Abstract
The thermophilic Geobacillus bacterium catalyzed the formation of 100-μm hexagonal crystals at 60°C in a hydrogel containing sodium acetate, calcium chloride, and magnesium sulfate. Under fluorescence microscopy, crystals fluoresced upon excitation at 365 ± 5, 480 ± 20, or 545 ± 15 nm. X-ray diffraction indicated that the crystals were magnesium-calcite in calcite-type calcium carbonate.
Biomineralization is defined as the synthesis of inorganic crystalline or amorphous mineral-like materials by living organisms. Most of the crystals formed through biomineralization consist of inorganic minerals, but they may also contain trace amounts of organic compounds, which are thought to regulate the biomineralization process. A Japanese researcher first coined the term biomineralization in the 1940s, and interest in this field of research soon began to grow (28, 29). Biomineralization is a universal process, occurring in both prokaryotes and eukaryotes, including mammals. Biomineralization research is also a diverse and multidisciplinary field, encompassing microscopic analyses of biominerals and tissues (21, 23), mineralogy (25), organic chemistry (30), paleontology (3), and cellular biochemistry (19).
Bacteria are capable of forming inorganic crystals either intracellularly (12, 14) or extracellularly (9). Calcite (calcium carbonate) precipitation is a well-known example of extracellular bacterial biomineralization. Certain species of marine bacteria have been shown to precipitate minerals in water supplemented with artificial marine salt media and differing ratios of Mg2+ to Ca2+ concentrations (20). Several groups have investigated the geobiochemical significance of the presence of calcium carbonate in seafloor sediments in terms of calcite formation by marine bacteria (17). Boquet et al. (4) reported calcite formation by 210 species of soil bacteria cultured on solid growth medium containing calcium acetate, yeast extract, and glucose. Sánchez-Román et al. (22) found that 19 species of moderately halophilic bacteria, grown in nutrient liquid media, catalyzed the precipitation of calcite, magnesium calcite, and struvite in variable proportions depending on the ratio of Mg2+ to Ca2+ in the media.
Researchers and engineers in materials science have only recently begun to focus on biomineralization (2). There is currently great interest in the development of mimic materials based on biomineralization. In our own research, we found that a moderately thermophilic bacterium isolated from thermophilically composted organic waste catalyzes the formation of inorganic crystals extracellularly under oligotrophic conditions. In this paper, we describe the physiological properties of this isolated thermophilic bacterium, the conditions under which it catalyzes the formation of crystals, the elemental composition and photoluminescent properties of the crystals, and the technological implications of our findings.
Composted organic waste (0.4 g) was suspended in 20 ml of sterilized water and streaked onto soybean-casein digest (SCD) agar (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan), and the agar plate was incubated at 60°C for 48 h. A single visible colony was picked and recultured by streaking on a fresh SCD agar plate incubated at 60°C. Another single colony was isolated from this plate and cultured in the same manner to obtain a pure culture of the thermophilic bacterium. Gram staining was carried out according to conventional methodology. Morphology and photoluminescence properties were observed using a fluorescence microscope (Axioskop 2 plus; Zeiss, Oberkochen, Germany). Growth under anaerobic conditions was evaluated by culturing the bacterium in an anaerobic chamber.
The organism we isolated from composted organic waste was a Gram-positive, rod-shaped (0.5 μm wide and 2 to 5 μm long), facultative aerobic bacterium. Oval-shaped endospores were readily discerned microscopically by their intracellular terminal sites. The bacterium was categorized as moderately thermophilic, as it grew maximally in SCD broth (pH 7.0) at 60°C but did not grow above 70°C or below 45°C (5, 32). The inability of the bacterium to grow at 37°C indicated that the organism was an obligate thermophile (16). The maximal pH for growth in SCD broth culture at 60°C was 6.0 to 7.0, but a pH of 5.0 strongly inhibited growth. There was no growth under acidic conditions (pH 3.0 to 4.0).
Total DNA was extracted from 1.5 ml of cultured thermophilic bacteria (approximately 0.04 g of cells) using a DNA extraction kit (InstaGene Matrix; Bio-Rad, CA) according to the manufacturer's instructions. Sense and antisense primers were designed (5′-GAGTTTGATCCTGGCTCAG-3′ and 5′-GGCTACCTTGTTACGA-3′, respectively) and used in PCR with total DNA serving as the template. The reaction mixture contained 5 μg of total DNA, 400 pmol of each oligonucleotide primer, and 0.1 U of Taq DNA polymerase in a volume of 50 μl. Thirty thermal cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min were carried out. PCR products were purified by agarose gel electrophoresis, and the resulting DNA fragment (approximately 1,500 bp) was ligated into a pCRII vector (Invitrogen, CA). The PCR-amplified fragment was sequenced using the dideoxy-chain termination method.
