 | Laboratory collection |
| PCC 8802 | Isolated from rice field soil (during spring), Ping-Tong District, southern Taiwan, as Synechococcus sp. strain RF-2 | Laboratory collection |
| Plasmids | ||
| pUC19 | Cloning vector | Laboratory collection |
| pRL1383a | RSF1010-derived broad-host-range vector, Spr and Smr, accession no. AF403426 | 10 |
| pAM1037 | Transposon Tn5 derivative (Kmr) | 19 |
| pRL448 | Plasmid carrying Kmr cassette | 8 |
| pRL453 | Plasmid carrying Sp/Sm Ω cassette | 8 |
| pHM54 | NifK knockout construct for PCC 7822 with Spr cassette going against nifK | This study |
| pHM55 | NifK knockout construct for PCC 7822 with Spr cassette going with nifK | This study |
| Primers | ||
| 7822 NifK1 | GCTATGACCATGATTACGCCAAGACCACGTTGAATTATTCC | This study |
| 7822 NifK2 | GTTGTAAAACGACGGCCAGTGTACGATCGATATCTTCAAACAGAG | This study |
| 7822 NifK3 | CGGCTGTCTTACCATGTAACCAAGC | This study |
| Sp/Up | CCAAGGATCGGGCCTTGATG | This study |
| Sp/11Up | CGTAACGCGCTTGCTGCTTG | This study |
The nifK gene was amplified by PCR using primers 7822 NifK1 and 7822 NifK2 (Table 1). Asymmetric PCR was carried out on pHM54 and pHM55 using the regular amount of 7822 NifK1 and 1/50 of the regular amount of 7822 NifK2 to produce single-stranded DNA (ss DNA) (37). The PCR product of ssDNA was boiled for 5 min and put into ice immediately for electroporation.
The putative ΔnifK mutant was analyzed for growth, acetylene reduction activity, and H2 production as previously described (17).
We made numerous unsuccessful attempts to construct specific knockout mutants of Cyanothece sp. strain ATCC 51142 (nifH, nifD, ntcA, and kaiA mutants) using single and double recombination procedures. The failure of single and double recombinations suggested to us that the Spr cassette was inserted randomly into Cyanothece sp. strain ATCC 51142 at a very high frequency. To confirm this, we used the Spr cassette alone, along with the kaiA knockout construct, to transform Cyanothece sp. strain ATCC 51142 in order to compare the levels of transformation. The Spr cassette alone gave rise to as many transformants as the kaiA knockout construct. The Spr cassette was recovered from the transformants that were produced by the Spr cassette (along with its flanking sequences) and sequenced. The sequencing results (see below) from three transformants indicated that the Spr cassette was inserted into the Cyanothece sp. strain ATCC 51142 genome randomly.
Sequencing results.
Three results, as follows, were found. (i) The Spr cassette was modified by direct repeats (TGCTTGCTGCTTGCTGCTTGCTGCTTGCTGCTTGCTGCTTGCTGCTTGCTGCTTGCTGCTTGC) and then inserted between amino acids (aa) 66 and 67 on cce_3669, a hypothetical protein. (ii) The Spr cassette was inserted between aa 192 and 193 on cce_4555, a putative polysaccharide pyruvyl transferase. (iii) The Spr cassette was inserted between aa 126 and 127 on cce_1116, a putative biopolymer transport protein.
From such experiments, we concluded that nonhomologous recombination was significantly more likely than homologous recombination and that Cyanothece sp. strain ATCC 51142 likely contained a recombination system that could insert such a cassette randomly throughout the genome.
Cyanothece sp. strains PCC 7424, PCC 7425, PCC 7822, PCC 8801, and PCC 8802 were successfully transformed by electroporation with a Tn5 derivative (pAM1037) and pRL448 to kanamycin resistance and by pRL1383a and pRL453 to Spr. All of the strains, except PCC 7822, yielded many Spr colonies by nonhomologous recombination (Table 2). Since Cyanothece sp. strain PCC 7822 had the best ratio of legitimate transformation versus nonhomologous illegitimate recombination, it was chosen for mutagenesis by insertional inactivation.
TABLE 2.
