Abstract
Although autochthonous vibrio densities are known to be influenced by water temperature and salinity, little is understood about other environmental factors associated with their abundance and distribution. Densities of culturable Vibrio vulnificus containing vvh (V. vulnificus hemolysin gene) and V. parahaemolyticus containing tlh (thermolabile hemolysin gene, ubiquitous in V. parahaemolyticus), tdh (thermostable direct hemolysin gene, V. parahaemolyticus pathogenicity factor), and trh (tdh-related hemolysin gene, V. parahaemolyticus pathogenicity factor) were measured in coastal waters of Mississippi and Alabama. Over a 19-month sampling period, vibrio densities in water, oysters, and sediment varied significantly with sea surface temperature (SST). On average, tdh-to-tlh ratios were significantly higher than trh-to-tlh ratios in water and oysters but not in sediment. Although tlh densities were lower than vvh densities in water and in oysters, the opposite was true in sediment. Regression analysis indicated that SST had a significant association with vvh and tlh densities in water and oysters, while salinity was significantly related to vibrio densities in the water column. Chlorophyll a levels in the water were correlated significantly with vvh in sediment and oysters and with pathogenic V. parahaemolyticus (tdh and trh) in the water column. Furthermore, turbidity was a significant predictor of V. parahaemolyticus density in all sample types (water, oyster, and sediment), and its role in predicting the risk of V. parahaemolyticus illness may be more important than previously realized. This study identified (i) culturable vibrios in winter sediment samples, (ii) niche-based differences in the abundance of vibrios, and (iii) predictive signatures resulting from correlations between environmental parameters and vibrio densities.
Vibrio spp. occur naturally in estuarine and marine environments, and two species of this genus, V. vulnificus and V. parahaemolyticus, are responsible for the majority of reported vibrio illnesses in the United States (2). V. vulnificus infections are most commonly associated with the Gulf of Mexico, either via consumption of raw oysters harvested from these waters or wound infections following exposure to seawater. On average, about 50 cases of V. vulnificus septicemia are reported in the United States each year, with a case fatality rate of approximately 50% (31), the highest of any food-borne pathogen. In contrast, V. parahaemolyticus is the most common cause of seafood-associated bacterial gastroenteritis in the United States, with an estimated annual rate of 4,500 cases per year according to the Centers for Disease Control and Prevention. V. parahaemolyticus also causes wound infections, though these are less frequent and less severe compared to those caused by V. vulnificus (5). Primary septicemia can occur following V. parahaemolyticus infection, but it is relatively rare for this pathogen. In the United States, V. parahaemolyticus illness most often results from consumption of raw or undercooked seafood, particularly oysters.
It is well established that vibrio densities correlate strongly with sea surface temperature (SST), with densities increasing as temperatures increase; however, with the exception of salinity, little is definitively known about the influence of other environmental parameters, such as turbidity and chlorophyll a (22, 33). Consequently, while SST has been estimated to explain approximately 50% of the annual variation of V. parahaemolyticus abundance in oysters harvested from the northern Gulf of Mexico (40), a considerable amount of variation remains unexplained. It is of interest to delineate the effects of other environmental parameters independent of SST, as these parameters may be associated with spatial and temporal variation of vibrio densities within seasonal periods when SST is relatively constant and risk of human exposure and illness is high. Moreover, the majority of what is known about V. parahaemolyticus in the environment is based on total populations; little information is available on the pathogenic subpopulations. Isolates containing genetic markers for pathogenicity factors, including the thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) typically constitute <1% of the population in marine or postharvest oyster samples, but they account for >90% of clinical isolates (12). The basis for V. vulnificus pathogenicity remains unclear, as few pathogenicity factors have been described definitively (31). To address these data gaps, we monitored densities of culturable V. vulnificus containing vvh (the V. vulnificus hemolysin gene) and V. parahaemolyticus containing tlh (the thermolabile hemolysin gene, ubiquitous in V. parahaemolyticus), tdh, and trh in water, oysters, and sediment collected from coastal waters of Mississippi and Alabama. Associations between bacterial densities and environmental parameters were analyzed by regressing observations against sea surface temperature, chlorophyll a, turbidity, and salinity.
MATERIALS AND METHODS
Sample collections and measurement of environmental parameters.
Samples were collected monthly from February 2006 to August 2007 at four oyster reefs within a ca.-70-mile stretch along the Mississippi Gulf Coast. Each oyster sample, consisting of 20 to 30 oysters, was collected by dredging. Each sample was stored in clean polypropylene bags and immediately placed on ice. Approximately 6 liters of surface water was collected in sterile 14-liter plastic buckets, and at least 100 g of sediment from the upper 2 cm of the sediment surface was collected using an Ekman dredge. In addition, monthly water samples were collected using sterile 4-liter bottles from eight sites in Alabama's Mobile Bay in February, March, and May of 2006. All samples were transported to the lab in large (∼120-liter) coolers within a thin layer of ice and analyzed within 5 h of collection. SST was measured in the field using a calibrated digital thermometer (Brooklyn Thermometer Company, Farmingdale, NY); salinity and turbidity were measured in the lab using a portable refractometer (VWR, West Chester, PA) and a LaMotte 2020 turbidity meter (Chestertown, MD), respectively. Chlorophyll a was determined by the filtering of 100 ml of water through a 45-mm GF/C filter (Whatman, Kent, ME), extraction with acetone, and measurement using a Turner TD-700 fluorometer (Sunnyvale, CA) as previously described (4).
Quantification of V. vulnificus and V. parahaemolyticus densities by direct plating/colony hybridization (DP/CH).