The small subunit rRNA gene of the isolated bacterium was amplified from bulk genomic DNA by PCR, and it constituted 1,483 bp of the 1,500 bp that were originally amplified by PCR. Searches for similar sequences were carried out using the BLAST program. According to the GenBank/DDBJ/EMBL database, the sequence of this gene showed 100% homology with Geobacillus thermoglucosidasius ACTT43742 (AB021197 in GenBank). The isolated bacterium was thus identified as Geobacillus thermoglucosidasius and designated strain NY05.
For crystal formation on a gel surface, bacteria were first inoculated onto an SCD agar plate and incubated at 60°C for 18 h. A total of 1 mg (wet weight) of bacterial cells were inoculated with a loop onto a crystal-promoting hydrogel (25 mM sodium acetate, 7.0 mM calcium chloride, 2.0 mM magnesium sulfate, and 1.5% [wt/vol] agar) and incubated at 60°C for 24 h (hydrogel method).
When cells were cultured using the hydrogel method, crystals of about 20 μm in size appeared in and around bacterial colonies during the course of a 3- to 4-h incubation. As shown in Fig. 1 B, 100- to 200-μm hexagon-like crystals appeared when incubation was extended to 24 h. The importance of metabolic activity for crystal formation was evidenced by the absence of crystallization in control experiments, i.e., those performed without bacteria, or with cultures that had been autoclaved. These results demonstrate that bacteria are not simply heterogeneous nuclei for crystallization but actively mediate the process.
FIG. 1.
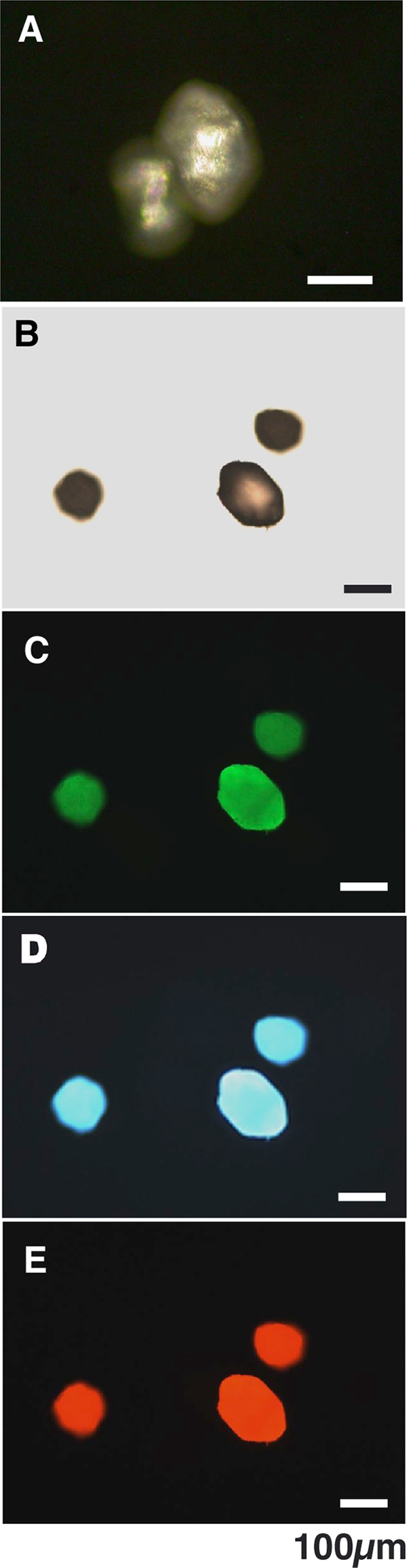
(A and B) Polarized (A) and light (B) microscopic observation of crystals formed using the hydrogel method. (C to E) Fluorescence microscopic image of crystals excited at 480 ± 20 nm, (C) 365 ± 5 nm (D), and 545 ± 15 nm (E).
To isolate crystals formed using the hydrogel method, the part of gel on which crystals formed was excised using a sterilized spatula and solubilized in buffer QX1 (Qiagen, CA) in a test tube at room temperature. Crystals were recovered at the bottom of the test tube and washed repeatedly with sterilized distilled water and ethanol and then dried at 60°C. Crystals were observed using an Eclipse-ME600 polarized microscope (Nikon, Tokyo, Japan) and an Axioskop 2 plus fluorescence microscope. For fluorescence microscopy, the following filter blocks were used: exciter, 360 to 370 nm, 460 to 500 nm, and 530 to 560 nm; dichroic mirror, 380 nm, 505 nm, and 570 nm; emitter, >399, 510 to 560 nm, and 573 to 684 nm.
