Abstract
Considerable experimental evidence is in support of several aspects of the mechanism that has been proposed for the catalytic activity of lysozyme. However, the enzymatically catalyzed hydrolysis of polysaccharides proceeds over 5 orders of magnitude faster than that of model compounds that mimic the configuration of the substrate in the active site of the enzyme. Although several possible explanations for this rate enhancement have been discussed elsewhere, a definitive mechanism has not emerged. Here we report striking results obtained by classical electrodynamics, which suggest that bond breakage and the consequent separation of charge in lysozyme is promoted by a large electrostatic field across the active site cleft, produced in part by a very asymmetric distribution of charged residues on the enzyme surface. Lysozymes unrelated in amino acid sequence have similar distributions of charged residues and electric fields. The results reported here suggest that the electrostatic component of the rate enhancement is greater than 9 kcal.mol-1. Thus, electrostatic interactions may play a more important role in the enzymatic mechanism than has generally been appreciated.
Full text
PDF
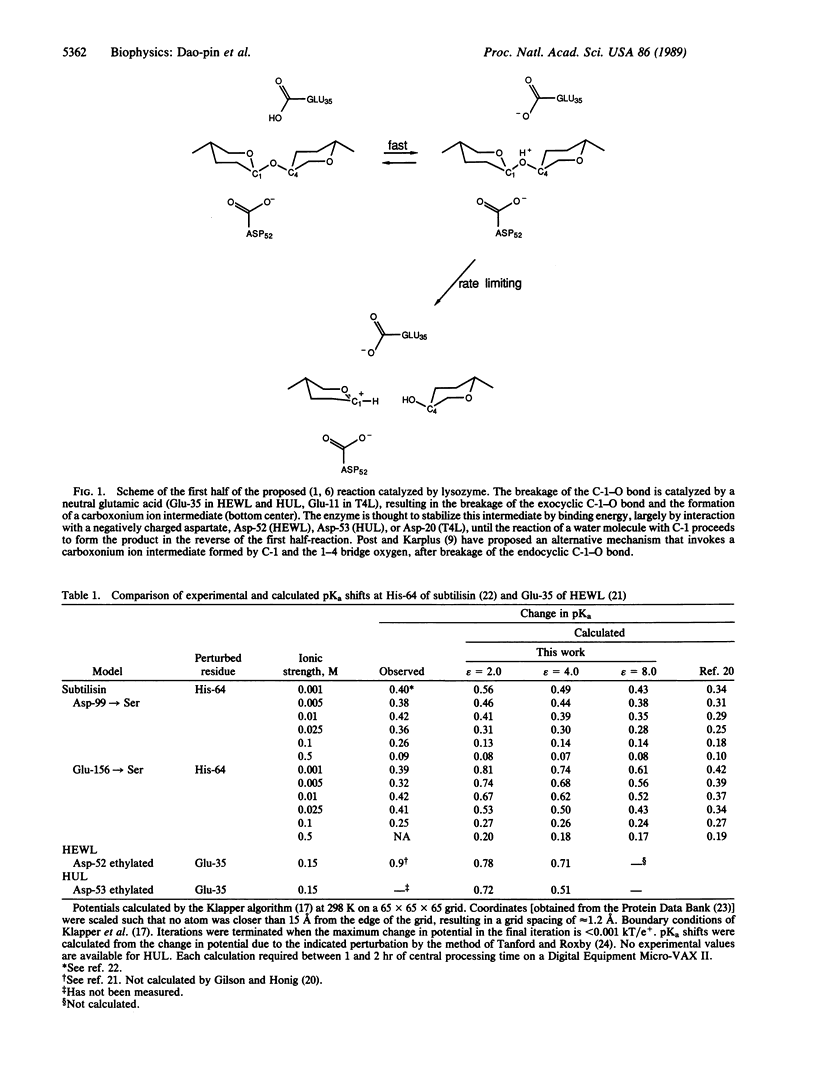
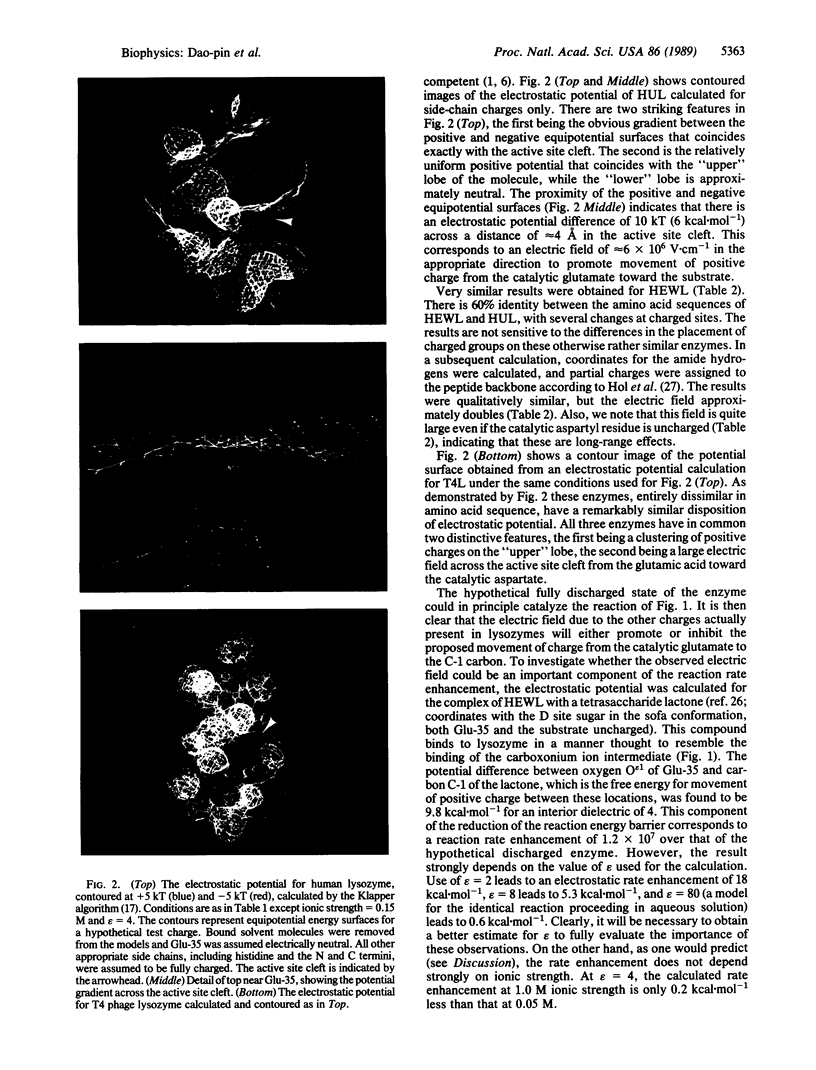


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T., Sun D. P., Wilson K., Wozniak J. A., Cook S. P., Matthews B. W. Contributions of hydrogen bonds of Thr 157 to the thermodynamic stability of phage T4 lysozyme. Nature. 1987 Nov 5;330(6143):41–46. doi: 10.1038/330041a0. [DOI] [PubMed] [Google Scholar]
- Anderson W. F., Grütter M. G., Remington S. J., Weaver L. H., Matthews B. W. Crystallographic determination of the mode of binding of oligosaccharides to T4 bacteriophage lysozyme: implications for the mechanism of catalysis. J Mol Biol. 1981 Apr 25;147(4):523–543. doi: 10.1016/0022-2836(81)90398-3. [DOI] [PubMed] [Google Scholar]
