Abstract
We detected flavins in the growth medium of the methanotrophic bacterium Methylocystis species strain M. Flavin secretion correlates with growth stage and increases under iron starvation conditions. Two other methanotrophs, Methylosinus trichosporium OB3b and Methylococcus capsulatus (Bath), secrete flavins, suggesting that flavin secretion may be common to many methanotrophic bacteria.
Methanotrophic bacteria utilize methane as their sole carbon source and play a key role in the global carbon cycle. The first step in methane metabolism, the oxidation of methane to methanol, is catalyzed by methane monooxygenase (MMO) metalloenzymes by either a copper-dependent membrane-bound particulate MMO (pMMO) (9) or an iron-dependent, soluble MMO (sMMO) (18). Therefore, metals are necessary for the growth of methanotrophic bacteria (12, 13). Despite the importance of metals in methanotroph physiology, uptake of metal ions by methanotrophic bacteria is not well understood. To study metal acquisition, we purified and characterized components of the spent growth medium from Methylocystis species strain M. Unexpectedly, we detected extracellular flavins (riboflavin and flavin mononucleotide [FMN]).
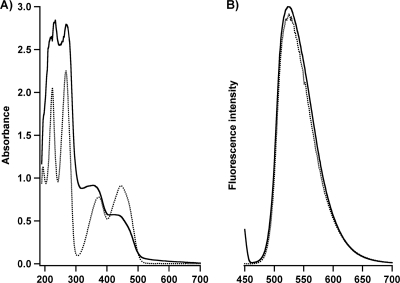
To perform these experiments, the type II methanotroph Methylocystis sp. strain M was grown using a procedure similar to that described for Methylosinus trichosporium OB3b (7, 8, 10) (see the supplemental material). The spent growth medium from Methylocystis sp. strain M was purified by fast protein liquid chromatography (FPLC) using a Discovery DSC-18 reverse-phase column. The eluted fractions exhibit UV-visible spectral features that suggest the presence of flavins (Fig. 1A). When the fractions were purified further using a Supelco C18 column, the purified flavins exhibited absorption features that were identical to those of commercial riboflavin (see Fig. S1 in the supplemental material). Upon excitation at 441 nm, the fluorescence emission spectrum of the purified fractions exhibited a broad peak between 450 and 700 nm, with a maximum at 523 nm (Fig. 1B). This feature is characteristic of the isoalloxazine ring in flavins (11, 21).
FIG. 1.
UV-visible (A) and fluorescence (B) spectra of extracellular compounds isolated from Methylocystis sp. strain M (solid lines) compared to those from commercial pure FMN (dashed lines). Upon excitation at 441 nm, the emission spectra were monitored between 450 and 700 nm.
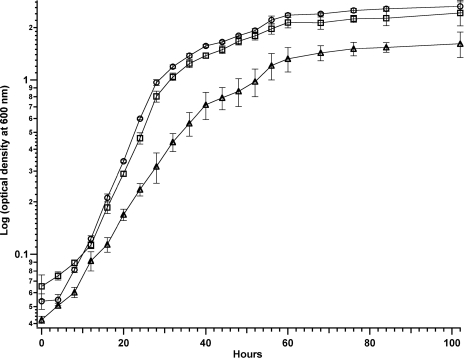
To characterize the amount of extracellular flavins secreted throughout growth, the cells were grown under three metal availability conditions: default growth (50 μM CuSO4·5H2O, 40 μM FeSO4·7H2O), copper starved (0 μM CuSO4·5H2O, 40 μM FeSO4·7H2O), and iron starved (50 μM CuSO4·5H2O, 2 μM FeSO4·7H2O). The growth curves are shown in Fig. 2, and the intracellular metal concentration of the cells is shown in Fig. S2 of the supplemental material. The growth rates, from an average of biological triplicates, determined for default, iron-starved, and copper-starved growth conditions between 12 and 28 h of growth are 0.043 ± 0.003 h−1, 0.014 ± 0.003 h−1, and 0.052 ± 0.002 h−1, respectively. These values imply that the availability of iron affects the growth of Methylocystis sp. strain M.
FIG. 2.
