Abstract
The importance of viruses in aquatic ecosystem functioning has been widely described. However, few studies have examined tropical aquatic ecosystems. Here, we evaluated for the first time viruses and their relationship with other planktonic communities in an Amazonian freshwater ecosystem. Coupling between viruses and bacteria was studied, focusing both on hydrologic dynamics and anthropogenic forced turbidity in the system (Lake Batata). Samples were taken during four hydrologic seasons at both natural and impacted sites to count virus-like particles (VLP) and bacteria. In parallel, virus-infected bacteria were identified and quantified by transmission electron microscopy (TEM). Viral abundance ranged from 0.5 × 107 ± 0.2 × 107 VLP ml−1 (high-water season, impacted site) to 1.7 × 107 ± 0.4 × 107 VLP ml−1 (low-water season, natural site). These data were strongly correlated with the bacterial abundance (r2 = 0.84; P < 0.05), which ranged from 1.0 × 106 ± 0.5 × 106 cells ml−1 (high water, impacted site) to 3.4 × 106 ± 0.7 × 106 cells ml−1 (low water, natural site). Moreover, the viral abundance was weakly correlated with chlorophyll a, suggesting that most viruses were bacteriophages. TEM quantitative analyses revealed that the frequency of visibly infected cells was 20%, with 10 ± 3 phages per cell section. In general, we found a low virus-bacterium ratio (<7). Both the close coupling between the viral and bacterial abundances and the low virus-bacterium ratio suggest that viral abundance tends to be driven by the reduction of hosts for viral infection. Our results demonstrate that viruses are controlled by biological substrates, whereas in addition to grazing, bacteria are regulated by physical processes caused by turbidity, which affect underwater light distribution and dissolved organic carbon availability.
Viruses are the most abundant and dynamic components of the aquatic microbial community (6, 31, 32). Viruses influence many biogeochemical and ecological processes, including nutrient cycling, system respiration, particle-size distribution, bacterial and algal biodiversity, species distribution, algal blooms, and genetic transfer between microorganisms (21, 49). In addition, viruses play a major role in aquatic microbial food webs by releasing carbon trapped in host cells to the dissolved organic carbon (DOC) pool and ultimately back to the bacterial community (11, 21). The action of viruses is an important mechanism of bacterial regulation in aquatic ecosystems, acting directly on bacterial populations and indirectly on bacterial diversity by decreasing the density of dominant bacterial species (31). Studies based on viral decay rates and electron microscopy analyses have shown that viruses can cause up to 40% of bacterial mortality and more than 10% of phytoplankton mortality in aquatic systems (11, 22, 48, 50, 54, 55). It also has been suggested that viral lysis and protistan grazing cause similar bacterial mortality in aquatic ecosystems (22, 40).
Several environmental factors, including solar radiation and temperature, can influence viral abundance. Exposure to solar radiation decreases viral abundance in aquatic ecosystems, while low temperatures decrease their virulence (33, 56). However, the majority of the studies on virus ecology have been performed in temperate or polar regions, where seasonal changes in solar radiation and water temperature are more pronounced (26, 30, 32). Viral abundances have been little investigated in tropical aquatic ecosystems (6, 39) and particularly in the Amazonian region, where the abundance and activity of aquatic viruses have not been studied.
The greatest watershed in the world is located in the Amazonian region. It is composed of clear-water, black-water, and turbid freshwater ecosystems, which are seasonally influenced by the flood pulse. The hydrologic pulse is characterized by a pronounced change in water level, defining the flood seasons. Nutrient sources and stocks and species dynamics vary according to the water level (25). During the high-water season (flood season), the tight connection between terrestrial and aquatic environments results in an increase in allochthonous DOC input and the dilution of inorganic nutrients and organisms. During the low-water season, there is an increase in nutrient concentrations, organism abundances, and the importance of autochthonous DOC. These seasonal changes differently impact ecosystem functions and aquatic community dynamics (5, 9, 18). For instance, bacterioplankton abundance is less changeable than phytoplankton abundance throughout the hydrological cycle due to the alternative sources of DOC (allochthonous in the high-water period and autochthonous in the low-water period) for bacterial communities (5, 24).
Lake Batata is a clear-water Amazonian floodplain lake located in the watershed of the Trombetas River, a tributary of the Amazon River. As a clear-water Amazonian ecosystem, it contains low concentrations of suspended particles and inorganic nutrients (47). Lake Batata is distinct because it was impacted by bauxite tailings for 10 years (1979 to 1989), affecting 30% of the lake's area. The tailings caused a huge increase in turbidity; large amounts of tailings settled on the sediment surface and often are resuspended by physical mixing or biotic movements (28). The presence of tailings resulted in a clear spatial variation in the lake, forming impacted and natural sites. Furthermore, tailing particles can directly act as a substrate for attaching bacteria and also can adsorb DOC (5).
Previous studies on bacterio-, phyto-, and zooplankton communities have shown that flood pulse acts as the primary driver of plankton community structure in Lake Batata (5, 9, 24). Bauxite tailings also affect microbial processes in impacted sites, such as bacterial growth and production (5), photosynthesis rates and primary production (43), or the availability of food for zooplankton (8). However, there still is no evidence indicating a complementary (flood pulse and forced turbidity) effect among these factors in any microbial community. Based on published data, we assume that (i) bacterioplankton abundance is less changeable through the hydrologic cycle than phytoplankton abundance (5, 24), and (ii) tailing particles can act as a substrate for attaching bacteria and also can adsorb organic matter, which is controlled by the flood pulse (5). Therefore, we hypothesized that the relationship between viruses and bacteria in Lake Batata is modulated by a synergistic effect between the hydrological cycle and turbidity.
MATERIALS AND METHODS
Study area.