- Artymiuk P. J., Blake C. C. Refinement of human lysozyme at 1.5 A resolution analysis of non-bonded and hydrogen-bond interactions. J Mol Biol. 1981 Nov 15;152(4):737–762. doi: 10.1016/0022-2836(81)90125-x. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Koenig D. F., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution. Nature. 1965 May 22;206(4986):757–761. doi: 10.1038/206757a0. [DOI] [PubMed] [Google Scholar]
- Dahlquist F. W., Rand-Meir T., Raftery M. A. Demonstration of carbonium ion intermediate during lysozyme catalysis. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1194–1198. doi: 10.1073/pnas.61.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. O., Johnson L. N., Machin P. A., Phillips D. C., Tjian R. Crystal structure of a lysozyme-tetrasaccharide lactone complex. J Mol Biol. 1974 Sep 15;88(2):349–371. doi: 10.1016/0022-2836(74)90487-2. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. H. Calculation of electrostatic potentials in an enzyme active site. Nature. 1987 Nov 5;330(6143):84–86. doi: 10.1038/330084a0. [DOI] [PubMed] [Google Scholar]
- Hol W. G., van Duijnen P. T., Berendsen H. J. The alpha-helix dipole and the properties of proteins. Nature. 1978 Jun 8;273(5662):443–446. doi: 10.1038/273443a0. [DOI] [PubMed] [Google Scholar]
- Klapper I., Hagstrom R., Fine R., Sharp K., Honig B. Focusing of electric fields in the active site of Cu-Zn superoxide dismutase: effects of ionic strength and amino-acid modification. Proteins. 1986 Sep;1(1):47–59. doi: 10.1002/prot.340010109. [DOI] [PubMed] [Google Scholar]
- Malcolm B. A., Rosenberg S., Corey M. J., Allen J. S., de Baetselier A., Kirsch J. F. Site-directed mutagenesis of the catalytic residues Asp-52 and Glu-35 of chicken egg white lysozyme. Proc Natl Acad Sci U S A. 1989 Jan;86(1):133–137. doi: 10.1073/pnas.86.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. M., Raftery M. A. Ionization behavior of the catalytic carboxyls of lysozyme. Effects of ionic strength. Biochemistry. 1972 Apr 25;11(9):1623–1629. doi: 10.1021/bi00759a013. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Dahlquist F. W. The chemistry of lysozyme. Fortschr Chem Org Naturst. 1969;27:340–381. [PubMed] [Google Scholar]
