Abstract
We cloned and purified the major family 10 xylanase (Xyn10A) from Acidothermus cellulolyticus 11B. Xyn10A was active on oat spelt and birchwood xylans between 60°C and 100°C and between pH 4 and pH 8. The optimal activity was at 90°C and pH 6; specific activity and Km for oat spelt xylan were 350 μmol xylose produced min−1 mg of protein−1 and 0.53 mg ml−1, respectively. Based on xylan cleavage patterns, Xyn10A is an endoxylanase, and its half-life at 90°C was approximately 1.5 h in the presence of xylan.
Xylanase enzymes are important in a wide variety of biotechnological and industrial applications (reviewed in references 5, 7, 14, 20, 29, and 31). Thermostable xylanases from diverse mesophilic and thermophilic microbes have been described (5, 9, 24, 28, 38). An area of intensifying industrial application for xylanases is in the deconstruction of plant cell walls to facilitate biofuel production from lignocellulose (8). With the current dependence on acid and heat pretreatment of lignocellulosic feedstocks, bioconversion enzymes from thermoacidophilic microbes are of particular value (25). Here we report the characterization of a thermostable glycoside hydrolase family 10 (GH10) xylanase (designated Xyn10A) from Acidothermus cellulolyticus 11B, a Gram-positive actinomycete that was isolated from acidic hot springs in Yellowstone National Park (4, 18).
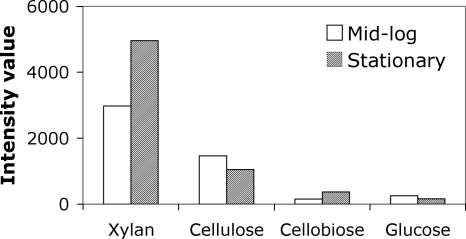
Transcriptional analyses of A. cellulolyticus 11B (ATCC 43068) grown in LPBM medium (4, 18) supplemented with a 0.5% concentration of either oat spelt xylan, cellulose, cellobiose, or glucose revealed that the xyn10A gene was more highly expressed in xylan-grown cultures (Fig. 1). Its expression was also detected in cellulose medium, but it was almost undetectable in cellobiose or glucose medium. Zymogram analysis (12) of oat spelt xylan- and cellulose-grown A. cellulolyticus culture supernatants showed a prominent clearing zone (using birchwood xylan as a substrate and Congo red staining) corresponding to the predicted molecular size of the Xyn10A protein; tandem mass spectrometry confirmed the presence of Xyn10A in the xylan medium (data not shown). These results suggested that Xyn10A hydrolyzes xylan and is the major xylanase produced on xylan by A. cellulolyticus.
FIG. 1.
Expression of xyn10A in A. cellulolyticus at mid-exponential (open bars) and stationary (filled bars) growth phases. Densitometry-based relative intensity values of reverse transcriptase PCR (RT-PCR) products are plotted along the y axis. RNA was extracted using the RNeasy plant minikit (Qiagen). Primers specific to the xyn10A gene (5′-CAAAGGAAAGATCTGGCAATG-3′ and 5′-TGAGCATCCCGTCGTAGTAGT-3′) were used to amplify a 485-bp product using the Qiagen OneStep RT-PCR kit. Expression of the housekeeping gyrB (Acel_0005) gene was used to normalize for the RNA (data not shown). Identical results were obtained in two independent experiments; data from one experiment are presented.
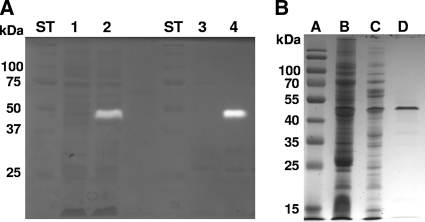
The xyn10A gene was cloned by isolating the A. cellulolyticus chromosomal DNA as described previously (4) and using it to PCR amplify the full-length Acel_0372 gene. From the A. cellulolyticus genome sequence (4), PCR primers (5′-GTGGTGGAGCTCGCAATTCGTTCACGTTGAGG-3′ and 5′-GTGGTGTCTAGAACCATCGAGTGGGAGTGACG-3′) containing SacI and XbaI restriction sites (underlined) were designed to facilitate cloning of the 1.4-kb PCR product into pK19 (21) for expression in Escherichia coli DH5α cells (26). Using hydroxyapatite column chromatography (19) following heat treatment (65°C, 15 min) of the crude cell extract, Xyn10A was purified to >90% purity based on the densitometry of SDS-PAGE gels (Fig. 2; Table 1).