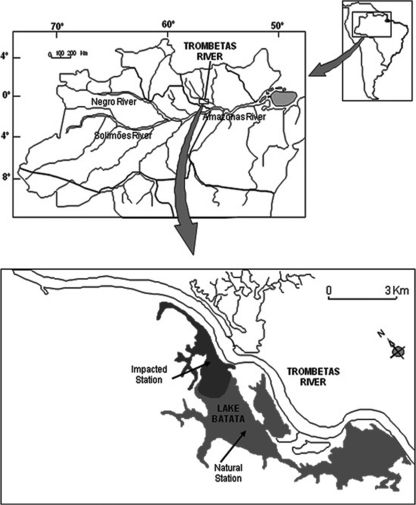
Our study was performed at Lake Batata (1o 28′ S, 56o 14′ W), a clear-water Amazonian lake situated at Porto Trombetas, Oriximiná Municipality, Pará, Brazil (Fig. 1). This lake is distinct from other floodplain lakes because it received by-products of bauxite processing for 10 years (from 1979 to 1989). Bauxite is processed by being washed with water jets, producing a liquid effluent (tailings) composed of 7 to 9% fine-grained solid particles (ca. 96% are smaller than 50 μm), mainly aluminum oxide, silicates, and iron oxides (27). The tailings spread into 30% of the lake area (impacted site), while the remaining lake area kept its natural conditions. The limnologic conditions of Lake Batata have been monitored since 1989, and the size of the impacted site has not been changed throughout these years. However, sporadically events of tailing resuspension related to the hydrological cycle have been observed during the last 20 years, keeping high concentrations of suspended sediments in the water column of the impacted site (28). Actually, they are quite rare nowadays. Only severe hydrodynamic forces can resuspend the particles deposited on the bottom of the lake (10). As a classic Amazonian floodplain lake, the ecology of Lake Batata is driven by the hydrologic cycle. The water level varies between 1 and 3 m during low water and 8 and 10 m during high water. The filling and drawdown seasons complete the annual flood pulse.
FIG. 1.
Map of the Amazon watershed and the location of Lake Batata, the sampling site.
Sampling.
Water samples were taken in the four seasons of the hydrologic cycle, filling, high water, drawdown, and low water, between September 2005 and July 2006. Samples were taken from the upper 0.5 m of the water column at two different sites, designated the impacted sites and natural sites of Lake Batata (Fig. 1). The impacted site is located in the area that received the bauxite tailings. One water sample was taken for limnologic analyses, and seven water samples were taken for virus and bacterium counts, considering both temporal and spatial scales. Samples for virus and bacterium counts were, immediately after sampling, fixed with glutaraldehyde solution (2%, final concentration; prefiltered on a 0.02-μm-pore-size filter). The dissolved oxygen concentration was measured at the subsurface with a portable oximeter (YSI-95). Subsamples were filtered through 0.7-μm glass microfiber filters (GF/F; Whatman), and the retained material was analyzed by gravimetry as a measurement of the suspended matter in the water column. Turbidity was measured using a turbidimeter (LaMotte 2008). The remaining water samples were acidified to pH 2.0 before they were transported to the analytical laboratory. The concentrations of total nitrogen and total phosphorus were estimated in the acidified water samples within 15 days of their collection using standard spectrophotometric techniques (53). The chlorophyll a concentration was determined by the colorimetric method after extraction with 90% acetone (29).
Virus and bacterium enumeration.
Bacteria and viruses were stained with SYBR green (Molecular Probes, Eugene, OR) (36). Samples (2 ml) were filtered on a 0.02-μm-pore-size Anodisc membrane filter (Whatman aluminum oxide) with a 0.45-μm-pore-size backing membrane filter. The filter was laid, sample side up, on a drop of SYBR green I solution (1:400) for 15 min in the dark. After being dried, the filter was placed on a glass slide and mounted with an antifade mounting solution (38). For each filter, more than 200 viruses and 100 bacteria were directly counted in 20 fields. The fields were selected randomly. Analyses were performed under ×1,000 magnification with an epifluorescence microscope (Provis AX-70; Olympus, Melville, NY) using light filters for blue excitation (450- to 490-nm-wide bandpass).
Transmission electron microscopy (TEM).
A one-liter sample was collected along the lake, during the low water period, and filtered through a 30-μm membrane mesh to remove large particles. Four hundred ml of filtered sample was centrifuged at 3,000 × g for 10 min. They were immediately fixed in a mixture of freshly prepared aldehydes (1% paraformaldehyde and 1% glutaraldehyde) in 1 M phosphate buffer, pH 7.3, for 1 h at room temperature (RT), washed twice in the same buffer at 3,000 rpm for 10 min, and stored at 4°C for subsequent use. After fixation, agar embedding was performed as before (35), so that uniformly distributed specimens could be processed as easily handled blocks of tissue. Samples were centrifuged at 1,500 × g for 1 min. They then were resuspended in molten 2% agar in 1 M sodium cacodylate buffer, pH 7.4, and quickly recentrifuged. The resulting agar pellets were kept in the same buffer at 4°C for further processing. Agar pellets containing water specimens were processed as described previously (35). Samples were postfixed in 1% osmium tetroxide in Sym-Collidine buffer, pH 7.4, for 2 h at room temperature (RT). After being washed with sodium maleate buffer, pH 5.2, they were stained en bloc in 2% uranyl acetate in 0.05 M sodium maleate buffer, pH 6.0, for 2 h at RT and washed in the same buffer as before, prior to dehydration in graded ethanol and infiltration and embedding with a propylene oxide-Epon sequence (Eponate 12 resin; Ted Pella, Redding, CA). After polymerization at 60°C for 16 h, thin sections were cut using a diamond knife on an LKB ultramicrotome (LKB Instruments, Gaithersburg, MD). Sections were mounted on uncoated 200-mesh copper grids (Ted Pella) before being stained with lead citrate. Organisms were examined using a transmission electron microscope (P300; Philips, Eidhoven, Netherlands) at 60 kV. To study the bacterial morphology and virus-bacterium interactions, electron micrographs from aquatic bacteria were randomly taken at magnifications of ×30,000 to 75,000. At least 35 cells of both infected and uninfected cells and the number of phage particles per cell were counted to assess the frequency of visibly infected cells (FVIC) and the mean number of viruses per cell section (41). Viruses within cells were characterized based on structure, size, electron density, and uniformity of structure (51). Cells were considered infected when a minimum of three phages were observed in each cell (51).
Statistical analysis.