The exceptionally strong polarizing property of crystals when examined under polarized microscopy confirmed that the material was composed of crystallized inorganic mineral (Fig. 1A). The remarkable fluorescent property of the crystals was demonstrated by fluorescence microscopy. As shown in Fig. 1C to E, the crystals fluoresced green, blue, and red when excited at 480 ± 20 nm, 365 ± 5 nm, and 545 ± 15 nm, respectively.
Geobacillus catalyzed the formation of crystals on gel media containing 25 mM sodium acetate, 7.0 mM calcium chloride, and 2.0 mM magnesium sulfate. Gels lacking magnesium sulfate did not prevent bacteria from catalyzing crystal formation, but the removal of sodium acetate or calcium chloride from media did preclude crystal formation. Thus, calcium and carboxyl carbon were both essential for crystal formation, while magnesium sulfate was inessential. Crystals formed when incubation temperatures were in the range of 60 to 70°C, but when the temperature was kept in the range of 40 to 50°C, fewer crystals formed. Crystallization was precluded by incubation above 80°C and below 30°C. The optimal temperature for crystal formation was therefore chosen to be 60°C. Crystal formation was observed only under aerobic conditions, suggesting that atmospheric oxygen and/or biological oxidative molecules are essential for the catalytic processes leading to crystal formation. The effect of sodium acetate concentration on crystal formation was also investigated using the hydrogel method. The concentrations of calcium chloride and magnesium sulfate were maintained at 7.0 mM and 2.0 mM, respectively, while the concentration of sodium acetate was varied. Sodium acetate in the range of 12.5 mM to 50 mM induced satisfactory crystal formation. However, at a concentration of 75 mM, sodium acetate decreased the efficiency of crystal formation, and no crystals were formed on gels with 100 mM sodium acetate. The effect of different sources of carbon on the ability of Geobacillus to catalyze crystal formation was investigated using the hydrogel method. The presence of sodium formate or acetate at 25 mM induced calcite formation, but 25 mM sodium propionate, sodium citrate, methyl alcohol, and ethyl alcohol did not promote crystal formation. These results indicated that Geobacillus-catalyzed crystal formation requires a carbon source derived from C1 and C2 carboxylates. The optimal concentration of calcium ion for crystal formation was also determined using the hydrogel method, in which the concentrations of sodium acetate and magnesium sulfate were 25 mM and 2.0 mM, respectively. More than 200 crystals per milligram (wet weight) of cells were obtained using 40 mM calcium chloride.
The effect of heavy metal ions on crystal formation was investigated by doping the hydrogel with heavy metal ions (CuCl2, NiCl2, CdCl2) at concentrations ranging from 0.01 to 0.5 mM. In general, results obtained for hydrogel doped with Cu2+ showed that a high concentration (0.5 mM) of heavy metal ions completely inhibited crystal formation. Doping hydrogel with 0.1 mM Cd2+ strongly inhibited crystal formation. On the other hand, low concentrations (0.01 mM) of metal ions did not impede crystal formation. The presence of Ni2+ at a concentration of 0.01 mM enhanced crystal formation.
Crystals were examined for fluorescence properties using an SPEX Fluorolog LF3-22-Tau3 fluorescence spectrophotometer (Horiba, Tokyo, Japan). Crystals were studied at room temperature, and matrix scanning was employed for the measurement of fluorescence spectra. Wavelength scans were performed at 250 to 400 nm at step intervals of 5 nm. The emission spectrum was measured for each excitation wavelength between 310 and 700 nm. Crystals were excited in the range of 260 to 400 nm and emitted energy in the range of 350 to 600 nm. The maximum excitation and emission wavelengths were 369.6 nm and 446.9 nm, respectively (Fig. 2 A). Figure 2B shows an example of a fluorescence spectrum for crystals excited at a wavelength of 350 nm, which were emitted at a wavelength of 375 to 550 nm with the maximum emission at 439 nm.
FIG. 2.