Oysters were shucked and homogenized; 0.01 g and 0.1 g of homogenate were plated onto V. vulnificus agar (VVA) plates (2% peptone, 3% NaCl, 1% cellobiose, 0.06% bromthymol blue [pH 8.2]) for detection of V. vulnificus, as described previously (37). In addition, oyster homogenate was plated onto triplicate T1N3 agar plates (1% tryptone, 3% NaCl [pH 7.2]) for detection of V. parahaemolyticus in three samples as previously described (11, 12, 19, 40). Water samples were shaken vigorously, and 1 ml was plated onto VVA and T1N3 plates as previously described (1, 19, 40). Sediment samples were diluted by half in an equal weight of phosphate-buffered saline (PBS; 3.72 mM NaH2PO4·2H2O, 14.0 mM Na2HPO4·2H2O, 0.145 M NaCl [pH 7.4]), and 1:1 and 1:10 dilutions of this mixture were prepared; 0.2 g of these dilutions were then plated onto three sets of T1N3 plates for enumeration of total (tlh) and pathogenic (tdh and trh) V. parahaemolyticus to yield final weights of 0.05 g and 0.01 g of sediment, respectively (19). Based on an expectation of higher densities of V. vulnificus in sediment samples, 0.01-g and 0.005-g final weights of sediment samples were plated onto VVA plates for enumeration of V. vulnificus. All plates were incubated overnight at 37°C. Colonies were lifted and probed with alkaline phosphatase-labeled oligonucleotide probes (DNA Technology A/S, Risskov, Denmark) specific for vvh, tdh, trh, and tlh, as described previously (26, 27, 30, 40). Probe sequences are as described previously (30, 37). When the colorimetric substrate nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) (Roche, Madison, WI) was added to probe-treated filters, the positive colonies, as indicated by development of a purple color, were counted on each filter using visual observation and a hand-held cell counter.
Quantification of total and pathogenic V. parahaemolyticus densities by real-time PCR in an MPN format.
Because levels of tdh+ and trh+ V. parahaemolyticus are usually below the limit of detection (LOD) of the DP/CH method, larger sample portions were enriched in an most-probable-number (MPN) format to improve the assay's sensitivity for tdh+ and trh+ V. parahaemolyticus. Alkaline peptone water (APW [10×], 10% peptone, 1% NaCl [pH 8.5]) was used to enrich three volumes of water (1 liter, 100 ml, and 10 ml) and two volumes of oyster homogenate (equal to 10 g and 1 g) in triplicate. Enrichments were incubated overnight at 37°C. Following incubation, 1-ml aliquots of each enrichment were boiled for 10 min, immediately plunged into ice, and stored at −20°C. The aliquots were then used as templates for multiplex real-time PCR using a SmartCycler II system (Cepheid, Sunnyvale, CA) as described previously (30). Primers and the tlh probe were obtained from Integrated DNA Technologies (Coralville, IA), tdh and trh probes were obtained from Applied Biosystems (Foster City, CA), and the DNA internal amplification control (IAC) template was obtained from BioGX (Birmingham, AL). The IAC was included in every PCR, as described previously (30), and lack of amplification from the IAC indicated inhibition; inhibited templates were diluted 1:10 and repeated. Primer and probe sequences are as described previously (30).
Statistical analyses.
Generalized linear mixed model (GLMM) regressions were used to estimate distributions of vibrio abundance in oyster, sediment, and water and their relationship to environmental parameters. The application of GLMM in the present context circumvents the calculation of density estimates on the level of individual samples, which can be problematic when vibrio densities (particularly tdh and trh) are frequently expected to be below the LOD. In GLMM regression analysis, continuous latent distributions underlying the discrete observations of abundance (e.g., plate counts and MPN outcomes) are assumed to follow a specified parametric distribution, which is then estimated based on the discrete observations considered the response variables in the regression. In the present context, based on observations from numerous environmental studies of vibrios and other pathogens, the latent distribution of vibrio abundance was taken to follow a log-normal distribution, with the mean log10 densities generally considered to be linearly related to environmental parameters. However, given the range of salinities observed, a quadratic polynomial was used to model the effect of salinity. Where applicable, plate counts and real-time PCR-MPN determinations for aliquots from the same sample were treated as repeated measurements, marginally distributed as either Poisson or binomial outcomes, respectively, conditional on the latent distribution of abundance and the volume of sample examined. The approach of using GLMM with discrete mixed-type response variables and latent (underlying) log-normal distribution was considered an appropriate method for combining the plate count and real-time PCR-MPN data in a manner that weighted the outcomes of the different methods in inverse proportion to their inherent measurement errors. Given apparent inhibition of certain PCRs at low dilution levels in some samples, PCR-MPN data were truncated to one dilution level, as has been described previously (10). The predictor variables for temperature and salinity were expressed in units of degrees Celsius and parts per thousand, respectively, while predictor variables for chlorophyll a and turbidity were expressed in base 10 logarithms of micrograms/liter and nephelometric turbidity units (NTU), respectively. Regression parameter estimates were determined by the maximum likelihood criteria. Because measurements of pathogenic V. parahaemolyticus (tdh and trh) densities were frequently below the LOD, the proportion of the variation in plate count and real-time PCR-MPN data explained by environmental parameters was evaluated using Nagelkerke's pseudo-R2 statistic (29) to estimate partial coefficients of determination, as has been described by Lipsitz et al. (24). Fitted regressions were evaluated by Akaike's information criteria and residual plots. Additional regression diagnostics were conducted to evaluate the possible effects of multicollinearity on estimated regression coefficients and partial coefficients of determination. All statistical analyses were conducted using SAS PROC NLMIXED (SAS Institute, Cary, NC). An alpha level of 0.05 was considered the minimum level for statistical significance.
For graphical presentation of data, densities of vvh+, tlh+, tdh+, and trh+ vibrios were determined by dividing the total number of CFU on one or more plates by the corresponding total volume of water or weight of oyster and sediment examined. Only values for CFU counts between 1 and 250 CFU/plate were plotted after accounting for dilution. The LOD ranges for V. vulnificus in water, oysters, and sediment were therefore 1 to 250 CFU/ml, 10 to 25,000 CFU/g, and 100 to 50,000 CFU/g, respectively. The LOD ranges for V. parahaemolyticus in water, oysters, and sediment were 1 to 250 CFU/ml, 10 to 25,000 CFU/g, and 20 to 25,000 CFU/g, respectively. To distinguish between detectable and nondetectable levels, nondetects (zeros) were replaced with 20% of the limits of detection when graphing the data.
RESULTS
Distribution and abundance of V. vulnificus and V. parahaemolyticus.