FIG. 2.
Heterologous expression and purification of recombinant Xyn10A. (A) Zymogram assay showing xylanase activity following electrophoresis of crude cell extracts. Lanes: ST, molecular weight standards; 1 and 3, crude cell extracts from DH5α(pK19) (vector control); 2 and 4, crude cell extracts from DH5α(pK19-xyn10A). Samples in lanes 3 and 4 were heated at 65°C for 15 min prior to loading. (B) SDS-PAGE (10% gel) showing purification of Xyn10A. Lanes: A, molecular weight standards; B, crude cell extract from DH5α(pK19-xyn10A); C, heat-treated extract from DH5α(pK19-xyn10A); D, concentrated fractions from the hydroxyapatite column.
TABLE 1.
Typical purification of Xyn10A from recombinant E. coli
| Purification step | Amt of protein (mg) | Activitya |
Yield (%) | Purification (fold) | |
|---|---|---|---|---|---|
| Units | Units/mg protein | ||||
| Crude cell extract | 3,840 | 14,800 | 3.85 | 100 | |
| Heat-treated extract | 838 | 13,100 | 15.6 | 88 | 4.1 |
| Hydroxyapatite | 4.63 | 1,740 | 376 | 12 | 97.7 |
Units, μmol xylose-reducing equivalents liberated from oat spelt xylan per min; activities measured at 90°C and pH 6.0.
The specific activity of purified Xyn10A was quantified using a reducing sugar assay with p-hydroxybenzoic acid hydrazide (16) and the Bradford assay (6). Xyn10A was active from 60 to 100°C and pH 4 to 8, with an optimum at 90°C and pH 6 under the conditions tested (Table 2). Other polysaccharides (Sigmacell cellulose, carboxymethylcellulose [Fluka], and xanthan gum [KELCO ZN 85192 A]) did not serve as substrates (data not shown). The specific activity and Km of purified Xyn10A (at 90°C and pH 6) on oat spelt xylan were 350 ± 27 U mg of protein−1 and 0.53 ± 0.18 mg ml−1, respectively, values comparable to those of XynA from Thermotoga maritima MSB8 (37). The optimal temperature for Xyn10A activity (Topt = 90°C at pH 6) was higher than that reported for the most thermostable cellulase (endoglucanase E1; Topt = 81°C) from Acidothermus (1, 3, 34), which has a growth optimum of 55°C (18). Relatively few xylanases described to date have temperature optima of ≥90°C; most of these were isolated from hyperthermophiles (growth Topt > 85°C) such as Thermotoga (27, 37) or from high-temperature environments where hyperthermophiles are found (32). Another exception is the high-temperature xylanase (Topt = 90°C) from a fungus (24).
TABLE 2.
Specific activity of purified Xyn10A at different temperatures and pH
| Temp (°C) | Sp acta at pH: |
||||
|---|---|---|---|---|---|
| 4.0 | 5.0 | 6.0 | 7.0 | 8.0 | |
| 100 | 37 ± 17 (12%) | 206 ± 35 (65%) | 91 ± 19 (28%) | — | — |
| 90 | 41 ± 9 (13%) | 187 ± 15 (58%) | 350 ± 27 (100%) | 158 ± 7 (49%) | — |
| 80 | — | 98 ± 44 (31%) | 210 ± 4 (66%) | 169 ± 9 (53%) | — |
| 70 | — | 68 ± 5 (21%) | 111 ± 7 (35%) | 72 ± 11 (22%) | 71 ± 1 (22%) |
| 60 | — | 63 ± 14 (20%) | 67 ± 1 (21%) | 68 ± 2 (21%) | 48 ± 2 (15%) |
Specific activity in μmol xylose-reducing equivalents min−1 mg protein−1 ± standard deviation. n ≥ 4. Values in parentheses are percent activities relative to the maximum activity (at 90°C and pH 6.0). Dashes indicate an activity value less than 10% of maximal; activities at 30 to 50°C, as well as at pH 3.0 and pH 9.0, were less than 10% of maximal (data not shown). The pH of the assay mixture was measured at the end of each assay, and no significant change in pH was found in assays in the pH 3 to 8 range. The pH at the end of the pH 9 assays was consistently 8.6. No significant differences were seen between measurements of pH at room temperature or at 90°C.