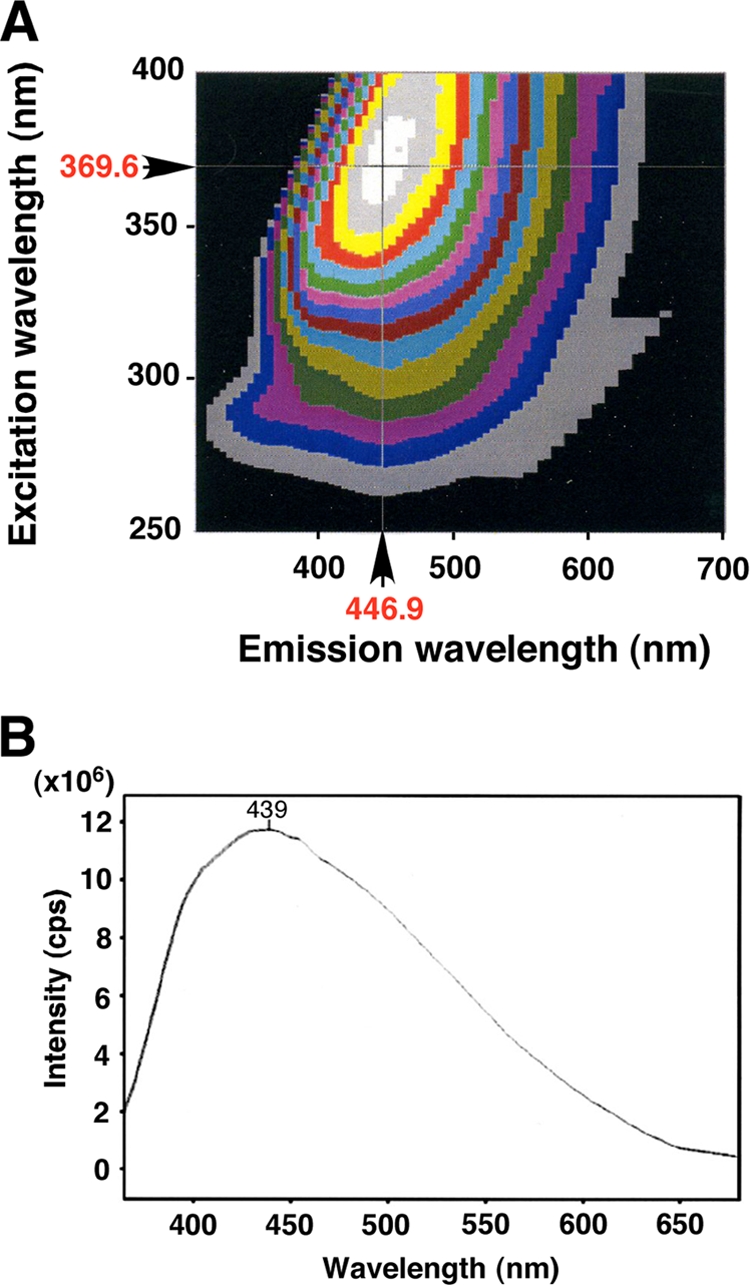
(A) Photoluminescence spectrum of a single crystal prepared using the gel method. (B) Emission fluorescence spectrum of a single crystal excited at 350 nm. cps, counts per second.
Energy-dispersive X-ray (EDX) elemental analyses of single purified crystals were performed using an EMAX-5770 electron microscope equipped with a microanalysis system (Horiba, Tokyo, Japan). The analyses were carried out at 20 kV of accelerating voltage and 0.26 nA of probe current. As shown in Fig. 3 A, crystals contained large amounts of carbon, oxygen, and calcium, with smaller amounts of magnesium, sodium, silica, sulfur, phosphorus, and chlorine. When the number of calcium atoms was converted to 100, the composition with respect to magnesium, sodium, silicon, sulfur, phosphorus, and chlorine was 19.1, 12.2, 5.10, 2.35, 1.22, and 1.83, respectively. We confirmed that the crystals react with hydrochloric acid using the Meingen reaction (4) and that they emit gas (CO2) upon melting. The crystal is possibly a calcium carbonate species, because its spectrum was similar to that of standard calcium carbonate (Fig. 3B). The inability of Geobacillus to catalyze crystal formation under anaerobic conditions suggests that the oxygen incorporated into the crystals may be derived from atmospheric oxygen.
FIG. 3.
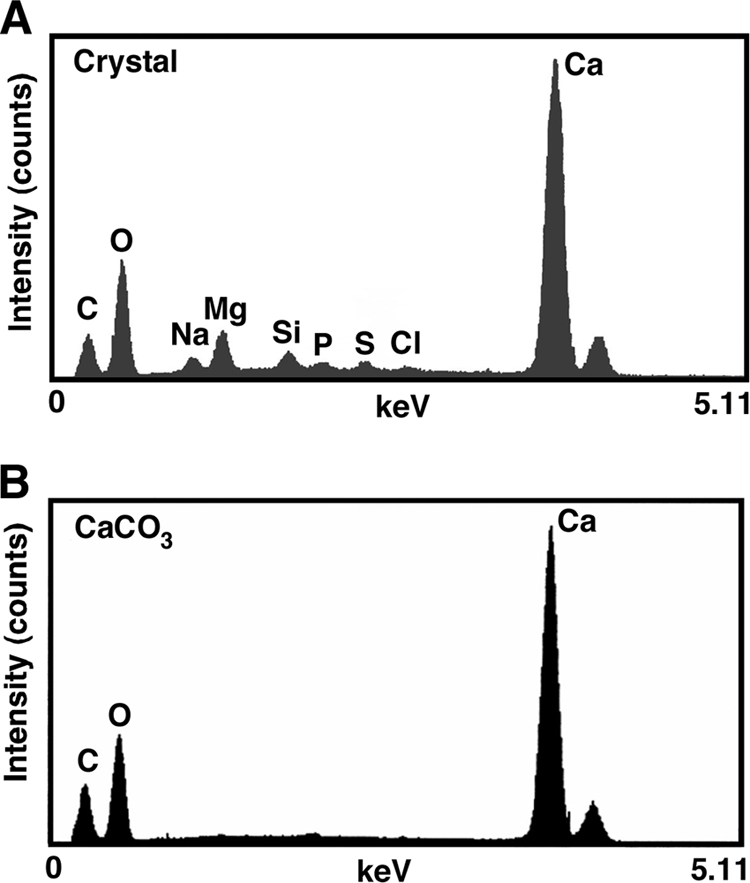
EDX spectrum of crystals. (A) Crystal formed through catalysis by G. thermoglucosidasius. (B) Purified calcium carbonate.