Models were selected using Akaike's information criterion (AIC) to investigate the relationships between viral abundances and explanatory variables. Model selection analyses such as AIC reduce the general complexity of the studied system by identifying the model that retains the most information while also being parsimonious, i.e., the best-predicting model is achieved by the balance of the number of variables and the explanatory power in the model. In contrast to stepwise multiple regressions, which often yield different results depending on the order in which models are computed, AIC yields consistent results and is independent of the order of computation (13). Because AIC values are relatively uninformative, the differences in AIC values between the best-fitting model and all other models (Δ) are calculated. Differences in AIC values of <2 indicate substantial evidence for alternative models, differences between 3 and 9 indicate alternative models have considerably less support, and differences of >10 indicate that alternative models are very unlikely (13). Viral abundance was the response variable, while bacterial abundance and concentrations of chlorophyll a, dissolved oxygen, suspended matter, total nitrogen, and total phosphorus were the explanatory variables. Model selection analysis was performed in SAM (Spatial Analysis in Macroecology) v3.0 (42). Because only bacterial abundance was selected in the AIC analysis, a further simple linear regression analysis between bacterial and viral abundance was conducted to confirm and graphically illustrate the result.
Temporal and spatial variances in viral and bacterial abundances were compared using two-way analysis of variance (two-way ANOVA), with hydrologic seasons and different sampling sites as the fixed factors. A virus-to-bacterium ratio (VBR) was calculated for each pair of virus and bacterium abundance data, and it was analyzed by the same two-way ANOVA procedures. Two-way ANOVAs were followed by Bonferroni's post hoc comparison tests. Whenever necessary, the variables were transformed to meet two-way ANOVA assumptions. The statistical tool pack SPSS software (version 11.5) was used to perform simple regression and two-way ANOVA. A maximum probability level of α = 0.05 was used throughout to determine statistical significance.
RESULTS
Limnological parameters.
Concentrations of suspended matter ranged from 1.1 to 14.0 mg liter−1. The highest concentrations were always found in the impacted site throughout the hydrologic cycle. As a consequence of the higher suspended-matter concentrations, turbidity also was higher at the impacted site (ANOVA; P < 0.05) (Table 1). On the other hand, higher nutrient concentrations (total nitrogen and total phosphorus) were found at the natural site (ANOVA; P < 0.05). Nutrient concentrations ranged from 147 to 632 μg liter−1 (total nitrogen) and 0.8 to 40.5 μg liter−1 (total phosphorus). We did not observe a clear seasonal pattern in nitrogen and phosphorus concentrations. As we observed for nutrient concentrations, the highest chlorophyll a concentrations were found at the natural site (ANOVA; P < 0.05) throughout the hydrologic cycle (Table 1). Dissolved oxygen was relatively constant between sites in the same hydrologic season, but slightly lower levels were observed during the filling and high-water seasons (Table 1).
TABLE 1.
Concentrations of suspended matter, turbidity, total nitrogen, total phosphorus, dissolved oxygen, and chlorophyll a at impacted and natural sites in Lake Batata through one hydrologic yeara
| Parameter | Impacted site |
Natural site |
||||||
|---|---|---|---|---|---|---|---|---|
| DD | LW | FL | HH | DD | LW | FL | HH | |
| Suspended matter (mg liter−1) | 7 ± 2 | 14 ± 7 | 10 ± 7 | 4 ± 0 | 5 ± 4 | 4 ± 2 | 2 ± 0 | 1 ± 0 |
| Turbidity (NTU) | 15 ± 3 | 45 ± 5 | 20 ± 5 | 4 ± 2 | 6 ± 1 | 4 ± 2 | 4 ± 1 | 5 ± 1 |
| Total nitrogen (μg liter−1) | 208 ± 34 | 148 ± 28 | 154 ± 49 | 158 ± 64 | 435 ± 300 | 527 ± 50 | 602 ± 103 | 632 ± 44 |
| Total phosphorus (μg liter−1) | 2 ± 1 | 1 ± 0 | 1 ± 0 | 2 ± 0 | 26 ± 23 | 38 ± 16 | 39 ± 13 | 41 ± 16 |
| Dissolved oxygen (mg liter−1) | 7 ± 0 | 8 ± 0 | 6 ± 0 | 6 ± 0 | 7 ± 0 | 7 ± 0 | 6 ± 0 | 7 ± 0 |
| Chlorophyll a (μg liter−1) | 6 ± 1 | 7 ± 1 | 2 ± 1 | 4 ± 2 | 6 ± 2 | 6 ± 1 | 7 ± 2 | 7 ± 2 |
DD, drawdown; LW, low water; FL, filling; and HH, high water. The numbers represent means ± standard deviations.
Viral and bacterial abundances.
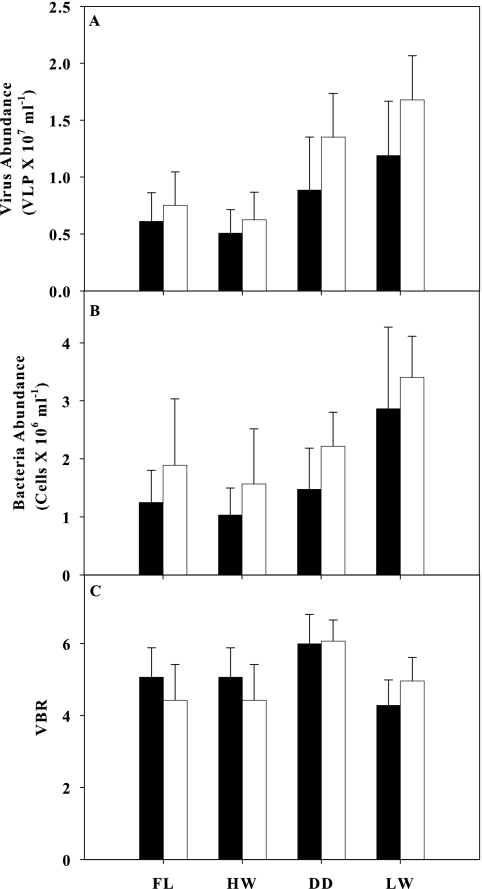
Viral abundance was higher than bacterial abundance irrespective of the sampling site or hydrologic season. Viral abundance ranged from 0.5 × 107 ± 0.2 × 107 VLP ml−1 (high-water season, impacted site) to 1.7 × 107 ± 0.4 × 107 VLP ml−1 (low-water season, natural site), while bacterial abundance ranged from 1.0 × 106 ± 0.5 × 106 cells ml−1 (high water, impacted site) to 3.4 × 106 ± 0.7 × 106 cells ml−1 (low water, natural site) (Fig. 2). Despite the differences in viral and bacterial abundances, there was no difference between the impacted and natural sites in the virus-to-bacterium ratio (VBR) (Table 2; Fig. 2C). On the other hand, temporal differences in the VBR were detected, and these were related to higher values in the drawdown period (Table 2; Fig. 2C).