Transformation efficiencya
| Cyanothece sp. strain | No. of transformants/CFU, 104 |
Kmr ratio (pAM1037/pRL448) | No. of transformants/CFU, 104 |
Spr ratio (pRL1383a/pRL453) | ||
|---|---|---|---|---|---|---|
| pAM1037 (Kmr) | pRL448 (Kmr) | pRL1383a (Spr) | pRL453 (Spr) | |||
| ATCC 51142 | 2.0 | 2.0 | 1.0 | 2.0 | 2.0 | 1.0 |
| PCC 7424 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| PCC 7425 | 2.0 | 2.0 | 1.0 | 2.0 | 2.0 | 1.0 |
| PCC 7822 | 1.0 | 0.2 | 5.0 | 1.0 | 0.01 | 100.0 |
| PCC 8801 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| PCC 8802 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
Six Cyanothece strains were transformed by transposon Tn5 derivative pAM1037 or broad-host-range plasmid pRL1383a in comparison with suicide vector pRL448 or pRL453, respectively. Only Cyanothece sp. strain PCC 7822 demonstrated a significantly lower background (transformation by suicide vectors) to evoke future mutagenesis by homologous recombination.
PCR-amplified nifK was cloned into pUC19, and Cyanothece sp. strain PCC 7822 was transformed by electroporation using ssDNA made from pHM54 and pHM55 (Table 1 and Fig. 1). Twenty Spr colonies were picked from each group for segregation by stepwise transfer onto fresh Sp plates three times, and 3 out of 20 colonies in each group demonstrated the mutant phenotype; i.e., they could not grow in the absence of combined nitrogen. We focused on three mutants from the pHM54 group. Colony PCR confirmed that two out of these three colonies had the Spr cassette inserted in nifK as shown in Fig. 1. DNA sequencing was performed on the PCR product from one colony, and the results (Fig. 2) indicated that the Spr cassette was inserted into the EcoRI site of nifK as designed and sketched in Fig. 1. Colony PCR on the ΔnifK mutant after 1 month of continuous growth in the presence of antibiotics indicated that chromosomal segregation was complete.
FIG. 1.
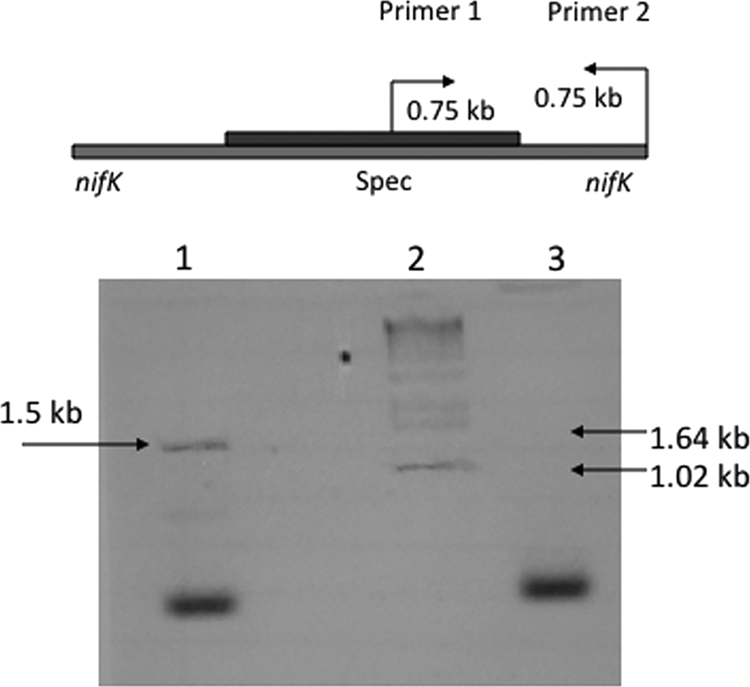
PCR confirmation of the Cyanothece sp. strain PCC 7822 ΔnifK mutant. PCR with primer 1 in the Spr cassette (Sp/11Up) and primer 2 in the nifK gene (7822 NifK2) produced a band of about 1.5 kb from ΔnifK mutant DNA (lane 1) but not from wild-type DNA (lane 3). Lane 2 is the 1-kb DNA ladder, and the 1.64- and 1.02-kb bands are highlighted. The 1.5-kb band in the ΔnifK mutant then was sequenced to demonstrate that the Spr cassette (Spec) was located within the nifK gene as shown in the scheme at the top.