Growth curves of Methylocystis sp. strain M, grown under default (squares), copper-starved (circles), and iron-starved (triangle) conditions. Data are represented in a semilogarithmic scale as averages and standard deviations of biological triplicates.
We then characterized the amount of extracellular flavins in the spent medium isolated from three different stages of growth. The samples used for quantitation were normalized based on optical density measured at 600 nm. The dry weight and cell length, normalized to the optical density, remained unaffected by growth under different metal concentrations (see Fig. S3 in the supplemental material). When cells were grown under default conditions to early exponential phase (36 h), they produced 0.050 ± 0.003 μM flavins, compared to 0.048 ± 0.003 μM produced by copper-starved cells and 0.054 ± 0.003 μM produced by iron-starved cells. For cells grown to late exponential phase (60 h), the iron-starved cells secreted 0.116 ± 0.004 μM flavins compared to 0.056 ± 0.004 μM and 0.053 ± 0.001 μM secreted by cells grown under default and copper-starved conditions, respectively. This trend was also observed for cells grown to stationary phase (102 h) with 0.102 ± 0.001 μM flavins produced by cells under default growth conditions, 0.078 ± 0.007 μM under copper-starved conditions, and 0.145 ± 0.003 μM under iron-starved conditions (see Fig. S4 in the supplemental material). The cells secreted approximately twice as much flavins when grown to stationary phase. Interestingly, cells that are iron starved also doubled the amount of secreted extracellular flavins during late exponential growth compared to cells grown under default growth conditions (see Fig. S4). These observations suggest that the secretion of flavins is dependent on the growth stage and iron availability.
To test this hypothesis, the amount of iron in the default growth condition was increased 3-fold (50 μM CuSO4, · 5H2O, 120 μM FeSO4·7H2O). The cells produced reduced amounts of flavins, 0.028 ± 0.008 μM compared to 0.050 ± 0.003 μM. Increased secretion of flavins was also observed for Helicobacter pylori under iron starvation conditions (21), and flavin secretion by Shewanella species is correlated with growth stages and oxygen availability (19).
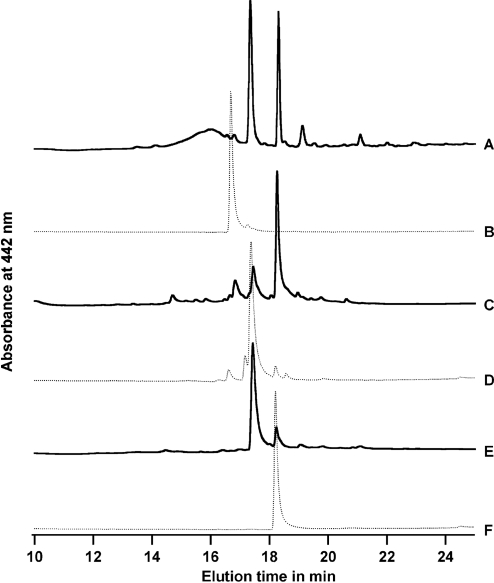
To determine if the secretion of flavins is common among culturable methanotrophs, we performed similar analyses on the spent media isolated from the type X methanotroph Methylococcus capsulatus (Bath) and the type II methanotroph M. trichosporium OB3b (Fig. 3). Interestingly, both M. trichosporium OB3b and M. capsulatus (Bath) secreted flavins as well.
FIG. 3.
Comparison of the DSC-18 elution profiles of extracellular flavins (FMN and riboflavin) secreted under default growth conditions by Methylocystis sp. strain M (A), M. trichosporium OB3b (C), and M. capsulatus (Bath) (E). The peaks that elute at ∼16.7 min, ∼17.4 min, and ∼18.2 min correspond to FAD (B), FMN (D), and riboflavin (F), respectively. The small peak at ∼16.8 min in line C may be due to contaminating cell lysis.
To confirm that the flavins detected did not result from cell lysis, control experiments were performed to detect FAD, which is primarily inside the cell (19). We detected negligible amounts of FAD in M. trichosporium OB3b spent medium and none in that from the other two methanotrophs, indicating that the flavins in the spent growth medium are indeed secreted (see Fig. S5 in the supplemental material).