FIG. 2.
Virus abundance (A), bacterial abundance (B), and virus-bacterium ratio (C) at the impacted (black bars) and natural (white bars) sites in different seasons of the flood pulse. FL, filling; HW, high water; D, drawdown; LW, low water. Bars and traces represent means and standard deviations, respectively.
TABLE 2.
Effects of flood pulse (season) and turbidity (site) on viral and bacterial abundances and virus-bacterium ratio samples from Lake Batataa
| Parameter | Virus |
Bacteria |
VBR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | DF | F | P | SS | DF | F | P | SS | DF | F | P | |
| Intercept | 47.5 | 1 | 380.1 | 0.000 | 203 | 1 | 258.1 | 0.000 | 1332 | 1 | 2018.5 | 0.000 |
| Season | 6.4 | 3 | 17.3 | 0.000 | 26.9 | 3 | 11.4 | 0.000 | 17.3 | 3 | 8.8 | 0.000 |
| Site | 1.2 | 1 | 9.7 | 0.003 | 4.9 | 1 | 6.3 | 0.016 | 0.2 | 1 | 0.3 | 0.558 |
| Season and site | 0.4 | 3 | 1.1 | 0.373 | 0.1 | 3 | 0 | 0.989 | 4 | 3 | 2.1 | 0.119 |
Samples were evaluated by two-way ANOVA. SS, sum of squares; DF, degrees of freedom; F, F-distribution value; P, P value.
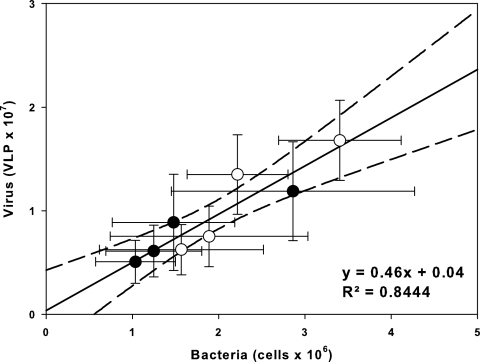
Bacterial abundance was the most important explanatory variable for viral abundance; i.e., this single variable comprised the most-parsimonious candidate model for positively predicting the viral abundance in Lake Batata (r2 = 0.844; AIC = 4.70; ΔAIC = 0; Akaike weight of model [wi] = 0.976). Adding the chlorophyll a concentration to the bacterial abundance only slightly enhanced the prediction of viral abundance, and therefore it was not included, although it comprised the second-best model (r2 = 0.855; AIC = 13.49; ΔAIC = 8.79; wi = 0.012). The linear regression analysis confirmed that bacterial and viral abundances were closely related (r2 = 0.844; P < 0.0001; n = 8) (Fig. 3). Both the hydrologic season and sampling site affected the bacterial and viral abundances in Lake Batata (two-way ANOVA; P < 0.05) (Table 2), but no interactions between the two factors were observed, either for viruses or for bacteria (two-way ANOVA; P > 0.05) (Table 2). Furthermore, the VBR was significantly affected by the flood pulse (two-way ANOVA; P < 0.05) (Table 2), while the sampling site and the interaction between factors had no effect on VBR (two-way ANOVA; P > 0.05) (Table 2).
FIG. 3.
Simple linear regression analysis between viral and bacterial abundances. Black and white points represent the impacted and natural sites, respectively. Lines around the points are standard errors, and the dashed lines represent 95% confidence interval for the linear regression.
TEM.
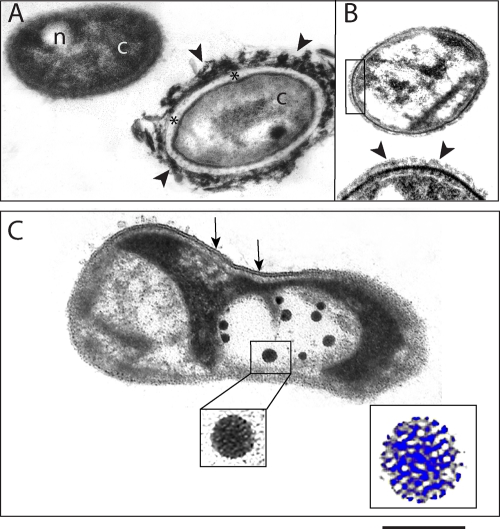
Because of the strong correlation between bacteria and viruses and the constant VBR values, we also investigated the occurrence of virus-bacterium interactions in samples randomly taken along the lake during the low-water period by TEM. Due to the thickness of the thin sections (∼80 nm), it was possible to clearly observe all bacterial structures, such as the cytoplasm, nucleoid (Fig. 4 A), periplasmic space (Fig. 4A, asterisks), capsule (Fig. 4A and B, arrowheads), cell wall (Fig. 4B), and cell membrane with the classical trilaminar organization (Fig. 4C, arrows). TEM revealed a morphological diversity of bacteria associated with the presence of different internal and external bacterial structures. Large variations in the extent and density of bacterial capsules were frequently observed (Fig. 4A and B). The TEM approach enabled us to clearly distinguish between uninfected (Fig. 4A and B) and virus-infected (Fig. 4C) bacteria. TEM revealed viruses with nearly spherical heads and without tails (Fig. 4C). The structure of the virus capsid with its repetitive morphological units occasionally could be observed in some cells (Fig. 4C, boxed areas). On the other hand, some infected bacteria lacked an intact cell membrane or were partially empty. A variable number of phages were present within virus-infected bacteria (Fig. 4C). Quantitative TEM analyses demonstrated that the FVIC was 20%, with 10.0 ± 3.5 (mean ± standard deviation) phages per cell section.
FIG. 4.