The maximum culturable vibrio densities in water were as high as >250 CFU/ml; minimum, maximum, and median densities are indicated in Table 1. The maximum vibrio densities in oysters and in sediment were up to 2.8 × 103 CFU/g and up to >2.5 × 104 CFU/g, respectively. Ranges for temperature, chlorophyll a, turbidity, and salinity were 9 to 34°C, 1 to 275 μg/liter, 0 to 47 nephelometric turbidity units, and 3 to 35 ppt, respectively.
TABLE 1.
Summary of statistics for V. parahaemolyticus and V. vulnificus densities in the Mississippi Sound and Dauphin Islanda
| Sample | Sampling period | No. of samples | Probe | Range of no. of CFU/ml or g for respective DP/CH probe (median) | No. of DP/CH detects/total no. of samples (%) | No. of DP/CH + MPN detects/total no. of samples (%) | Range of SST (°C) (median) | Range of Chl (μg/liter) (median) | Range of Turb (NTU) (median) | Range of salinity (ppt) (median) | Mean of log vibrios/g or mlb | SD of log vibrios/g or mlb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | Feb. 2006-Aug. 2007 | 115 | vvh | <1->250 (1) | 70/115 (61) | NA | 9-34 (21) | 1-275 (8) | 0-47 (7) | 3-35 (22) | 0.14 (−0.036, 0.32) | 0.89 (0.75, 1.1) |
| tlh | <1-132 (<1) | 56/115 (49) | 82/115 (71) | −0.34 (−0.54, −0.15) | 1.3 (1.2, 1.5) | |||||||
| tdh | <1-2 (<1) | 9/115 (8) | 55/115 (48) | −3.6 (−4.5, −2.7) | 3.5 (2.8, 4.5) | |||||||
| trh | <1-4 (<1) | 3/115 (3) | 36/115 (31) | −4.2 (−4.8, −3.6) | 2.2 (1.9, 2.4) | |||||||
| Oysters | Mar. 2006-Aug. 2007 | 70 | vvh | <10-2,555 (368) | 61/70 (87) | NA | 9-34 (26) | 1-20 (7) | 1-47 (8) | 9-32 (24) | 2.2 (2, 2.4) | 0.84 (0.68, 1) |
| tlh | <10-2,791 (236) | 61/70 (87) | 66/70 (94) | 2 (1.7, 2.2) | 0.96 (0.8, 1.2) | |||||||
| tdh | <10-36 (<10) | 14/70 (20) | 49/70 (70) | −0.9 (−1.3, −0.52) | 1.4 (1.1, 1.8) | |||||||
| trh | <10-36 (<10) | 12/70 (17) | 42/70 (60) | −1.5 (−2, −1) | 1.5 (1.1, 2) | |||||||
| Sediment | July 2006-Aug. 2007 | 54 | vvh | <100-13,800 (367) | 44/54 (81) | NA | 9-34 (26) | 1-20 (7) | 1-29 (8) | 9-32 (24) | 2.5 (2.2, 2.8) | 0.86 (0.67, 1.1) |
| tlh | <20->25,000 (1,408) | 51/54 (94) | NA | 3 (2.8, 3.3) | 0.87 (0.7, 1.1) | |||||||
| tdh | <20-467 (<20) | 22/54 (41) | NA | 0.79 (0.35, 1.2) | 0.96 (0.65, 1.4) | |||||||
| trh | <20-483 (<20) | 20/54 (37) | NA | 0.6 (0.048, 1.1) | 1 (0.67, 1.6) |
tlh, thermolabile hemolysin gene; tdh, thermostable direct hemolysin gene; trh, tdh-related hemolysin gene; vvh, V. vulnificus hemolysin gene; NA, not applicable; SST, sea surface temperature; Chl, chlorophyll a; Turb, turbidity; NTU, nephelometric turbidity units; ppt, parts per thousand; DP/CH, direct plating/colony hybridization; MPN, most probable number. Data for numbers of CFU/ml and CFU/g are from DP/CH. Data for percent detects are from combined DP/CH and MPN/PCR data for water and oysters and from DP/CH for sediment; no MPN/PCR was carried out for vvh or for sediment samples.
Mean and standard deviation of number of log10 CFU/ml or CFU/g estimated by GLMM (best estimate, 95% confidence interval).
Overall, sampling detected both V. vulnificus and V. parahaemolyticus in most water, oyster, and sediment samples. Specifically, based on DP/CH measurements alone, V. parahaemolyticus detection rates were highest in sediment samples (94%), followed by oysters (87%) and water (49%) (Table 1). On the other hand, V. vulnificus detection rates were highest in oysters (87%), followed by sediment (81%) and water (61%). Detection rates and densities exhibited appreciable site-to-site variation (data not shown) in addition to an overall seasonal variation (Fig. 1), with higher detection rates and densities associated with warmer SST. Both V. parahaemolyticus and V. vulnificus remained culturable from sediment, even as temperatures dropped below 15°C, but in water and oysters, they fell below levels detectable by DP/CH; this phenomenon was more pronounced for V. parahaemolyticus.
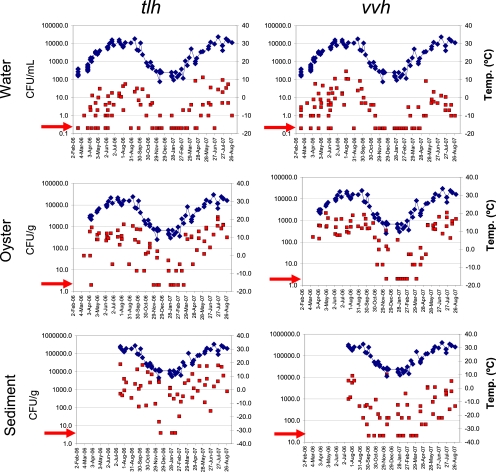
FIG. 1.