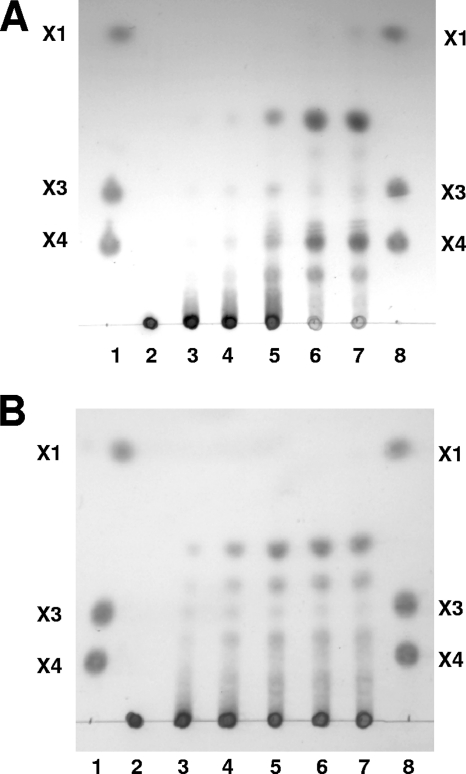
Xylan hydrolysis products produced by Xyn10A were analyzed using thin-layer chromatography (TLC) (Fig. 3) as described previously (15). The major products from oat spelt and birchwood xylan had retention factor (Rf) values between those of xylose and xylopentaose (Fig. 3), indicating that Xyn10A functions primarily as an endoxylanase. The differences in the pattern of degradation products from the two xylans likely reflect the known differences in the structures of these xylans (13, 17). Oat spelt xylan consists of arabinoxylan with trace glucose substituents, while birchwood xylan is primarily an unsubstituted xylose polymer with traces of uronic acids as side groups (17, 22, 30).
FIG. 3.
Time course of Xyn10A product formation with birchwood (A) and oat spelt (B) xylans, using TLC. Xylan substrates (2% in 10 mM phosphate buffer, pH 6) were incubated with purified Xyn10A at 90°C. Lanes 1 and 8, standards: xylotetraose (X4) and xylotriose (X3) (Megazyme, Wicklow, Ireland) and xylose (X1). Lane 2, unreacted xylan; lanes 3 to 7, xylan with increased incubation times in the presence of purified Xyn10A (10, 20, 40, 60, and 240 min, respectively).
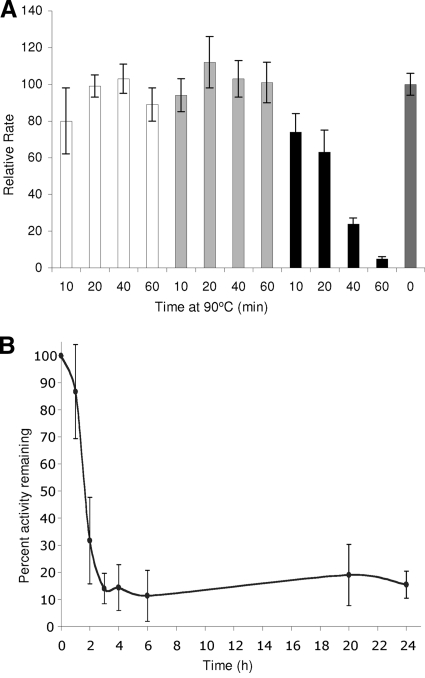
Xylans protected Xyn10A against thermal inactivation. While Xyn10A had a half-life of 12 min at 90°C in buffer, negligible loss in activity occurred in 1 h in the presence of either oat spelt or birchwood xylans (Fig. 4 A). Nonsubstrate polysaccharides (listed above) did not stabilize the enzyme (data not shown). In the presence of oat spelt xylan, approximately 15% of the activity was retained beyond 3 h and up to 24 h of 90°C heat treatment (Fig. 4B). These results indicate that xylans stabilize purified Xyn10A at high temperature; presumably, substrate-enzyme interactions prevent conformational changes at higher temperatures. It should be noted that Xyn10A is actively degrading xylan during the incubation at 90°C, and loss of activity after longer incubation periods may be due to the depletion of the stabilizing substrate. Certain other xylanases are known to be stabilized by substrate or by immobilization on glass beads at high temperature (24, 27), and in some cases, carbohydrate-binding domains were shown to contribute to thermostability (2, 11, 33, 36). Xyn10A lacks predicted carbohydrate-binding domains, and therefore how Xyn10A interacts with xylans for stabilization is unknown.
FIG. 4.