FIG. 2.
The Spr cassette was inserted into nifK at the EcoRI site as designed. The PCR product from the ΔnifK mutant was sequenced from a primer within the Spr cassette, Sp/Up (Table 1). The sequence underlined is the Spr cassette, and the rest is nifK. The GAATTC sequence shown in bold is the EcoRI site. Another sequencing result using a primer with nifK (7822 NifK3, Table 1) confirmed the insertion of the Spr cassette into nifK as well (data not shown).
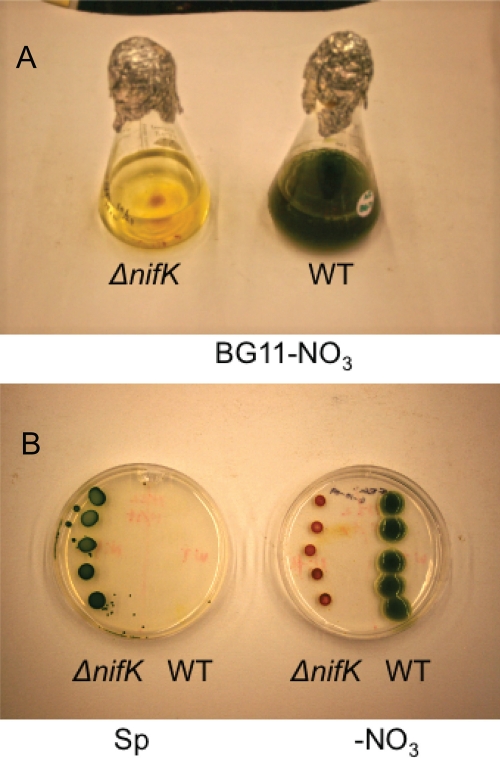
The phenotype of the ΔnifK mutant was demonstrated both on plates and in liquid culture (Fig. 3A and B). When the ΔnifK mutant and the wild type were spotted onto plates containing Sp, the wild type died, as seen after 5 weeks (Fig. 3B, left plate), whereas the ΔnifK mutant always grew well. When the two strains were spotted onto plates lacking combined nitrogen, the wild type always grew whereas the ΔnifK mutant slowly died (Fig. 3B, right plate). The phenotype was noticeable by 1 week, and by 5 weeks, the culture was completely bleached (Fig. 3A). This phenotype was demonstrated numerous times and has remained stable for >1 year. These results strongly suggested that the ΔnifK mutant was incapable of growth on media lacking combined nitrogen and presumably was defective in N2 fixation. We then checked the mutant for both hydrogen production and nitrogenase activity. As shown in Table 3, the ΔnifK mutant produced little hydrogen when incubated either in air or under argon.
FIG. 3.
Growth of the Cyanothece sp. strain PCC 7822 wild type (WT) and the ΔnifK mutant in liquid media (A) and on plates (B). (A) The wild type and the ΔnifK mutant were grown in BG11 medium without combined nitrogen for 5 weeks. The mutant was unable to fix nitrogen and began to appear bleached after 1 week. (B) The wild type and the ΔnifK mutant were spotted onto BG11 plates with Sp (left) to demonstrate that the mutant, but not the wild type, was antibiotic resistant. The plate on the right contained no combined nitrogen and demonstrated that the wild type, but not the ΔnifK mutant, could fix nitrogen.
TABLE 3.