Densities of V. parahaemolyticus and V. vulnificus, as quantified by DP/CH for tlh and vvh markers, respectively, over the course of the 19-month study. Red squares, density of tlh or vvh in number of CFU/ml (water) or CFU/g (oysters and sediment); blue diamonds, sea surface temperature in °C. Red arrow identifies nondetects, in which the number of CFU were below the limits of detection by DP/CH analysis. For graphing purposes, nondetects (zeros) were replaced with 20% of the limits of detection, which were 0.2 CFU/ml water, 2 CFU/g oyster, and 4 (V. parahaemolyticus) or 20 (V. vulnificus) CFU/g sediment. Note that V. parahaemolyticus and V. vulnificus remain culturable from sediment, even as temperatures drop below 15°C.
Estimated mean vibrio densities (in log10 CFU/ml or log10 CFU/g) determined by regression analysis were highest in sediment and lowest in water for all four gene targets (Table 1). Detection of tdh or trh in water samples was very infrequent, but detection of tdh was more frequent than detection of trh by DP/CH (8% versus 3%) and by MPN/PCR (48% versus 31%). In oysters, tdh was detected by DP/CH and/or MPN/PCR in 70% of samples, and trh was detected in 60% of samples. DP/CH detection of all four gene targets exhibited greatest variability in water followed by oysters and sediment. The tdh and trh densities exhibited more variability than tlh densities in all three sample types.
Over the course of the sampling period, V. vulnificus and V. parahaemolyticus densities decreased in water and oysters as SST decreased and were frequently below the minimum LOD for the DP/CH method during cooler months, when water temperatures fell below 15°C (Fig. 1). Both V. vulnificus and V. parahaemolyticus remained culturable in sediment samples when they could not be cultured from water or oysters.
Relationships of vvh, tlh, tdh, and trh.
The tdh and trh markers were detected at similar frequencies in water, in oysters, and in sediment; indeed, when a subset of individual isolates from this study were examined separately, the majority (78%) contained both tdh and trh (19).
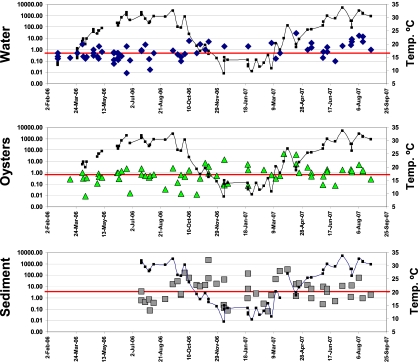
The median values for abundance of tlh relative to vvh (tlh-to-vvh ratio) in water and oysters were 0.5 (n = 73) and 0.7 (n = 65), respectively, whereas the median tlh-to-vvh ratio in sediment was 3.5 (n = 50). Thus, on a sample-to-sample basis, V. parahaemolyticus densities tended to be lower than V. vulnificus densities in water and in oysters, but V. parahaemolyticus densities were higher than V. vulnificus densities in the sediment, and this is illustrated in Fig. 2.
FIG. 2.
Relative abundance of tlh compared to vvh (tlh-to-vvh ratio) during the 19-month sampling period in water (diamonds), oysters (triangles), and sediment (squares). Temperature is indicated by the connected black squares. Median ratios for water, oysters, and sediment (red lines) were 0.5, 0.7, and 3.5, respectively. Ratios were calculated as tlh densities (number of CFU/g or ml) divided by vvh densities (number of CFU/g or ml); thus, values of greater than 1.00 indicate that the tlh density was higher than the vvh density in that particular sample, while values of less than 1.00 indicate the opposite.
When the differences between latent distributions of vibrio densities, [log(tlh) − log(vvh)] and [log(tdh) − log(tlh)], were analyzed by regression of observed data against environmental parameters in GLMM analysis, turbidity was identified as a major factor associated with these differences. Regression coefficients (b) describing the relationship between [log(tlh) − log(vvh)] and turbidity were estimated to be 0.89, 1.65, and 1.46 in water, oysters, and sediment, respectively. Those values describing the relationship between [log(tdh) − log(tlh)] and turbidity were 2.52 and 1.87 in water and oysters, respectively; no significant relationship existed with these levels in sediment. In addition, chlorophyll a levels were associated with [log(tlh) − log(vvh)] in water (b = −1.13) and with [log(tdh) − log(tlh)] in oysters (b = −2.12). Temperature was not associated with any differences in relative abundance between tlh and vvh, but it was associated with differences in tdh versus tlh abundance [log(tdh) − log(tlh)] in both water (b = −0.10) and in oysters (b = −0.06).
Associations of V. vulnificus and V. parahaemolyticus densities with environmental determinants.
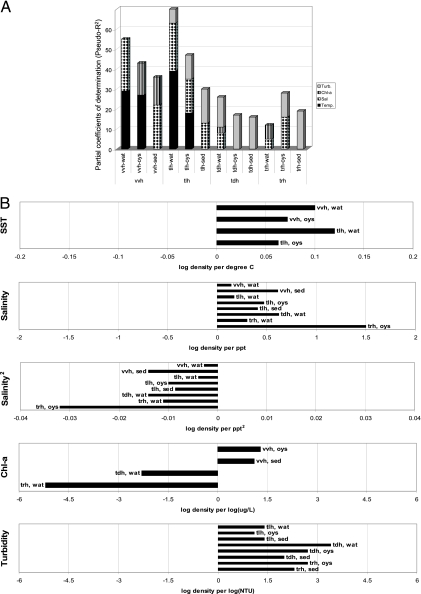
GLMM regression analysis indicated different patterns in the association between environmental parameters and the abundance of vibrios by type (gene marker) (Fig. 3). In general, temperature and salinity had a significant association with densities of vvh and tlh in water samples, while salinity, chlorophyll a, and turbidity had a significant association with the abundance of vibrios with pathogenicity markers tdh and trh; in particular, turbidity was found to be strongly related to tdh and trh densities in all sample types. For oyster and sediment samples, the association with environmental parameters was more variable depending on the target. Results are summarized in Tables S2 to S5 in the supplemental material for the vibrio markers vvh, tlh, tdh, and trh, respectively.
FIG. 3.
Partial coefficients of determination (Pseudo-R2) for temperature (Temp., black), salinity (Sal, diamonds), log10 chlorophyll a (Chl-a, stripes), and log10 turbidity (Turb., gray) (A) and regression coefficients for temperature, salinity, quadratic salinity (Salinity2), log10 chlorophyll a (Chl-a), and log10 turbidity (B). Densities of tlh, tdh, trh, and vvh were measured in water (wat), oysters (oys), and sediment (sed); only statistically significant partial coefficients of determination and regression coefficients (P < 0.05) are presented.