The structure of purified crystals was analyzed by the Bragg-Brentano method by using a RINT-2000 powder X-ray diffractometer (Rigaku, Tokyo, Japan) with a graphite monochromator, a scintillation counter scanning rate of 4°/min, and a rotary stage. The X-ray generator was operated at 40 kV and 100 to 200 mA. The divergence and receiving slits for the diffracted beam were set at 0.01° and 0.15 mm, respectively. The scanning regions of the diffraction angle (2 theta) were 20° to 90° at a step interval of 0.02° and a scan rate of 2.0°/min. Spectra were collected by using a diffractometer with a graphite monochromator and Cu Kα radiation (Fig. 4). The peak pattern of crystals was consistent with that of CaCO3 as a reference at up to 60° in 2 theta degrees, but the peak intensity of crystals was not consistent with that of the CaCO3 reference at over 40° in 2 theta degrees. A comparison of the X-ray diffraction pattern with the powder diffraction file (PDF-2) provided by the International Centre for Diffraction Data suggested that the crystals are magnesian calcite [(Ca, Mg)CO3] (PDF identification number 00-043-0697). Therefore, the data indicate that the crystals formed by Geobacillus nucleation are calcite-type calcium carbonate, in which some of the calcium in the crystal structure has been replaced by magnesium, sodium, sulfur, chlorine, and phosphorus.
FIG. 4.
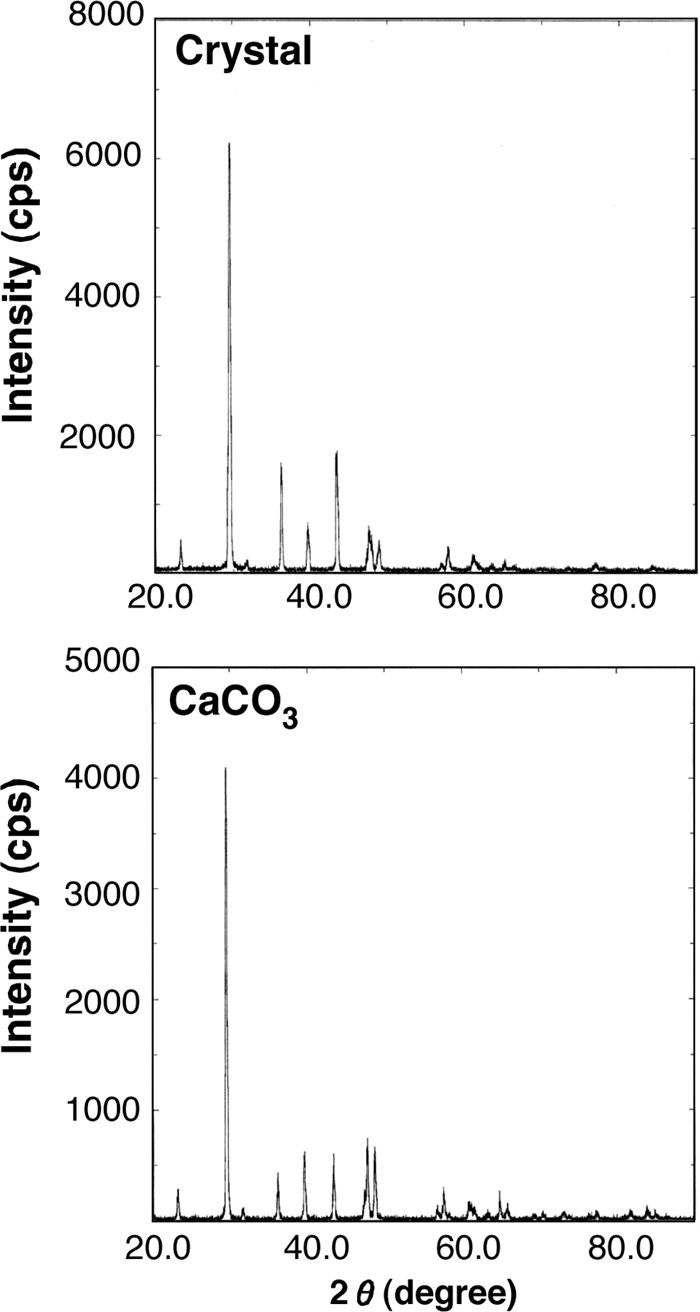
Powder X-ray diffraction patterns of the CaCO3 reference (bottom) and the unidentified crystal formed by G. thermoglucosidasius catalysis (top).