Acetylene reduction activity and hydrogen production of Cyanothece sp. strain PCC 7822 and the ΔnifK mutant after incubation in air or argon
| Cyanothece sp. strain PCC 7822 | Incubation conditiona | Avg relative acetylene reduction activityb ± SD | Avg hydrogen production rateb ± SD |
|---|---|---|---|
| Wild type | Air | 1 | 5.1 ± 1.8 |
| ΔnifK mutant | Air | 2.7 ± 0.9 | 1.7 ± 2.5 |
| Wild type | Argon | 31.5 ± 10.6 | 58 ± 16 |
| ΔnifK mutant | Argon | 1,139 ± 363 | 2.4 ± 3.4 |
Cultures were grown in medium containing N (2.5 mM NH4NO3) under low-light conditions for 10 days, washed with N-free medium twice, and grown in N-free medium for 3 days under low-light conditions. Then, 50 ml was added to 66-ml bottles. Some bottles were sparged with argon. Acetylene reduction assays were performed after the bottles were shaken under low-light conditions (30 μmol photons m−2 s−1) for 22 h. After injection of 3 ml of acetylene, the bottles were also kept under light for 2 h.
Activities were computed as milligrams of chlorophyll a per hour, and the acetylene reduction activities were normalized to the wild-type value obtained in air. Hydrogen production rates are in micromoles of H2 per milligram of chlorophyll a per hour.
Similarly, the nitrogenase activity of the ΔnifK mutant differed from that of the wild type, but in an interesting fashion. As shown in Table 3, acetylene reduction by the ΔnifK mutant was actually higher than that of the wild type when cells were incubated with either acetylene in air or acetylene in argon. The rate of acetylene reduction by the ΔnifK mutant under argon was ∼1,000-fold greater than that by the wild type with acetylene in air.
This nitrogenase phenotype is not unique, and we must consider the relationship of H2 evolution and acetylene reduction to the nitrogenase enzyme (1, 2). Hydrogen is always produced when nitrogenase reduces N2 to NH3, indicating that H2 evolution is integral to the enzyme mechanism (23). Importantly, reduction of acetylene to ethylene is not accompanied by H2 evolution and it is possible that nitrogenase reduces acetylene when only partially activated with no H2 evolution. Acetylene reduction discharges nitrogenase before it ever reaches full activation. The current seven-stage mechanism for the fixation of N2 to the production of 2NH3 also explains why acetylene is a competitive inhibitor of N2 fixation, whereas N2 is a noncompetitive inhibitor of acetylene reduction (2, 5, 15). Consistent with this feature, air (79% N2) inhibited acetylene reduction in the wild type and the ΔnifK mutant, but to a greater extent in the ΔnifK mutant. This result suggested that the MoFe center is present in the mutant (1, 2). Finally, N2 cannot fully stop H2 evolution, as we have also demonstrated in Cyanothece sp. strain PCC 7822 (17), whereas acetylene can (1). Thus, the phenotype of the ΔnifK mutant indicated that the metal cofactors (e.g., MoFe) were assembled and were likely poorly integrated into the abnormal nitrogenase complex in the mutant.
Cyanothece sp. strain PCC 7822 represents an excellent organism for further studies. It demonstrates cycling behavior of photosynthesis and nitrogen fixation, and like Cyanothece sp. strain ATCC 51142, it produces large quantities of organic acids, lipids, and polyhydroxyalkanoates (pHAs) and copious levels of hydrogen (17). The genomic sequence has been completed, and we have the opportunity to use this strain for metabolic enhancement of one or more of these important compounds.
Acknowledgments
We thank Peter Wolk (Michigan State University) and Susan Golden (University of California—San Diego) for graciously supplying key constructs used in this study.
This work was supported in part by a grant from the DOE Genomics:GTL program (DE 09-19 PO 2905402N, Himadri Pakrasi, principal investigator) and in part by a grant from the Membrane Biology EMSL Scientific Grand Challenge project at the W. R. Wiley Environmental Molecular Science Laboratory, a national scientific user facility sponsored by the U.S. DOE Office of Biological and Environmental Research program located at the Pacific Northwest National Laboratory. The Pacific Northwest National Laboratory is operated for the DOE by Battelle.
Footnotes
Published ahead of print on 17 September 2010.