- Remington S. J., Anderson W. F., Owen J., Ten Eyck L. F., Grainger C. T., Matthews B. W. Structure of the lysozyme from bacteriophage T4: an electron density map at 2.4 A resolution. J Mol Biol. 1978 Jan 5;118(1):81–98. doi: 10.1016/0022-2836(78)90245-0. [DOI] [PubMed] [Google Scholar]
- Russell A. J., Thomas P. G., Fersht A. R. Electrostatic effects on modification of charged groups in the active site cleft of subtilisin by protein engineering. J Mol Biol. 1987 Feb 20;193(4):803–813. doi: 10.1016/0022-2836(87)90360-3. [DOI] [PubMed] [Google Scholar]
- Smith L. E., Mohr L. H., Raftery M. A. Mechanism for lysozyme-catalyzed hydrolysis. J Am Chem Soc. 1973 Oct 31;95(22):7497–7500. doi: 10.1021/ja00803a046. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Hayes F. R., Russell A. J., Thomas P. G., Fersht A. R. Prediction of electrostatic effects of engineering of protein charges. Nature. 1987 Nov 5;330(6143):86–88. doi: 10.1038/330086a0. [DOI] [PubMed] [Google Scholar]
- Tanford C., Roxby R. Interpretation of protein titration curves. Application to lysozyme. Biochemistry. 1972 May 23;11(11):2192–2198. doi: 10.1021/bi00761a029. [DOI] [PubMed] [Google Scholar]
- Vernon C. A. The mechanisms of hydrolysis of glycosides and their revelance to enzyme-catalysed reactions. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):389–401. doi: 10.1098/rspb.1967.0036. [DOI] [PubMed] [Google Scholar]
- Warshel A. Energetics of enzyme catalysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5250–5254. doi: 10.1073/pnas.75.11.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A., Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976 May 15;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]
- Warwicker J., Watson H. C. Calculation of the electric potential in the active site cleft due to alpha-helix dipoles. J Mol Biol. 1982 Jun 5;157(4):671–679. doi: 10.1016/0022-2836(82)90505-8. [DOI] [PubMed] [Google Scholar]