Electron micrographs of bacteria from Lake Batata. (A and B) Uninfected bacteria. Bacterial structures such as cytoplasm (c), nucleoid (n), periplasmic space (*), and external capsule (arrowheads) are clearly observed. (A) Two morphologically distinct types of bacteria are seen in the same field. Note the variable thickness of the bacterial capsule, which is thicker and denser in panel A (arrowheads) than in panel B (arrowheads.) The boxed area in panel B is shown at a higher magnification at the bottom of the same figure. Note the strongly electron-dense cell wall below the capsule (arrowheads). (C) Virus-infected bacterium with several phages. The boxed area shows the virus capsid structure at high magnification. Note that the capsid is composed of repetitive morphological units (highlighted in blue at a higher magnification). The cell membrane is partially observed (arrows). Scale bars, 460 nm (A and B), 300 nm (B, high magnification), 266 nm (C), 80 nm (C, virus at high magnification), and 40 nm (C, virus at higher magnification, highlighted in blue).
DISCUSSION
Two main results of this study are noteworthy: (i) viral and bacterial abundances were strongly correlated, and (ii) both flood pulse and forced turbidity significantly influenced viral and bacterial abundances in Lake Batata. The strong positive correlation between viral and bacterial abundances (Fig. 3) indicates that most viruses present in Lake Batata are bacteriophages, i.e., viruses that infect bacteria (26, 40). Indeed, we did not find any relationship between viral abundance and chlorophyll a concentration, which was considered a surrogate of phytoplankton abundance, suggesting a weak link between viruses and phytoplankton in Lake Batata (see Fig. S1 in the supplemental material). However, it is worth noting that we did not evaluate the impact of viruses on planktonic algae, which might be high in terms of viral infection or the percentage of nutrients provided to phytoplankton by the viral lysis of bacteria.
Bacterial abundance was higher and more stable throughout the year than was phytoplankton abundance. However, occasionally in the low-water season, algal growth was strongly stimulated by the resuspension of nutrients trapped in the sediment into the water column. For instance, in Lake Batata, bacterial abundance varied only from 1.04 × 106 to 2.86 × 106 cells ml−1, respectively, in the high- and low-water seasons, during this study, and elsewhere from 1.31 × 106 to 2.61 × 106 cells ml−1 (18), while phytoplankton abundance varied from 1,543 to 11,081 individuals ml−1 in the high- and low-water seasons, respectively (15). The encounter between virus and host cell is mediated by random drift in the water column; it depends directly on the abundance of the latter (12). Therefore, considering that bacteria are more abundant than phytoplankton in Lake Batata, it was expected that viral abundance is more correlated with bacterial abundance than chlorophyll a concentrations. We suggest that this pattern also must be found in other oligotrophic aquatic ecosystems where the relative importance of bacteria to phytoplankton in the structure and functioning of the ecosystem is greater (14). Finally, because most viruses seem to be bacteriophages, they must be susceptible to temporal and spatial environmental changes affecting the bacterial community in Lake Batata, such as (i) the water temperature, which drives bacterial metabolism (1), (ii) the hydrologic dynamic, which defines the dilution/concentration effect (24), and (iii) the turbidity, promoting reduction on primary production and, hence, the availability of autochthonous organic carbon (43).
Overall, it is difficult to determine if bacterial abundance controls viral abundance or if it is controlled by viruses through infection, but some clues can be obtained from the abundance of viruses and bacteria and the virus-to-bacterium ratio (VBR). Both viruses and bacteria showed low abundances in Lake Batata (Fig. 2A and B); low bacterial abundances also were reported previously in this lake (2, 5, 18). However, most surprising were the low virus-to-bacterium ratios found in Lake Batata (4.28 to 6.06) irrespective of site or flood-pulse season (Fig. 2C), mainly when these values are compared to those of other aquatic ecosystems. The VBR is always higher than 1, ranging between 5 and 83 in marine ecosystems (52) and between 5 and 20 in freshwater ecosystems (34). Therefore, the VBR values found in Lake Batata are in the lower range of those reported in the literature. On average, 10 viruses per bacterial cell were observed (Fig. 4), in agreement with the low VBR observed for Lake Batata, and also lower than the numbers reported elsewhere (22, 46). Viruses were reported in host-specific parasites that infect one or a few species of the same genus. However, there are few examples of bacteriophage and cyanophage with relatively broad host ranges (3, 16, 17). In fact, high abundances of host species are necessary to promote the random virus-host cell encounter. We hypothesize that the low bacterial abundance limits the rate of virus-host bacterium encounters. This fact implies (i) low virus-to-bacterium ratios, (ii) low frequencies of visible infected cells, and (iii) a low level of viral predation in Lake Batata. Therefore, bacterial abundance is regulated by other environmental factors at Lake Batata, such as the availability of dissolved phosphorus (19), the presence of bauxite tailings in the water column (5), and the source and composition of DOC (18), rather than viral predation pressure. Hence, viral abundance is limited by the low concentration of bacterial host cells.
Other factors could be related to low viral abundances in Lake Batata. The loss of viral particles is caused primarily by solar radiation in aquatic systems (37, 56, 57). Viruses could be less abundant in tropical than in temperate freshwater ecosystems due to the effect of solar radiation, which is more intense in the tropical zones (6). However, this response is not supported yet, because there are only a few studies regarding virioplankton in tropical ecosystems. Our results provide evidence of lower viral abundance in tropical freshwaters, although our data do not suggest a definitive cause other than low bacterial host cell abundance.
The impacted site showed lower abundances of bacteria and viruses than the natural one, irrespective of the hydrologic cycle (Fig. 2A and B). Several factors might contribute to the lower bacterial abundances, and consequently viral abundances, at the impacted site, such as the high sedimentation rates of suspended matter, the low phytoplankton primary production rates, and the higher abundance of zooplankton. The high concentration of suspended matter at the impacted site increases the sedimentation rates of inorganic and organic particles, and most of the attached bacteria sink with these particles (5). Also, phosphorus is the main limiting nutrient for bacterioplankton growth in Lake Batata (19). Furthermore, higher turbidity at the impacted site decreases the PAR attenuation and plankton primary production (44). Low primary production decreases the input of autochthonous DOC, which is the main source of labile carbon for the bacterial community in Lake Batata (18). Despite the sedimentation rate and the photosynthetic active radiation (PAR) attenuation effect, zooplankton abundance was higher at the impacted site, suggesting a top-down pressure on the bacterial community. Pieces of evidence of planktonic grazing in Lake Batata were discussed previously by Bozelli (7); the study reported that densities of two cladocerans (Bosmina hagmanni and Moina minuta) had a significant positive correlation with bacterial densities in Lake Batata. Furthermore, a study focusing on bacterial dynamics also demonstrated grazing pressure driving the abundance of heterotrophic pelagic bacteria (5). Considering that the abundance of phytoplankton and free-living bacteria are lower in the impacted site, the higher zooplankton abundance observed at this site might have been supported by the availability of aggregates. The tailing particles adsorb organic matter, which are substrates for bacteria (5).