Regression diagnostics were conducted to evaluate the possibility of adverse effects of collinearity between predictors on the estimated regression coefficients and measures of partial association. For each sample type (water, sediment, and oyster), the amount of collinearity in the environmental variables was quantified by determining the amount of variation in each predictor variable that was associated with that of the other three. Corresponding variance inflation factors (VIF) for each predictor variable were determined and found to range from 1.2 to 2.1. This range of values indicates that the probable effects of collinearity are relatively small, as the variance of regression parameter estimates is inflated by at most a factor of 2.1, which would not be considered indicative of a severe effect (6). Stability of regression coefficient estimates was further evaluated by systematic removal of predictor variables. Parameter estimates were found to be reasonably robust and particularly so with respect to temperature and salinity.
Effects of salinity were summarized by two regression coefficients corresponding to a second-degree polynomial rather than a simple linear term. The regression coefficients, written as a combination (b1, b2), express both the linear and quadratic terms, respectively. This factor is referred to simply as salinity throughout Results and Discussion, without reference to the individual linear and quadratic components of the polynomial. Also, as indicated previously, regression variables for chlorophyll a and turbidity are log transformations of the original measurement values, and therefore, regression coefficient estimates are in units of log10 micrograms/liter and log10 NTU, for chlorophyll a and turbidity, respectively.
Abundance of vvh in water samples was significantly associated with temperature (regression coefficient [b] = 0.10; partial coefficient of determination [pseudo-R2] = 29%) and salinity (b1 = 0.14, b2 = −0.0027; R2 = 26%). Detection of tlh in water samples was significantly associated with temperature (b = 0.12; R2 = 39%), salinity (b1 = 0.17, b2 = −0.0039; R2 = 24%), and turbidity (b = 1.4; R2 = 15%). Temperature was not a significant predictor of tdh or trh density in water samples, but salinity was a significant predictor of tdh (b1 = 0.62, b2 = −0.014; R2 = 8%) and trh (b1 = 0.3, b2 = −0.011; R2 = 5%) densities, respectively.
Detection of vvh in oyster samples was significantly associated with temperature (b = 0.072; R2 = 27%) and by chlorophyll a levels in the water column (b = 1.30; R2 = 16%). Temperature (b = 0.063; R2 = 18%), salinity (b1 = 0.47, b2 = −0.0099; R2 = 17%), and turbidity (b = 1.10; R2 = 12%) were all significant predictors of tlh density in oysters. Salinity (b1 = 1.50, b2 = −0.032; R2 = 16%) and turbidity (b = 2.70; R2 = 12%) were significant predictors of trh density in oyster samples, while only turbidity was a significant predictor of tdh density in oysters (b = 2.70; R2 = 17%).
Detection of vvh in sediment was significantly affected by salinity (b1 = 0.61, b2 = −0.014; R2 = 22%) and chlorophyll a levels (b = 1.1; R2 = 14%) in the water column. Detection of tlh in sediment was significantly associated with salinity (b1 = 0.41, b2 = −0.0085; R2 = 13%) and turbidity (b = 1.4; R2 = 17%) levels in the water column, while only turbidity was a significant predictor of tdh (b = 2.0; R2 = 16%) and trh (b = 2.3; R2 = 19%) in the sediment.
DISCUSSION
The role of temperature in determining the abundance of vibrios in marine and estuarine environments has been well documented, but temperature accounts for only a portion of the variability in V. vulnificus and V. parahaemolyticus densities (2, 12, 22, 28, 33, 40). In this study, we have monitored temperature and other environmental parameters that may affect V. vulnificus and V. parahaemolyticus abundance in water, oysters, and sediment collected from coastal waters of Mississippi and Alabama over the course of 19 months. Few studies have addressed the occurrence of total and pathogenic V. parahaemolyticus as well as V. vulnificus at this level of spatial and temporal detail.
Temperature was strongly associated with vibrio densities, but unlike previous reports of up to 50% (2, 9), partial coefficients of determination were 39% and 29% for tlh and vvh, respectively. The association of vvh with temperature here is in contrast to the work of Motes et al., in which the R2 value was 60% when using an antibody-based MPN method (28). The absence of a relationship between temperature and pathogenic V. parahaemolyticus, despite its strong association with total V. parahaemolyticus, was also surprising. Previous studies (12) have demonstrated that the ratios of pathogenic to total V. parahaemolyticus are higher when temperatures are lower. Thus, it is possible that pathogenic V. parahaemolyticus responds differently to temperature than total V. parahaemolyticus.
The relationship between salinity and vibrio densities was consistent with observations of previous studies indicating a nonlinear relationship when salinities vary over a sufficiently wide range (2, 40). The fitted coefficients (linear and quadratic) indicate that, where an association with salinity was found to be significant, vibrio densities tended to increase with salinity up to an optimal point (27 ppt for vvh in water, 22 ppt for tlh in water, 24 ppt for tlh in oysters, 22 ppt for tdh in water, and 14 ppt for trh in water) and then decreased as salinities increased beyond the optimum level. No significant trend versus salinity was apparent for V. vulnificus densities in oysters, which is surprising in light of previous studies (28, 31, 34). In comparing these observations to the findings of previous studies, it should be recognized that a nonlinear relationship will not be identified when salinities do not vary over a sufficiently wide range or the range of variation lies on only one side of the optimum salinity level. This was the case in our previous study (40). At one site in Alabama, salinity varied over a narrow range and there was no identifiable association between salinity and vibrio densities. At the second site, in Mississippi, where salinity varied over a wider range but below the optimum level, a significant positive linear relationship with salinity was identified (40). Other studies have also identified the importance of salinity to vibrio densities (23, 25, 28, 32). The salinity ranges in these studies varied, but all were narrower than that of the current study, which had a wide salinity range of 3 to 35 ppt.