REFERENCES
- 1.Barney, B. M., H. I. Lee, P. C. Dos Santos, B. M. Hoffman, D. R. Dean, and L. C. Seefeldt. 2006. Breaking the N2 triple bond: insights into the nitrogenase mechanism. Dalton Trans. 21:2277-2284. [DOI] [PubMed] [Google Scholar]
- 2.Barney, B. M., T. C. Yang, R. Y. Igarashi, P. C. Dos Santos, M. Laryukhin, H. I. Lee, B. M. Hoffman, D. R. Dean, and L. C. Seefeldt. 2005. Intermediates trapped during nitrogenase reduction of N triple bond N, CH3-N=NH, and H2N-NH2. J. Am. Chem. Soc. 127:14960-14961. [DOI] [PubMed] [Google Scholar]
- 3.Colón-López, M. S., D. M. Sherman, and L. A. Sherman. 1997. Transcriptional and translational regulation of nitrogenase in light-dark- and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 179:4319-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colón-López, M. S., and L. A. Sherman. 1998. Transcriptional and translational regulation of photosystem I and II genes in light-dark- and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 180:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dos Santos, P. C., R. Y. Igarashi, H. I. Lee, B. M. Hoffman, L. C. Seefeldt, and D. R. Dean. 2005. Substrate interactions with the nitrogenase active site. Acc. Chem. Res. 38:208-214. [DOI] [PubMed] [Google Scholar]
- 6.Elhai, J., T. Thiel, and H. B. Pakrasi. 1990. DNA transfer into cyanobacteria, p. 1-23. In S. B. Gelvin and R. A. Schilperoort (ed.), Plant molecular biology manual. Kluwer Academic Publishers, Dordrecht, Netherlands.
- 7.Elhai, J., A. Vepritskiy, A. M. Muro-Pastor, E. Flores, and C. P. Wolk. 1997. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 179:1998-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhai, J., and C. P. Wolk. 1988. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 167:747-754. [DOI] [PubMed] [Google Scholar]
- 9.Elhai, J., and C. P. Wolk. 1988. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene 68:119-138. [DOI] [PubMed] [Google Scholar]
- 10.Golden, S. S. 1988. Mutagenesis of cyanobacteria by classical and gene-transfer-based methods. Methods Enzymol. 167:714-727. [DOI] [PubMed] [Google Scholar]
- 11.Golden, S. S., and L. A. Sherman. 1984. Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J. Bacteriol. 158:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haselkorn, R. 1991. Genetic systems in cyanobacteria. Methods Enzymol. 204:418-430. [DOI] [PubMed] [Google Scholar]
- 13.Haselkorn, R. 1995. Molecular genetics of nitrogen fixation in photosynthetic prokaryotes, p. 29-36. In I. A. Tikhonovich, N. A. Provorov, V. I. Romanov, and W. E. Newton (ed.), Nitrogen fixation: fundamentals and applications. Kluwer Academic Publishers, Dordrecht, Netherlands.
- 14.Koksharova, O. A., and C. P. Wolk. 2002. Genetic tools for cyanobacteria. Appl. Microbiol. Biotechnol. 58:123-137. [DOI] [PubMed] [Google Scholar]
- 15.Liang, J., and R. H. Burris. 1988. Interactions among N2, N2O, and C2H2 as substrates and inhibitors of nitrogenase from Azotobacter vinelandii. Biochemistry 27:6726-6732. [Google Scholar]
- 16.Mackey, S. R., and S. S. Golden. 2007. Winding up the cyanobacterial circadian clock. Trends Microbiol. 15:381-388. [DOI] [PubMed] [Google Scholar]
- 17.Min, H., and L. A. Sherman. 2010. Hydrogen production by the unicellular, diazotrophic cyanobacterium Cyanothece sp. strain ATCC 51142 under conditions of continuous light. Appl. Environ. Microbiol. 76:4293-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parrish, J. R., T. Limjindaporn, J. A. Hines, J. Liu, G. Liu, and R. L. Finley, Jr. 2004. High-throughput cloning of Campylobacter jejuni ORFs by in vivo recombination in Escherichia coli. J. Proteome Res. 3:582-586. [DOI] [PubMed] [Google Scholar]
- 19.Reddy, K. J., J. B. Haskell, D. M. Sherman, and L. A. Sherman. 1993. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J. Bacteriol. 175:1284-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneegurt, M. A., D. M. Sherman, S. Nayar, and L. A. Sherman. 1994. Oscillating behavior of carbohydrate granule formation and dinitrogen fixation in the cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 176:1586-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneegurt, M. A., D. M. Sherman, and L. A. Sherman. 1997. Composition of the carbohydrate granules of the cyanobacterium, Cyanothece sp. strain ATCC 51142. Arch. Microbiol. 167:89-98. [PubMed] [Google Scholar]
- 22.Sherman, L. A., P. Meunier, and M. S. Colón-López. 1998. Diurnal rhythms in metabolism: a day in the life of a unicellular, diazotrophic cyanobacterium. Photosynth. Res. 58:25-42. [Google Scholar]
- 23.Simpson, F. B., and R. H. Burris. 1984. A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase. Science 224:1095-1097. [DOI] [PubMed] [Google Scholar]
- 24.Stöckel, J., E. A. Welsh, M. Liberton, R. Kunnvakkam, R. Aurora, and H. B. Pakrasi. 2008. Global transcriptomic analysis of Cyanothece 51142 reveals robust diurnal oscillation of central metabolic processes. Proc. Natl. Acad. Sci. U. S. A. 105:6156-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiel, T. 1994. Genetic analysis of cyanobacteria, p. 581-611. In D. A. Brant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publisher, Dordrecht, Netherlands.