Stabilization of purified Xyn10A by xylans. (A) Purified Xyn10A was diluted 1:20 with 4% oat spelt xylan (white), 4% birchwood xylan (gray), or phosphate buffer (black) and incubated at 90°C for the times indicated. Rates of reducing sugar formation were determined and were linear for all samples. Activities relative to unheated Xyn10A (dark gray) are reported. Results are averages of three independent experiments; error bars indicate standard deviations. (B) Long-term thermostability of Xyn10A at 90°C in the presence of 4% oat spelt xylan. Experiments were performed as described for panel A.
The closest structurally characterized GH10 homolog of Xyn10A is from Clostridium thermocellum, whose homolog shares only 39% identity over 86% of the length of Xyn10A. Therefore, structural deductions for understanding intrinsic and substrate-associated thermostability of Xyn10A are difficult and warrant the structural characterization of the enzyme. With its thermoacidic range for enzyme activity, the A. cellulolyticus Xyn10A xylanase should be highly suitable for use in the hydrolysis of acid- and heat-pretreated lignocellulose in bioenergy applications (10, 35) and would be particularly compatible for use in combination with thermoacidic cellulases such as the highly thermostable endoglucanase E1 from A. cellulolyticus (3, 23). In addition, the broad effective temperature and pH ranges make it attractive for other emerging biotechnological processes.
Acknowledgments
This research was primarily supported by Chevron Technology Ventures (grant number RSO#21) to A.M.B. and partially supported by a University of California, Davis, Committee on Research New Funding Initiative grant to R.E.P.
Footnotes
Published ahead of print on 17 September 2010.
REFERENCES
- 1.Adney, W. S., M. P. Tucker, R. A. Nieves, S. R. Thomas, and M. E. Himmel. 1995. Low molecular weight thermostable β-D-glucosidase from Acidothermus cellulolyticus. Biotechnol. Lett. 17:49-54. [Google Scholar]
- 2.Araki, R., S. Karita, A. Tanaka, T. Kimura, and K. Sakka. 2006. Effect of family 22 carbohydrate-binding module on the thermostability of Xyn10B catalytic module from Clostridium stercorarium. Biosci. Biotechnol. Biochem. 70:3039-3041. [DOI] [PubMed] [Google Scholar]
- 3.Baker, J. O., W. S. Adney, R. A. Nieves, S. R. Thomas, D. B. Wilson, and M. E. Himmel. 1994. A new thermostable endoglucanase, Acidothermus cellulolyticus E1. Appl. Biochem. Biotechnol. 45/46:245-256. [Google Scholar]
- 4.Barabote, R. D., G. Xie, D. H. Leu, P. Normand, A. Necsulea, V. Daubin, C. Médigue, W. S. Adney, X. C. Xu, A. Lapidus, R. E. Parales, C. Detter, P. Pujic, D. Bruce, C. Lavire, J. F. Challacombe, T. S. Brettin, and A. M. Berry. 2009. Complete genome of the cellulolytic thermophile Acidothermus cellulolyticus 11B provides insights into its ecophysiological and evolutionary adaptations. Genome Res. 19:1033-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beg, Q. K., M. Kapoor, L. Mahajan, and G. S. Hoondal. 2001. Microbial xylanases and their industrial applications: a review. Appl. Microbiol. Biotechnol. 56:326-338. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Butt, M. S., M. Tahir-Nadeem, Z. Ahmad, and M. T. Sultan. 2008. Xylanases and their applications in baking industry. Food Technol. Biotechnol. 46:22-31. [Google Scholar]
- 8.Dodd, D., and I. O. Cann. 2009. Enzymatic deconstruction of xylan for biofuel production. Glob. Change Biol. Bioenergy 1:2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George, S. P., A. Ahmad, and M. B. Rao. 2001. A novel thermostable xylanase from Thermomonospora sp.: influence of additives on thermostability. Bioresour. Technol. 78:221-224. [DOI] [PubMed] [Google Scholar]
- 10.Haki, G. D., and S. K. Rakshit. 2003. Developments in industrially important thermostable enzymes: a review. Bioresour. Technol. 89:17-34. [DOI] [PubMed] [Google Scholar]