Regression analysis indicated that chlorophyll a, like temperature, also had a different association with total V. parahaemolyticus than with pathogenic V. parahaemolyticus, further suggesting that these two subpopulations may respond differently to environmental cues. The relationship between vibrios and chlorophyll a levels has been explored previously (8, 13, 33, 34, 38). Studies of Chesapeake Bay (18) and in Peru (14), however, reported no correlation between V. cholerae and chlorophyll a. A 2004 study in New Jersey reported that potential correlation between total chlorophyll a and total vibrios measured by PCR using vibrio genus primers was masked by a relatively strong correlation between temperature and total chlorophyll a (36). The correlation between temperature and chlorophyll a in our study would appear to be much less, as regression diagnostics did not suggest any problematical issues.
Turbidity was positively associated with total V. parahaemolyticus in all sample types, but it was not significantly associated with vvh in any sample type. Turbidity, as it existed spatially and temporally in this study, was a stronger driving force with respect to the rarer pathogenic V. parahaemolyticus rather than with respect to total V. parahaemolyticus.
The median abundance of tlh relative to vvh (tlh-to-vvh ratio) in water and oysters was <1, indicating that V. parahaemolyticus was outnumbered by V. vulnificus, while the median tlh-to-vvh ratio in sediment was 3.5, indicating that the opposite was true in sediment. As turbidity increased, the relative abundance of tlh versus vvh (tlh-to-vvh ratio) increased in all sample types. This indicates a possibility that these two vibrio populations may react differently to the components of turbidity or to the events that cause increased turbidity. Chlorophyll a had the opposite association, in which the relative abundance ratio decreased as chlorophyll a levels increased. The relationship between the tlh-to-vvh ratios and temperature was minimal. The difference between tdh and tlh (tdh-to-tlh ratio) was found to be associated equally with turbidity and chlorophyll a. Thus, in one possible scenario, changes in turbidity may cause selection of one vibrio over the other, while changes in chlorophyll a levels may drive the densities closer to one another.
This study is unique in several aspects. First, turbidity had a significant relationship with V. parahaemolyticus density in both the sediment and the water column but not with density of V. vulnificus in the sediment. Potentially, this indicates a close association of V. parahaemolyticus with sedimentary environments. Sediment resuspension is an important contributor to turbidity in the water column. Therefore, resuspension of sediment during storm and wind events or by any activities that disturb the bottom sediment are potentially redistributing bacterial reservoirs into the water column. This is especially true in the Mississippi Sound, where the mean water depth is 2.98 m (15). It is unclear why turbidity in the water column would be a significant predictor of bacterial density in the sediment, but it may be related to composition of turbidity (resuspensions, blooms, and runoff) and the frequency and proximity of suspension events increasing turbidity in the water column and mixing the sediment-water interface, creating a deeper aerobic layer in the sediment. Additionally, sedimentation of water column particulates, including organic matter, such as diatoms, dinoflagellates, fungi, and zooplankton, provides additional attachment surfaces and nutrient resources in the sediment matrix (7, 17, 21). Thus, turbidity may be more important than previously realized, with respect to the intraseasonal variation of V. parahaemolyticus abundance, when water temperatures are relatively constant.
Second, this study demonstrated that V. parahaemolyticus outnumbered V. vulnificus in the sediment but not in oysters or in the water column. A comparison of two previous studies indicates that V. vulnificus outnumbers V. parahaemolyticus when temperatures are warm, but the reverse is true when temperatures are cold (12, 28). There have been no published reports suggesting that V. parahaemolyticus is better adapted for sediment than V. vulnificus. Our study suggests that some factor(s) associated with either the sediment, the bacteria, or the interaction of the two causes this phenomenon. It is possible that there is some buffering or protective component in sediment. Indeed, there are many attachment sites in sediment that may serve as microhabitats for vibrios, and these sites may be more heavily colonized by or more readily available to V. parahaemolyticus than to V. vulnificus. The ability of vibrios to attach to chitin (7, 16), surfaces of edaphic and epiphytic diatoms (A. R. Flowers, personal communication), and organic detritus in the sediment may also contribute to their protection from shock, death, or predation.
Remote sensing has demonstrated that our sampling areas in the Mississippi Sound and Dauphin Island have some of the highest turbidity levels in the Gulf of Mexico, likely because of the Mississippi River and Mobile Bay plumes (NOAA CoastWatch; http://coastwatch.noaa.gov, accessed 20 January 2009) and because the coastal waters of Mississippi and Alabama are very shallow and therefore subject to sediment resuspension. These plumes and resuspended sediment are sources of nutrient influx that could support V. parahaemolyticus growth.
The majority of the pathogenic V. parahaemolyticus isolates characterized in this study contained both tdh and trh, and a smaller group contained tdh alone. This is in contrast to other studies in which these distributions vary (19, 20, 35). A subset of the tdh+ and trh+ V. parahaemolyticus identified in this study was collected for phylogenetic studies, resulting in a collection of 98 tdh+, trh+, or tdh+ trh+ isolates of V. parahaemolyticus. Of these 98 isolates, 18 contained tdh only, 3 contained trh only, and 77 were tdh+ trh+ (19). This also has implications in light of the fact that we subjected 41 of these tdh+ and trh+ strains to multilocus sequence analysis (19), and none of these matched the O3:K6 pandemic clone that likely was introduced into the United States in 1998, including the 7/41 that were tdh positive and trh negative, like the pandemic clone.
Assessing the risk of V. parahaemolyticus infection from consumption of oysters or other seafood requires an extensive understanding of the environmental predictors of V. parahaemolyticus. However, more data have been collected on the densities of total V. parahaemolyticus than on potentially pathogenic V. parahaemolyticus because the latter is less numerous in the environment and methods for its accurate enumeration in environmental samples have only recently become available. Unfortunately, total V. parahaemolyticus levels do not completely reflect levels of pathogenic V. parahaemolyticus in oysters because of the inconsistent relationship between these two populations. While temperature appears to be the most important factor influencing the level of total V. parahaemolyticus, our results suggest that turbidity may be more influential than temperature or other environmental factors for predicting the abundance of pathogenic V. parahaemolyticus. This has implications, with respect to human health risk, as the role of turbidity may make the distribution of exposure to pathogens more variable, especially when one takes into account postharvest effects in oysters. Of note, this study relied on culture-dependent methods to quantify vibrios in the environment. One shortcoming of this study is the fact that nonculturable vibrios are potentially pathogenic (3), and the abundance or risk may be underestimated because of this.