Variations in the water level of the lake also influenced bacterial and, consequently, viral abundances (Table 2; Fig. 2A and B). Several environmental factors are related to the changes in the water level and can equally influence the plankton communities. Planktonic organisms and inorganic nutrients are more diluted during the high-water season and are more concentrated during the low-water season. Thus, differences in bacterial and viral abundances over time may be related to the dilution/concentration effect of the flood pulse on organisms and limiting nutrients. The water column during the high-water season can reach 9 m in Lake Batata; during the low-water period the maximum depth reaches 2 m. The change in depths throughout the hydrologic cycle concentrates viral and bacterial communities almost 3-fold. In addition to the variation in the lake volume, there is a difference in the main carbon source during the year. The connectivity between terrestrial and aquatic environments increases during the filling and high-water periods, enhancing the contribution of allochthonous DOC to the water column. Conversely, during the low-water period, the influence of terrestrial carbon is reduced and the importance of autochthonous DOC increases due to greater plankton primary production. Allochthonous DOC is more refractory, and in microcosm experiments it sustained lower bacterial abundances than did autochthonous DOC produced by algae (18). Therefore, differences in both bacterial abundances and production between hydrologic seasons (high and low water) may be related to different sources of allochthonous and autochthonous DOC in the water column. During low-water conditions, bacterial growth appeared to be driven mainly by the DOC released by phytoplankton (45). Further, bacterial abundance was reduced during the high-water season due to the dilution effect and the input of less-labile DOC from floodplains (5).
Despite the differences in viral and bacterial abundances, there was no difference between the impacted and natural sites in terms of the VBR (Table 2). On the other hand, temporal differences in the VBR were detected, and these seem to be related to higher VBR values in the drawdown period (Table 2; Fig. 2C). The decreasing water depth enhances the resuspension of inorganic nutrients through the action of wind and benthic macroinvertebrates, which in turn would promote the growth of the plankton communities (28). Fast-growing plankton cells would dominate the lake, and this scenario is propitious for viral infection and multiplication inside these cells. Therefore, the input of autochthonous inorganic nutrients in the drawdown season could promote a cascade effect, resulting in greater viral abundances and greater VBR than those in other seasons of the flood pulse.
Our TEM approach enabled us to observe the ultrastructure of aquatic bacteria and clearly identify virus-infected bacteria. Because we used thin-sectioned samples prepared for optimal morphology and contrast rather than preparations of whole cells, it was possible to observe bacterial features such as the limiting membrane, bacterial wall, and nucleoid. Thin sections revealed a structural heterogeneity of aquatic bacteria characterized mainly by variations in the extent and density of bacterial capsules. The production of these capsules may be associated with mechanisms for bacterial attachment to surfaces and also may represent a major source of dissolved organic matter (DOM) after cell damage or death (23). On the other hand, thin sectioning is not the approach of choice to assess classes of bacteria based on shape, because the different planes of sectioning may make difficult an accurate classification. Our data unambiguously showed a variable number of viruses within the bacterial cytoplasm, demonstrating a clear interaction between these organisms. This interaction cannot be directly appreciated when a negative stain of whole cells is used. The FVIC (20%) represents the same reduction in organic matter processing by the bacterial community in Lake Batata. On the other hand, the DOM released after the bacteria lyse increases the amount of DOM that is labile for the bacteria to consume (4). The burst size was 10 ± 3 phages per cell, which is lower than values from temperate systems (46). However, most studies are based on VLP quantification using whole cells (6, 20), whereas our results represent the mean number of viruses observed in cell sections. Because there are potential differences between the analysis of thin sections (∼80 nm thickness) and whole cells (which can be >1 μm in size), we did not extrapolate our quantitative analysis with the use of additional equations. Although the number of viruses may be underestimated, our results showing a low burst size support the low viral abundance detected in Lake Batata.
We concluded that there is a strong correlation between viral and bacterial abundances in Lake Batata, indicating that most viruses are bacteriophages. Due to low bacterial and viral abundances and particularly the low virus-to-bacterium ratios, we suggest that viral abundances are controlled by the abundances of specific host bacteria, which are in turn regulated by other environmental factors, such as nutrient availability and substrate quality. Both the flood pulse and the forced turbidity affected viral and bacterial abundances in Lake Batata. As far as we know, this study provides the first virus assessment of an Amazonian freshwater ecosystem.
Supplementary Material
Acknowledgments
This study was supported by the Mineração Rio do Norte (MRN) and by grants from the National Council of Research and Development (CNPq) of Brazil.
We are grateful to F. A. Esteves, R. L. Bozelli, and M. P. F. Barros, whose criticisms and insights greatly improved the manuscript. We also thank C. H. Duque-Estrada, R. Picanço, G. Miranda, and M. Tose for fieldwork assistance.
Footnotes
Published ahead of print on 10 September 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
This is a contribution from the Laboratory of Aquatic Ecology and Ecology Program of the Federal University of Juiz de Fora.