We observed that visible calcite crystals formed within 4 h in the area around colonies of G. thermoglucosidasius cultured on crystal formation gel. This observation supports the contention that G. thermoglucosidasius secretes nano-sized nucleus-like factors that are associated with extracellular crystal formation. Addadi et al. (1) reported that acidic matrix macromolecules of polystyrene film, which are involved in regulating calcite growth, often contain aspartic acid-rich domains and covalently bound sulfated polysaccharides. They proposed that sulfates and beta-sheet-structured carboxylates cooperate in oriented calcite crystal nucleation (1). In our study, the rate of crystal formation may have been enhanced due to the gel matrices that regulated the dispersion of calcium ions.
G. thermoglucosidasius was unable to catalyze calcite formation under anaerobic conditions, indicating that oxygen is required for the formation of calcite crystals. Accordingly, we proposed that the following two reactions are involved in G. thermoglucosidasius-catalyzed calcite formation when either formate, 2Ca2+ + 2HCOO− + O2 → 2CaCO3 + 2H+, or acetate, 2Ca2+ + 2CH3COO− + 3O2 → 2CaCO3 + 2CO2 + 2H+, is involved.
The precise role G. thermoglucosidasius-catalyzed calcite formation plays in the environment has not been elucidated. Reports demonstrating that bacteria adsorbed onto inorganic minerals are resistant to various chemical and physical stressors suggest a possible role for bacterially catalyzed calcite formation (7, 13, 26). We found endospore-like substances adsorbed on the surface of purified calcite. Purified calcite formed by G. thermoglucosidasius nucleation and exposed to extreme conditions (i.e., washing with 70% ethyl alcohol, treatment with protease and DNase, UV irradiation at 254 nm, boiling for 15 min, freezing at −80°C for 48 h) was incubated on the surface of SCD nutrient agar at 60°C for 14 to 18 h, resulting in the formation of a bacterial colony around the calcite. Though the calcite crystals are inorganic substances and were completely dehydrated, G. thermoglucosidasius cells and/or endospores could have been adsorbed to the surface and protected from the extreme conditions in a cryptobiotic state (6, 10). We confirmed that the colony was growing concentrically around the calcite that had been exposed to the extreme environment. These results indicated that calcite-bound cells were more resistant to physicochemical stressors.
The calcite formation was initiated under oligotrophic conditions in which formate or acetate was present, as were trace amounts of calcium. If G. thermoglucosidasius cells were exposed to conditions unsuitable for their growth in the natural environment, they would be expected to catalyze the formation of calcite and adsorb onto it, living in a cryptobiotic state until conditions improved. It is assumed that G. thermoglucosidasius cells adsorb onto calcite as a means of surviving unfavorable conditions but remain there and continue to grow under favorable oligotrophic conditions. Calcite formation may therefore be one of the means these organisms use to survive in extreme environments.
G. thermoglucosidasius-catalyzed calcite crystals may have a number of applications that can be exploited. Metal oxide-type phosphors, which are conventionally used in fluorescent lamps and cathode ray tubes, are generally prepared by doping high-purity and precious rare earth with an activator (31). Different CaCO3 modifications doped with Eu3+, Tb3+, or Ce3+ have been synthesized as phosphor hosts, and their luminescent properties have been determined (8). The maximum excitation and emission wavelengths for Eu3+-doped calcite-type red phosphor are 393 nm and 611 nm, respectively (18). Vaterite-type CaCO3 doped with Ce3+ and Tb3+ emits green light, and its maximum excitation and emission wavelengths are 275 nm and 545 nm, respectively (11). However, the drastic loss of fluorescence intensity these phosphors display at room temperature is disadvantageous. In addition, these phosphors have a very narrow emission wavelength interval of 50 nm. The calcite crystals formed by G. thermoglucosidasius nucleation, on the other hand, are excited by a wavelength interval of 260 to 400 nm, and their emission wavelengths are from 350 to 600 nm. The wide emission wavelength interval is a novel fluorescence property of G. thermoglucosidasius-catalyzed calcite crystals. Unlike conventional phosphors, calcite crystals formed by G. thermoglucosidasius nucleation can be prepared without rare earth, and they accommodate magnesium, sodium, sulfur, and phosphorus atoms in the carbonate host lattice. We may expect new industrial applications for G. thermoglucosidasius-catalyzed calcite phosphor, owing to its fluorescence stability without loss of intensity.