In conclusion, our results demonstrate that V. parahaemolyticus and V. vulnificus tend to distribute differently between the water column and the sediment, with V. vulnificus favoring the water column and V. parahaemolyticus favoring the sediment. Higher levels of V. vulnificus in the water may account for the higher levels in oysters relative to V. parahaemolyticus. The affinity of V. parahaemolyticus for sediment is probably the underlying factor resulting in the positive correlation between turbidity and V. parahaemolyticus levels in the overlying waters and oysters. Sediment appears to serve as a reservoir of pathogenic V. parahaemolyticus in the water and oysters when resuspended. Incorporation of the current data on the relationship between turbidity and total or pathogenic V. parahaemolyticus into risk assessment models would improve their accuracy. The ability to measure turbidity, temperature, and other relevant environmental factors on a global scale using remote sensing technology will also greatly enhance the power of this approach.
Supplementary Material
Acknowledgments
Special thanks go to Dawn Rebarchik, Veronica C. Young, Jessica Jones, Dan Holiday, Adrienne Stutes, and the boat captains for technical assistance.
This research was supported by a NOAA Oceans and Human Health Initiative grant (NA-04-OAR4600214), by NSF grant EF-0813285 as part of the joint NSF-NIH Ecology of Infectious Diseases program, and by a NASA grant (NNX09AR57G).
Footnotes
Published ahead of print on 3 September 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Andrews, W. H., and T. S. Hammack. April 2003, posting date. Food sampling and preparation of sample homogenate. In G. J. Jackson et al. (ed.), Bacteriological analytical manual online. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Gaithersburg, MD.
- 2.Anonymous. 2005. Quantitative risk assessment on the public health impact of pathogenic Vibrio parahaemolyticus in raw oysters. U.S. Food and Drug Administration, Washington, DC.
- 3.Baffone, W., B. Citterio, E. Vittoria, A. Casaroli, R. Campana, L. Falzano, and G. Donelli. 2003. Retention of virulence in viable but non-culturable halophilic Vibrio spp. Int. J. Food Microbiol. 89:31-39. [DOI] [PubMed] [Google Scholar]
- 4.Biber, P. D., C. L. Gallegos, and W. J. Kenworthy. 2008. Calibration of a bio-optical model in the North River, NC: a tool to evaluate water-quality impacts on seagrasses. Estuaries Coasts 31:177-191. [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2006. Surveillance for foodborne-disease outbreaks—United States, 1998-2002. MMWR Morb. Mortal. Wkly. Rep. 55:1-42.16410759 [Google Scholar]
- 6.Chatterjee, S., and B. Price. 1991. Regression diagnostics. John Wiley, New York, NY.
- 7.Colwell, R. R., A. Huq, M. S. Islam, K. M. Aziz, M. Yunus, N. H. Khan, A. Mahmud, R. B. Sack, G. B. Nair, J. Chakraborty, D. A. Sack, and E. Russek-Cohen. 2003. Reduction of cholera in Bangladeshi villages by simple filtration. Proc. Natl. Acad. Sci. U. S. A. 100:1051-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constantin de Magny, G., R. Murtugudde, M. R. Sapiano, A. Nizam, C. W. Brown, A. J. Busalacchi, M. Yunus, G. B. Nair, A. I. Gil, C. F. Lanata, J. Calkins, B. Manna, K. Rajendran, M. K. Bhattacharya, A. Huq, R. B. Sack, and R. R. Colwell. 2008. Environmental signatures associated with cholera epidemics. Proc. Natl. Acad. Sci. U. S. A. 105:17676-17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantin de Magny, G., C. Paroissin, B. Cazelles, M. de Lara, J. Delmas, and J. Guegan. 2005. Modeling environmental impacts of plankton reservoirs on cholera population dynamics. ESAIM Proc. 14:156-173. [Google Scholar]
- 10.Cook, D. W., P. O'Leary, J. C. Hunsucker, E. M. Sloan, J. C. Bowers, R. J. Blodgett, and A. DePaola. 2002. Vibrio vulnificus and Vibrio parahaemolyticus in U.S. retail shell oysters: a national survey from June 1998 to July 1999. J. Food Prot. 65:79-87. [DOI] [PubMed] [Google Scholar]
- 11.Deepanjali, A., H. S. Kumar, I. Karunasagar, and I. Karunasagar. 2005. Seasonal variation in abundance of total and pathogenic Vibrio parahaemolyticus bacteria in oysters along the southwest coast of India. Appl. Environ. Microbiol. 71:3575-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DePaola, A., J. A. Nordstrom, J. Bowers, J. G. Wells, and D. W. Cook. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl. Environ. Microbiol. 69:1521-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eiler, A., M. Johansson, and S. Bertilsson. 2006. Environmental influences on Vibrio populations in northern temperate and boreal coastal waters (Baltic and Skagerrak Seas). Appl. Environ. Microbiol. 72:6004-6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil, A. I., V. R. Louis, I. N. Rivera, E. Lipp, A. Huq, C. F. Lanata, D. N. Taylor, E. Russek-Cohen, N. Choopun, R. B. Sack, and R. R. Colwell. 2004. Occurrence and distribution of Vibrio cholerae in the coastal environment of Peru. Environ. Microbiol. 6:699-706. [DOI] [PubMed] [Google Scholar]
- 15.Higgins, G. G., and C. K. Eleutrius. 1978. Mississippi Sound: volume, surface area and bathymetric statistics. J. Miss. Acad. Sci. 23:39-45. [Google Scholar]
- 16.Huq, A., and R. R. Colwell. 1995. Vibrios in the marine and estuarine environments. J. Mar. Biotechnol. 3:60-63. [Google Scholar]
- 17.Huq, A., E. B. Small, P. A. West, M. I. Huq, R. Rahman, and R. R. Colwell. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 45:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, S. C., V. Louis, N. Choopun, A. Sharma, A. Huq, and R. R. Colwell. 2000. Genetic diversity of Vibrio cholerae in Chesapeake Bay determined by amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 66:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, C. N., A. R. Flowers, V. C. Young, N. Gonzalez-Escalona, A. Depaola, N. F. Noriea III, and D. J. Grimes. 2009. Genetic relatedness among tdh+ and trh+ Vibrio parahaemolyticus cultured from Gulf of Mexico oysters (Crassostrea virginica) and surrounding water and sediment. Microb. Ecol. 57:437-443. [DOI] [PubMed] [Google Scholar]