REFERENCES
- 1.Amado, A. M., J. B. Cotner, A. L. Suhett, F. Assis Esteves, R. L. Bozelli, and V. F. Farjalla. 2007. Contrasting interactions mediate dissolved organic matter decomposition in tropical aquatic ecosystems. Aquat. Microb. Ecol. 49:25-34. [Google Scholar]
- 2.Amado, A. M., F. F. Vinicius, A. E. Francisco de, L. B. Reinaldo, R. Fábio, and E.-P. Alex. 2006. Complementary pathways of dissolved organic carbon removal pathways in clear-water Amazonian ecosystems: photochemical degradation and bacterial uptake. FEMS Microbiol. Ecol. 56:8-17. [DOI] [PubMed] [Google Scholar]
- 3.Ammann, A., H. Neve, A. Geis, and K. J. Heller. 2008. Plasmid transfer via transduction from Streptococcus thermophilus to Lactococcus lactis. J. Bacteriol. 190:3083-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amon, R. M. W., and R. Benner. 1996. Bacterial utilization of different size classes of dissolved organic matter. Limnol. Oceanogr. 41:41-51. [Google Scholar]
- 5.Anesio, A. M., P. C. Abreu, and F. D. Esteves. 1997. Influence of the hydrological cycle on the bacterioplankton of an impacted clear water Amazonian lake. Microb. Ecol. 34:66-73. [DOI] [PubMed] [Google Scholar]
- 6.Bettarel, Y., M. Bouvy, C. Dumont, and T. Sime-Ngando. 2006. Virus-bacterium interactions in water and sediment of West African inland aquatic systems. Appl. Environ. Microbiol. 72:5274-5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozelli, R. L. 1996. The influence of bauxite tailings on the cladoceran populations of Lake Batata, Amazonia, Brazil. Int. Rev. Gesamten Hydrobiol. 81:621-634. [Google Scholar]
- 8.Bozelli, R. L. 1998. Influences of suspended inorganic matter on carbon ingestion and incorporation rates of two tropical cladocerans, Diaphanosoma birgei and Moina minuta. Arch. Hydrobiol. 142:451-465. [Google Scholar]
- 9.Bozelli, R. L. 1994. Zooplankton community density in relation to water-level fluctuations and inorganic turbidity in an Amazonian lake, Lago-Batata, state of Para, Brazil. Amazoniana-Limnol. Oecol. Region. Syst. Fluminis Amazonas 13:17-32. [Google Scholar]
- 10.Bozelli, R. L., A. Caliman, R. D. Guariento, L. S. Carneiro, J. M. Santangelo, M. P. Figueiredo-Barros, J. J. F. Leal, A. M. Rocha, L. B. Quesado, P. M. Lopes, V. F. Farjalla, C. C. Marinho, F. Roland, and F. A. Esteves. 2009. Interactive effects of environmental variability and human impacts on the long-term dynamics of an Amazonian floodplain lake and a South Atlantic coastal lagoon. Limnologica 39:306-313. [Google Scholar]
- 11.Bratbak, G., and M. Heldal. 2000. Viruses rule the waves—the smallest and most abundant members of marine ecosystems. Microbiol. Today 27:171-173. [Google Scholar]
- 12.Brussaard, C. P. D. 2004. Viral control of phytoplankton populations—a review. J. Eukaryot. Microbiol. 51:125-138. [DOI] [PubMed] [Google Scholar]
- 13.Burnham, K. P., and D. Anderson. 2002. Model selection and multimodel inference: a practical information—theoretic approach, 2nd ed. Springer-Verlag, New York, NY.
- 14.Cotner, J. B., and B. A. Biddanda. 2002. Small players, large role: microbial influence on biogeochemical processes in pelagic aquatic ecosystems. Ecosystems 5:105-121. [Google Scholar]
- 15.de Melo, S., and V. L. M. Huszar. 2000. Phytoplankton in an Amazonian flood-plain lake (Lago Batata, Brasil): diel variation and species strategies. J. Plankton Res. 22:63-76. [Google Scholar]
- 16.Deng, L., and P. K. Hayes. 2008. Evidence for cyanophages active against bloom-forming freshwater cyanobacteria. Freshwater Biol. 53:1240-1252. [Google Scholar]
- 17.Evans, T. J., M. A. Crow, N. R. Williamson, W. Orme, N. R. Thomson, E. Komitopoulou, and G. P. C. Salmond. 2010. Characterization of a broad-host-range flagellum-dependent phage that mediates high-efficiency generalized transduction in, and between, Serratia and Pantoea. Microbiology 156:240-247. [DOI] [PubMed] [Google Scholar]
- 18.Farjalla, V. F., D. A. Azevedo, F. A. Esteves, R. L. Bozelli, F. Roland, and A. Enrich-Prast. 2006. Influence of hydrological pulse on bacterial growth and DOC uptake in a clear-water Amazonian lake. Microb. Ecol. 52:334-344. [DOI] [PubMed] [Google Scholar]
- 19.Farjalla, V. F., F. A. Esteves, R. L. Bozelli, and F. Roland. 2002. Nutrient limitation of bacterial production in clear water Amazonian ecosystems. Hydrobiologia 489:197-205. [Google Scholar]
- 20.Filippini, M., N. Buesing, Y. Bettarel, T. Sime-Ngando, and M. O. Gessner. 2006. Infection paradox: high abundance but low impact of freshwater benthic viruses. Appl. Environ. Microbiol. 72:4893-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. [DOI] [PubMed] [Google Scholar]
- 22.Fuhrman, J. A., and R. T. Noble. 1995. Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol. Oceanogr. 40:1236-1242. [Google Scholar]
- 23.Heissenberger, A., G. G. Leppard, and G. J. Herndl. 1996. Relationship between the intracellular integrity and the morphology of the capsular envelope in attached and free-living marine bacteria. Appl. Environ. Microbiol. 62:4521-4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huszar, V. L. M., and C. S. Reynolds. 1997. Phytoplankton periodicity and sequences of dominance in an Amazonian flood-plain lake (Lago Batata, Pará, Brasil): responses to gradual environmental change. Hydrobiologia 346:169-181. [Google Scholar]
- 25.Junk, W. J., P. B. Bayley, and R. E. Sparks. 1989. The flood pulse concept in river-floodplain system. Can. Spec. Publ. Fish Aquat. Sci. 106:110-127. [Google Scholar]
- 26.Kepner, R. L., R. A. Wharton, and C. A. Suttle. 1998. Viruses in antartic lakes. Limnol. Oceanogr. 43:1754-1761. [DOI] [PubMed] [Google Scholar]
- 27.Lapa, R. P. 2000. A bauxita e o rejeito de bauxita, p. 25-36. In F. R. Bozelli, F. A. Esteves, and F. Roland (ed.), Lago Batata: impacto e recuperação de um ecossistema amazônico. IB-UFRJ/ Sociedade Brasileira de Limnologia, Rio de Janeiro, RJ, Brazil.