G. thermoglucosidasius calcite phosphor could have potential advantages as a filler in rubber and plastics, fluorescent particles in stationery ink, and a fluorescent marker for biochemistry applications (15) and also with uses in other biodevices (24). Our study may provide novel inorganic materials with a range of photoluminescent properties that can be fabricated from calcite doped with various heavy-metal ions (e.g., Cu2+, Ni2+, Cd2+). In materials engineering, environmentally friendly systems with minimal energy consumption and resource depletion are required for producing materials and composites. Biological processes serve as impressive archetypes of sustainable materials technologies. Because of its potential benefits in this regard, the study of biominerals has gained recognition as an important area of biomimetic materials science (27).
Acknowledgments
We acknowledge Kentaro Sakai of the Cooperative Research Center and Hirosumi Yokoyama of the Department of Applied Physics, University of Miyazaki, for their technical assistance in EDX analysis and X-ray diffraction.
Footnotes
Published ahead of print on 17 September 2010.
REFERENCES
- 1.Addadi, L., J. Moradian, E. Shay, N. G. Maroudas, and S. A. Weiner. 1987. Chemical model for the cooperation of sulfates and carboxylates in calcite crystal nucleation: relevance to biomineralization. Proc. Natl. Acad. Sci. U. S. A. 84:2732-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aizenberg, J., D. A. Muller, J. L. Grazul, and D. R. Hamann. 2003. Direct fabrication of large micropatterned single crystals. Science 299:1205-1208. [DOI] [PubMed] [Google Scholar]
- 3.Benzerara, K., N. Menguy, F. Guyot, C. Dominici, and P. Gillet. 2003. Nanobacteria-like calcite single crystals at the surface of the Tataouine meteorite. Proc. Natl. Acad. Sci. U. S. A. 100:7438-7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boquet, E., A. Boronat, and A. Ramos-Cormenzana. 1973. Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature 246:527-529. [Google Scholar]
- 5.Cheng, Y., Z. Guo, X. Liu, H. Yin, G. Qiu, F. Pan, and H. Liu. 2009. The bioleaching feasibility for Pb/Zn smelting slag and community characteristics of indigenous moderate-thermophilic bacteria. Bioresour. Technol. 100:2737-2740. [DOI] [PubMed] [Google Scholar]
- 6.Clegg, J. S. 2001. Cryptobiosis—a peculiar state of biological organization. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 128:613-624. [DOI] [PubMed] [Google Scholar]
- 7.Demanèche, S., L. Jocteur-Monrozier, H. Quiquampoix, and P. Simonet. 2001. Evaluation of biological and physical protection against nuclease degradation of clay-bound plasmid DNA. Appl. Environ. Microbiol. 67:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang, M., J. Liu, G. Yin, and R. Sun. 2009. Preparation and characterization of Eu3+-doped CaCO3 phosphor by microwave synthesis. Rare Met. 28:439-444. [Google Scholar]
- 9.Kashefi, K., J. M. Tor, K. P. Nevin, and D. R. Lovley. 2001. Reductive precipitation of gold by dissimilatory Fe(III)-reducing Bacteria and Archaea. Appl. Environ. Microbiol. 67:3275-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keilin, D. 1959. The problem of anabiosis or latent life: history and current concept. Proc. R. Soc. Lond. B Biol. Sci. 150:149-191. [DOI] [PubMed] [Google Scholar]
- 11.Kojima, Y., S. Doi, and T. Yasue. 2002. Synthesis of cerium (III) and terbium (III) codoped vaterite phosphor emitting by black light irradiation and its fluorescence property. J. Ceramic Soc. Jpn. 110:755-760. [Google Scholar]
- 12.Konishi, Y., T. Tsukiyama, K. Ohno, N. Saitoh, T. Nomura, and S. Nagamine. 2006. Intracellular recovery of gold by microbial reduction of AuCl4-ions using the anaerobic bacterium Shewanella algae. Hydrometallurgy 81:24-29. [Google Scholar]
- 13.Kubota, M., T. Nakabayashi, Y. Matsumoto, T. Shiomia, Y. Yamada, K. Ino, H. Yamanokuchi, M. Matsui, T. Tsunoda, F. Mizukami, and K. Sakaguchi. 2008. Selective adsorption of bacterial cells onto zeolites. Colloids Surf. B Biointerfaces 64:88-97. [DOI] [PubMed] [Google Scholar]