To place extracellular flavins in the context of metal uptake molecules such as siderophores and methanobactins (15, 22), we performed assays to detect these compounds in methanotrophic bacteria. Assays performed using Fe-chrome azurol S (Fe-CAS) plates on the spent media isolated from Methylocystis sp. strain M and M. capsulatus (Bath) grown under iron-starved conditions indicate that they do not secrete siderophores. M. trichosporium OB3b does secrete siderophores (22) (see Fig. S6 in the supplemental material), however. Methylocystis sp. strain M secretes methanobactin (see Fig. S7 in the supplemental material), and the purified copper-loaded methanobactin from this species is similar to that isolated and characterized from M. trichosporium OB3b (see Fig. S7) (4, 10, 14, 15).
Riboflavin is capable of metal coordination and in fact has a higher affinity for iron than for any other metal in the Irving-Williams series (1). FMN can bind iron and copper (see Fig. S8 in the supplemental material). In Shewanella species, flavins are proposed to chelate iron (17) and to act as electron shuttles that aid in an increased reduction of Fe(III) oxides into Fe(II) for cellular usage and respiration (3, 6, 17, 19). In methanotrophs, as with methanobactin for copper, extracellular flavins may chelate iron and transfer electrons from surface reductases to insoluble Fe(III) sources (2, 15, 16). To test if extracellular flavins play a role in iron acquisition, experiments comparing the effects of iron, Fe-FMN, or apo-FMN addition to iron-starved Methylocystis sp. strain M were performed. However, addition of apo-FMN or Fe-FMN did not alter the growth rates (see Fig. S9 in the supplemental material).
In conclusion, our results suggest that extracellular flavins may have a function distinct from those of methanobactin and siderophores. Extracellular flavins may also play a role in the reduction and chelation of other transition metals, such as manganese (17). Discerning the explicit role(s) of extracellular flavins in methanotrophs will require further investigation, and the link between flavin secretion and metal ion homeostasis in multiple bacteria as well as plants and yeasts (5, 20) represents an important area for future research.
Supplementary Material
Acknowledgments
This work was supported by NIH grant GM070473 and NSF grant MCB0842366.
Portions of this work were performed in the Keck Biophysics Facility and the IMSERC at Northwestern University. We thank Paul Thomas for pointing us to the presence of flavins in our preparations and Jeffrey A. Gralnick for useful discussions. The Methylocystis sp. strain M culture was obtained originally from Hiroo Uchiyama. A stock of Pseudomonas aeruginosa was obtained from Dianne K. Newman.
Footnotes
Published ahead of print on 10 September 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Albert, A. 1950. The metal-binding properties of riboflavin. Biochem. J. 47:xxvii. [PubMed] [Google Scholar]
- 2.Balasubramanian, R., and A. C. Rosenzweig. 2008. Copper methanobactin: a molecule whose time has come. Curr. Op. Chem. Biol. 12:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron, D., E. LaBelle, D. Coursolle, J. A. Gralnick, and D. R. Bond. 2009. Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1. J. Biol. Chem. 284:28865-28873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behling, L. A., S. C. Hartsel, D. E. Lewis, A. A. Dispirito, D. W. Choi, L. R. Masterson, G. Veglia, and W. H. Gallagher. 2008. NMR, mass spectrometry and chemical evidence reveal a different chemical structure for methanobactin that contains oxazolone rings. J. Am. Chem. Soc. 130:12604-12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boretsky, Y. R., O. V. Protchenko, T. M. Prokopiv, I. O. Mukalov, D. V. Fedorovych, and A. A. Sibirny. 2007. Mutations and environmental factors affecting regulation of riboflavin synthesis and iron assimilation also cause oxidative stress in the yeast Pichia guilliermondii. J. Basic Microbiol. 47:371-377. [DOI] [PubMed] [Google Scholar]