- 20.Julie, D., L. Solen, V. Antoine, C. Jaufrey, D. Annick, and H. H. Dominique. 2010. Ecology of pathogenic and non-pathogenic Vibrio parahaemolyticus on the French Atlantic coast. Effects of temperature, salinity, turbidity and chlorophyll a. Environ. Microbiol. 12:929-937. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko, T., and R. R. Colwell. 1975. Adsorption of Vibrio parahaemolyticus onto chitin and copepods. Appl. Microbiol. 29:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko, T., and R. R. Colwell. 1973. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J. Bacteriol. 113:24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipp, E. K., C. Rodriguez-Palacios, and J. B. Rose. 2001. Occurrence and distribution of the human pathogen Vibrio vulnificus in a subtropical Gulf of Mexico estuary. Hydrobiologia 460:165-173. [Google Scholar]
- 24.Lipsitz, S. R., T. Leong, J. Ibrahim, and S. Lipschultz. 2001. A partial correlation coefficient and coefficient of determination for multivariate normal repeated measures data. J. Royal Stat. Soc. D. 50:87-95. [Google Scholar]
- 25.Martinez-Urtaza, J., A. Lozano-Leon, J. Varela-Pet, J. Trinanes, Y. Pazos, and O. Garcia-Martin. 2008. Environmental determinants of the occurrence and distribution of Vibrio parahaemolyticus in the rias of Galicia, Spain. Appl. Environ. Microbiol. 74:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy, S. A., A. DePaola, D. W. Cook, C. A. Kaysner, and W. E. Hill. 1999. Evaluation of alkaline phosphatase- and digoxigenin-labelled probes for detection of the thermolabile hemolysin (tlh) gene of Vibrio parahaemolyticus. Lett. Appl. Microbiol. 28:66-70. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy, S. A., A. DePaola, C. A. Kaysner, W. E. Hill, and D. W. Cook. 2000. Evaluation of nonisotopic DNA hybridization methods for detection of the tdh gene of Vibrio parahaemolyticus. J. Food Prot. 63:1660-1664. [DOI] [PubMed] [Google Scholar]
- 28.Motes, M. L., A. DePaola, D. W. Cook, J. E. Veazey, J. C. Hunsucker, W. E. Garthright, R. J. Blodgett, and S. J. Chirtel. 1998. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl. Environ. Microbiol. 64:1459-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagelkerke, N. J. D. 1991. A note on a general definition of the coefficient of determination. Biometrika 78:691-692. [Google Scholar]
- 30.Nordstrom, J. L., M. C. Vickery, G. M. Blackstone, S. L. Murray, and A. DePaola. 2007. Development of a multiplex real-time PCR assay with an internal amplification control for the detection of total and pathogenic Vibrio parahaemolyticus bacteria in oysters. Appl. Environ. Microbiol. 73:5840-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver, J. D. 2005. Vibrio vulnificus. In S. S. Belkin and R. R. Colwell (ed.), Oceans and health: pathogens in the marine environment. Springer, New York, NY.
- 32.O'Neill, K. R., S. H. Jones, and D. J. Grimes. 1992. Seasonal incidence of Vibrio vulnificus in the Great Bay estuary of New Hampshire and Maine. Appl. Environ. Microbiol. 58:3257-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips, A. M. B., A. DePaola, J. Bowers, S. Ladner, and D. J. Grimes. 2007. An evaluation of the use of remotely sensed parameters for prediction of incidence and risk associated with Vibrio parahaemolyticus in Gulf Coast oysters (Crassostrea virginica) J. Food Prot. 70:879-884. [DOI] [PubMed] [Google Scholar]
- 34.Randa, M. A., M. F. Polz, and E. Lim. 2004. Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl. Environ. Microbiol. 70:5469-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robert-Pillot, A., A. Guenole, J. Lesne, R. Delesmont, J. M. Fournier, and M. L. Quilici. 2004. Occurrence of the tdh and trh genes in Vibrio parahaemolyticus isolates from waters and raw shellfish collected in two French coastal areas and from seafood imported into France. Int. J. Food Microbiol. 91:319-325. [DOI] [PubMed] [Google Scholar]
- 36.Thompson, J. R., M. A. Randa, L. A. Marcelino, A. Tomita-Mitchell, E. Lim, and M. F. Polz. 2004. Diversity and dynamics of a north Atlantic coastal Vibrio community. Appl. Environ. Microbiol. 70:4103-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Food and Drug Administration and Center for Food Safety and Applied Nutrition. 2001. Vibrio cholerae, V. parahaemolyticus, V. vulnificus, and other Vibrio spp. In Bacteriological analytical manual online. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Gaithersburg, MD. http://www.cfsan.fda.gov/∼ebam/bam-9.html.
- 38.Watkins, W. D., and V. J. Cabelli. 1985. Effect of fecal pollution on Vibrio parahaemolyticus densities in an estuarine environment. Appl. Environ. Microbiol. 49:1307-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright, A. C., G. A. Miceli, W. L. Landry, J. B. Christy, W. D. Watkins, and J. G. Morris, Jr. 1993. Rapid identification of Vibrio vulnificus on nonselective media with an alkaline phosphatase-labeled oligonucleotide probe. Appl. Environ. Microbiol. 59:541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmerman, A. M., A. DePaola, J. C. Bowers, J. A. Krantz, J. L. Nordstrom, C. N. Johnson, and D. J. Grimes. 2007. Variability of total and pathogenic Vibrio parahaemolyticus densities in northern Gulf of Mexico water and oysters. Appl. Environ. Microbiol. 73:7589-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.