- 26.Thiel, T., and H. Poo. 1989. Transformation of a filamentous cyanobacterium by electroporation. J. Bacteriol. 171:5743-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toepel, J., J. E. McDermott, T. C. Summerfield, and L. A. Sherman. 2009. Transcriptional analysis of the unicellular, diazotrophic cyanobacterium Cyanothece sp. ATCC 51142 grown under short day/night cycles. J. Phycol. 45:610-620. [DOI] [PubMed] [Google Scholar]
- 28.Toepel, J., E. Welsh, T. C. Summerfield, H. B. Pakrasi, and L. A. Sherman. 2008. Differential transcriptional analysis of the cyanobacterium Cyanothece sp. strain ATCC 51142 during light-dark and continuous-light growth. J. Bacteriol. 190:3904-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Oost, J., B. A. Bulthuis, S. Feitz, K. Krab, and R. Kraayenhof. 1989. Fermentation metabolism of the unicellular cyanobacterium Cyanothece PCC 7822. Arch. Microbiol. 152:415-419. [Google Scholar]
- 30.Vermaas, W. 1996. Molecular genetics of the cyanobacterium Synechocystis sp. PCC 6803: principles and possible biotechnology applications. J. Appl. Phycol. 8:263-273. [Google Scholar]
- 31.Vermaas, W. F. 1998. Gene modifications and mutation mapping to study the function of photosystem II. Methods Enzymol. 297:293-310. [DOI] [PubMed] [Google Scholar]
- 32.Welsh, E. A., M. Liberton, J. Stockel, T. Loh, T. Elvitigala, C. Wang, A. Wollam, R. S. Fulton, S. W. Clifton, J. M. Jacobs, R. Aurora, B. K. Ghosh, L. A. Sherman, R. D. Smith, R. K. Wilson, and H. B. Pakrasi. 2008. The genome of Cyanothece 51142, a unicellular diazotrophic cyanobacterium important in the marine nitrogen cycle. Proc. Natl. Acad. Sci. U. S. A. 105:15094-15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams, J. G., and A. A. Szalay. 1983. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene 24:37-51. [DOI] [PubMed] [Google Scholar]
- 34.Wolk, C. P. 1996. Heterocyst formation. Annu. Rev. Genet. 30:59-78. [DOI] [PubMed] [Google Scholar]
- 35.Wolk, C. P., and J. Kraus. 1982. Two approaches to obtaining low, extracellular deoxyribonuclease activity in cultures of heterocyst-forming cyanobacteria. Arch. Microbiol. 131:302-307. [Google Scholar]
- 36.Wolk, C. P., A. Vonshak, P. Kehoe, and J. Elhai. 1984. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 81:1561-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zorin, B., P. Hegemann, and I. Sizova. 2005. Nuclear-gene targeting using single-stranded DNA avoids illegitimate DNA integration in Chlamydomonas reinhardtii. Eukaryot. Cell 4:1264-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]