- 6.Coursolle, D., D. B. Baron, D. R. Bond, and J. A. Gralnick. 2010. The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J. Bacteriol. 192:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox, B. G., W. A. Froland, D. R. Jollie, and J. D. Lipscomb. 1990. Methane monooxygenase from Methylosinus trichosporium OB3b. Methods Enzymol. 188:191-202. [DOI] [PubMed] [Google Scholar]
- 8.Hakemian, A. S., K. C. Kondapalli, J. Telser, B. M. Hoffman, T. L. Stemmler, and A. C. Rosenzweig. 2008. The metal centers of particulate methane monooxygenase from Methylosinus trichosporium OB3b. Biochemistry 47:6793-6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakemian, A. S., and A. C. Rosenzweig. 2007. The biochemistry of methane oxidation. Annu. Rev. Biochem. 76:223-241. [DOI] [PubMed] [Google Scholar]
- 10.Hakemian, A. S., C. E. Tinberg, K. C. Kondapalli, J. Telser, B. M. Hoffman, T. L. Stemmler, and A. C. Rosenzweig. 2005. The copper chelator methanobactin from Methylosinus trichosporium OB3b binds Cu(I). J. Am. Chem. Soc. 127:17142-17143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harbury, H. A., and K. A. Foley. 1958. Molecular interaction of isoalloxazine derivatives. Proc. Natl. Acad. Sci. U. S. A. 44:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao, W.-C., Y.-R. Chen, E. C. Yi, H. Lee, Q. Tian, K.-M. Wu, S.-F. Tsai, S. S.-F. Yu, Y.-J. Chen, R. Aebersold, and S. I. Chan. 2004. Quantitative proteomic analysis of metabolic regulation by copper ions in Methylococcus capsulatus (Bath). J. Biol. Chem. 279:51554-51560. [DOI] [PubMed] [Google Scholar]
- 13.Karlsen, O. A., F. S. Berven, G. P. Stafford, Ø. Larsen, J. C. Murrell, H. B. Jensen, and A. Fjellbirkeland. 2003. The surface-associated and secreted MopE protein of Methylococcus capsulatus (Bath) responds to changes in the concentration of copper in the growth medium. Appl. Environ. Microbiol. 69:2386-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, H. J., D. W. Graham, A. A. DiSpirito, M. A. Alterman, N. Galeva, C. K. Larive, D. Asunskis, and P. M. A. Sherwood. 2004. Methanobactin, a copper-aquisition compound from methane oxidizing bacteria. Science 305:1612-1615. [DOI] [PubMed] [Google Scholar]
- 15.Knapp, C. W., D. A. Fowle, E. Kulczycki, J. A. Roberts, and D. W. Graham. 2007. Methane monooxygenase gene expression mediated by methanobactin in the presence of mineral copper sources. Proc. Natl. Acad. Sci. U. S. A. 104:12040-12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulczycki, E., D. A. Fowle, C. Knapp, D. W. Graham, and J. A. Roberts. 2007. Methanobactin-promoted dissolution of Cu-substituted borosilicate glass. Geobiology 5:251-263. [Google Scholar]
- 17.Marsili, E., D. B. Baron, I. D. Shikhare, D. Coursolle, J. A. Gralnick, and D. R. Bond. 2008. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. U. S. A. 105:3968-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merkx, M., D. A. Kopp, M. H. Sazinsky, J. L. Blazyk, J. Müller, and S. J. Lippard. 2001. Dioxygen activation and methane hydroxylation by soluble methane monooxygenase: a tale of two irons and three proteins. Angew. Chem. Int. Ed. Engl. 40:2782-2807. [DOI] [PubMed] [Google Scholar]
- 19.von Canstein, H., J. Ogawa, S. Shimizu, and J. R. Lloyd. 2008. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microbiol. 74:615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vorwieger, A., C. Gryczka, A. Czihal, D. Douchkov, J. Tiedemann, H. P. Mock, M. Jakoby, B. Weisshaar, I. Saalbach, and H. Baumlein. 2007. Iron assimilation and transcription factor controlled synthesis of riboflavin in plants. Planta 226:147-158. [DOI] [PubMed] [Google Scholar]
- 21.Worst, D. J., M. M. Gerrits, C. Vandenbroucke-Grauls, and J. G. Kusters. 1998. Helicobacter pylori ribBA-mediated riboflavin production is involved in iron acquisition. J. Bacteriol. 180:1473-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon, S., S. M. Kraemer, A. A. Dispirito, and J. D. Semrau. 2010. An assay for screening microbial cultures for chalkophore production. Environ. Microbiol. Rep. 2:295-303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.