- 14.Langley, S., and T. J. Beveridge. 1999. Effect of O-side-chain-lipopolysaccharide chemistry on metal binding. Appl. Environ. Microbiol. 65:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling, H., Z. Mao, and C. Gao. 2009. Fabrication of fluorescent microparticles by doping water-soluble CdTe nanocrystals into calcium carbonate for monitoring intracellular uptake. Colloids Surf. A Physicochem. Eng. Asp. 336:115-122. [Google Scholar]
- 16.Loginova, L. G., T. I. Bogdanova, and L. M. Seregina. 1981. Growth of obligate-thermophilic bacteria on a medium with paraffin. Mikrobiologiia 50:49-54. (In Russian.) [PubMed] [Google Scholar]
- 17.Paerl, H. W., T. F. Steppe, and R. P. Reid. 2001. Bacterially mediated precipitation in marine stromatolites. Environ. Microbiol. 3:123-130. [DOI] [PubMed] [Google Scholar]
- 18.Pan, Y., M. Wu, and Q. Su. 2003. Synthesis of Eu3+-doped calcium and strontium carbonate phosphors at room temperature. Mater. Res. Bull. 38:1537-1544. [Google Scholar]
- 19.Reith, F., B. Etschmann, C. Grosse, H. Moors, M. A. Benotmane, P. Monsieurs, G. Grass, C. Doonan, S. Vogt, B. Lai, G. Martinez-Criado, G. N. George, D. H. Nies, M. Mergeay, A. Pring, G. Southam, and J. Brugger. 2009. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc. Natl. Acad. Sci. U. S. A. 106:17757-17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivadeneyra, M. A., R. Delgado, R. J. Párraga, A. Ramos-Cormenza, and G. Delgado. 2006. Precipitation of minerals by 22 species of moderately halophilic bacteria in artificial marine salts media: influence of salt concentration. Folia Microbiol. (Praha) 51:445-453. [DOI] [PubMed] [Google Scholar]
- 21.Samata, T., N. Hayashi, M. Kono, K. Hasegawa, C. Horita, and S. Akera. 1999. A new matrix protein family related to the nacreous layer formation of Pinctada fucata. FEBS Lett. 462:225-229. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Román, M., M. A. Rivadeneyra, C. Vasconcelos, and J. A. McKenzie. 2007. Biomineralization of carbonate and phosphate by moderately halophilic bacteria. FEMS Microbiol. Ecol. 61:273-284. [DOI] [PubMed] [Google Scholar]
- 23.Saruwatari, K., T. Matsui, H. Mukai, H. Nagasawa, and T. Kogure. 2009. Nucleation and growth of aragonite crystals at the growth front of nacres in pearl oyster, Pinctada fucata. Biomaterials 30:3028-3034. [DOI] [PubMed] [Google Scholar]
- 24.Sato, H., G. Yamanaka, and M. Ikeya. 2003. Basic study on electrically stimulated luminescence (ESL) as a dosimetry and dating method. Radiat. Meas. 37:335-339. [Google Scholar]
- 25.Tourney, J., and B. T. Ngwenya. 2009. Bacterial extracellular polymeric substances (EPS) mediate CaCO3 morphology and polymorphism. Chem. Geol. 262:138-146. [Google Scholar]
- 26.Vettori, C., E. Gallori, and G. Stotzky. 2000. Clay minerals protect bacteriophage PBS1 of Bacillus subtilis against inactivation and loss of transducing ability by UV radiation. Can. J. Microbiol. 46:770-773. [PubMed] [Google Scholar]
- 27.Wakayama, H., S. R. Hall, and S. Mann. 2005. Fabrication of CaCO3-biopolymer thin films using supercritical carbon dioxide. J. Mater. Chem. 15:1134-1136. [Google Scholar]
- 28.Watabe, N., D. G. Sharp, and K. M. Wilbur. 1958. Studies on shell formation. VIII. Electron microscopy of crystal growth of the nacreous layer of the oyster Crassostrea virginica. Biophys. Biochem. Cytol. 4:281-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watabe, N., and K. M. Wilbur. 1960. Influence of the organic matrix on crystal type in mollusks. Nature 188:334. [Google Scholar]
- 30.Wynn, A., and T. H. Shafer. 2005. Four differentially expressed cDNAs in Callinectes sapidus containing the Rebers-Riddiford consensus sequence. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 141:294-306. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita, N. 1993. Coexistence of Eu2+ and Eu3+ centers in the CaO:Eu powder phosphor. J. Electrochem. Soc. 140:840-843. [Google Scholar]
- 32.Zeinali, M., M. Vossoughi, and S. K. Ardestani. 2007. Characterization of a moderate thermophilic Nocardia species able to grow on polycyclic aromatic hydrocarbons. Lett. Appl. Microbiol. 45:622-628. [DOI] [PubMed] [Google Scholar]