- 28.Leal, J. J. F., F. D. Esteves, V. F. Farjalla, and A. Enrich-Prast. 2003. Effect of Campsurus notatus on NH444, DOC fluxes, O2 uptake and bacterioplankton production in experimental microcosms with sediment-water interface of an Amazonian lake impacted by bauxite tailings. Int. Rev. Hydrobiol. 88:167-178. [Google Scholar]
- 29.Lorenzen, C. J. 1967. Determination of chlorophyll and phaeopigments: spectrophotometric equations. Limnol. Oceanogr. 12:342-345. [Google Scholar]
- 30.Madan, N. J., W. A. Marshall, and J. Laybourn-Parry. 2005. Virus and microbial loop dynamics over an annual cycle in three contrasting Antarctic lakes. Freshwater Biol. 50:1291-1300. [Google Scholar]
- 31.Maranger, R., and D. F. Bird. 1995. Viral abundance in aquatic systems—a comparison between marine and fresh-waters. Mar. Ecol. Progr. Ser. 121:217-226. [Google Scholar]
- 32.Maranger, R., D. F. Bird, and S. K. Juniper. 1994. Viral and bacterial dynamics in arctic sea-ice during the spring algal bloom near Resolute, NWT, Canada. Mar. Ecol. Progr. Ser. 111:121-127. [Google Scholar]
- 33.Maranger, R., P. A. del Giorgio, and D. F. Bird. 2002. Accumulation of damaged bacteria and viruses in lake water exposed to solar radiation. Aquat. Microb. Ecol. 28:213-227. [Google Scholar]
- 34.Mathias, C. B., A. K. T. Kirschner, and B. Velimirov. 1995. Seasonal-variations of virus abundance and viral control of the bacterial production in a backwater system of the Danube River. Appl. Environ. Microbiol. 61:3734-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melo, R. C. N., L. A. Spencer, S. A. C. Perez, I. Ghiran, A. M. Dvorak, and P. F. Weller. 2005. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic 6:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noble, R. T., and J. A. Fuhrman. 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 14:113-118. [Google Scholar]
- 37.Noble, R. T., and J. A. Fuhrman. 1997. Viral decay and its causes in coastal waters. Appl. Environ. Microbiol. 63:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel, A., R. T. Noble, J. A. Steele, M. S. Schwalbach, I. Hewson, and J. A. Fuhrman. 2007. Virus and prokaryote enumeration from planktonic aquatic environments by epifluorescence microscopy with SYBR Green I. Nat. Protoc. 2:269-276. [DOI] [PubMed] [Google Scholar]
- 39.Peduzzi, P., and F. Schiemer. 2004. Bacteria and viruses in the water column of tropical freshwater reservoirs. Environ. Microbiol. 6:707-715. [DOI] [PubMed] [Google Scholar]
- 40.Proctor, L. M., and J. A. Fuhrman. 1992. Mortality of marine bacteria in response to enrichments of the virus size fraction from seawater. Mar. Ecol. Progr. Ser. 87:283-293. [Google Scholar]
- 41.Proctor, L. M., A. Okubo, and J. A. Fuhrman. 1993. Calibrating estimates of phage-induced mortality in marine bacteria—ultrastructural studies of marine bacteriophage development from one-step growth experiments. Microb. Ecol. 25:161-182. [DOI] [PubMed] [Google Scholar]
- 42.Rangel, T. F. L. V. B., J. A. F. Diniz-Filho, and L. M. Bini. 2006. Towards an integrated computational tool for spatial analysis in macroecology and biogeography. Global Ecol. Biogeogr. 15:321-327. [Google Scholar]
- 43.Roland, F., and F. D. Esteves. 1998. Effects of bauxite tailing on PAR attenuation in an Amazonian crystalline water lake. Hydrobiologia 377:1-7. [Google Scholar]
- 44.Roland, F., F. D. Esteves, and F. A. R. Barbosa. 2002. Relationship between antropogenically caused turbidity and phytoplankton production in a clear Amazonian floodplain lake. Amazoniana-Limnol. Oecol. Region. Syst. Fluminis Amazonas 17:65-77. [Google Scholar]
- 45.Roland, F., L. M. Lobão, L. O. Vidal, E. Jeppesen, R. Paranhos, and V. Huszar. 2010. Relationships between pelagic bacteria and phytoplankton abundances in contrasting tropical freshwaters. Aquat. Microb. Ecol. 60:261-272. [Google Scholar]
- 46.Säwström, C., W. Graneli, J. Laybourn-Parry, and A. M. Anesio. 2007. High viral infection rates in Antarctic and Arctic bacterioplankton. Environ. Microbiol. 9:250-255. [DOI] [PubMed] [Google Scholar]
- 47.Sioli, H. 1984. The Amazon. D. W. J. Publishers, Dordrecht, Netherlands.
- 48.Suttle, C. A. 1994. The significance of viruses to mortality in aquatic microbial communities. Microb. Ecol. 28:237-243. [DOI] [PubMed] [Google Scholar]
- 49.Suttle, C. A. 2005. Viruses in the sea. Nature 437:356-361. [DOI] [PubMed] [Google Scholar]
- 50.Weinbauer, M. G. 2004. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 28:127-181. [DOI] [PubMed] [Google Scholar]
- 51.Weinbauer, M. G., and M. G. Hofle. 1998. Size-specific mortality of lake bacterioplankton by natural virus communities. Aquat. Microb. Ecol. 15:103-113. [Google Scholar]
- 52.Weinbauer, M. G., and C. A. Suttle. 1997. Comparison of epifluorescence and transmission electron microscopy for counting viruses in natural marine waters. Aquat. Microb. Ecol. 13:225-232. [Google Scholar]
- 53.Wetzel, R. G., and G. E. Likens. 1991. Limnological analyses, 2nd ed. Springer-Verlag, New York, NY.
- 54.Wilhelm, S. W., and R. E. H. Smith. 2000. Bacterial carbon production in Lake Erie is influenced by viruses and solar radiation. Can. J. Fish. Aquat. Sci. 57:317-326. [Google Scholar]
- 55.Wilhelm, S. W., and C. A. Suttle. 1999. Viruses and nutrient cycles in the sea—viruses play critical roles in the structure and function of aquatic food webs. Bioscience 49:781-788. [Google Scholar]
- 56.Wilhelm, S. W., M. G. Weinbauer, C. A. Suttle, and W. H. Jeffrey. 1998. The role of sunlight in the removal and repair of viruses in the sea. Limnol. Oceanogr. 43:586-592. [Google Scholar]
- 57.Wommack, K. E., R. T. Hill, T. A. Muller, and R. R. Colwell. 1996. Effects of sunlight on bacteriophage viability and structure. Appl. Environ. Microbiol. 62:1336-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.