- 11.Jun, H., Y. Bing, Z. Keying, D. Xuemei, and C. Daiwen. 2009. Thermostable carbohydrate binding module increases the thermostability and substrate-binding capacity of Trichoderma reesei xylanase 2. Nat. Biotechnol. 26:53-59. [DOI] [PubMed] [Google Scholar]
- 12.Jung, K.-H., and M. Y. Pack. 1983. Expression of a Clostridium thermocellum xylanase gene in Bacillus subtilis. Biotechnol. Lett. 15:115-120. [Google Scholar]
- 13.Kabel, M. A., H. Borne, J. P. van den Vincken, A. G. J. Voragen, and H. A. Schols. 2007. Structural differences of xylans affect their interaction with cellulose. Carbohydr. Polym. 69:94-105. [Google Scholar]
- 14.Kulkarni, N., A. Shendye, and M. Rao. 1999. Molecular and biotechnological aspects of xylanases. FEMS Microbiol. Rev. 23:411-456. [DOI] [PubMed] [Google Scholar]
- 15.Lee, J.-W., J.-Y. Park, M. Kwon, and I.-G. Choi. 2009. Purification and characterization of thermostable xylanase from the brown-rot fungus Laetiporus sulphureus. J. Biosci. Bioeng. 107:33-37. [DOI] [PubMed] [Google Scholar]
- 16.Lever, M. 1973. Colorimetric and fluorometric carbohydrate determination with p-hydroxybenzoic acid hydrazide. Biochem. Med. 7:274-281. [DOI] [PubMed] [Google Scholar]
- 17.Li, K., P. Azadic, R. Collins, J. Toland, J. S. Kim, and K. E. Eriksson. 2000. Relationships between activities of xylanase and xylan structures. Enzyme Microbial. Technol. 27:89-94. [DOI] [PubMed] [Google Scholar]
- 18.Mohagheghi, A., K. Grohmann, M. Himmel, L. Leighton, and D. M. Updegraff. 1986. Isolation and characterization of Acidothermus celluloyticus gen. nov., sp. nov., a new genus of thermophilic, acidophilic, cellulolytic bacteria. Int. J. Syst. Bacteriol. 36:435-443. [Google Scholar]
- 19.Parales, R. E., R. Huang, C.-L. Yu, J. V. Parales, F. K. N. Lee, M. M. Ivkovic-Jensen, W. Liu, D. J. Lessner, R. Friemann, S. Ramaswamy, and D. T. Gibson. 2005. Purification, characterization, and crystallization of the components of the nitrobenzene and 2-nitrotoluene dioxygenase enzyme systems. Appl. Environ. Microbiol. 71:3806-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polizeli, M. L. T. M., A. C. S. Rizzatti, R. Monti, H. F. Terenzi, J. A. Jorge, and D. S. Amorim. 2005. Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotechnol. 67:577-591. [DOI] [PubMed] [Google Scholar]
- 21.Pridmore, R. D. 1987. New and versatile cloning vectors with kanamycin-resistance marker. Gene 56:309-312. [DOI] [PubMed] [Google Scholar]
- 22.Puls, J., N. Schroder, A. Stein, R. Janzon, and B. Saake. 2006. Xylans from oat spelts and birch kraft pulp. Macromol. Symp. 232:85-92. [Google Scholar]
- 23.Ransom, C., V. Balan, G. Biswas, B. Dale, E. Crockett, and M. Sticklen. 2007. Heterologous Acidothermus cellulolyticus 1,4-beta-endoglucanase E1 produced within the corn biomass converts corn stover into glucose. Appl. Biochem. Biotechnol. 137-140:207-219. [DOI] [PubMed] [Google Scholar]
- 24.Ratanachomsri, U., R. Sriprang, W. Sornlek, B. Buaban, V. Champreda, S. Tanapongpipat, and L. Eurwilaichitr. 2006. Thermostable xylanase from Marasmius sp.: purification and characterization. J. Biochem. Mol. Biol. 39:105-110. [DOI] [PubMed] [Google Scholar]
- 25.Rubin, E. M. 2008. Genomics of cellulosic biofuels. Nature 454:841-845. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor, NY.
- 27.Simpson, H. D., U. R. Haufler, and R. M. Daniel. 1991. An extremely thermostable xylanase from the thermophilic eubacterium Thermotoga. Biochem. J. 277:413-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh, S., A. M. Madlala, and B. A. Prior. 2003. Thermomyces lanuginosus: properties of strains and their hemicellulases. FEMS Microbiol. Rev. 27:3-16. [DOI] [PubMed] [Google Scholar]
- 29.Subramaniyan, S., and P. Prema. 2002. Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Crit. Rev. Biotechnol. 22:33-64. [DOI] [PubMed] [Google Scholar]
- 30.Sun, H.-J., S. Yoshida, N.-H. Park, and I. Kusakabe. 2002. Preparation of (1->4)-beta-D-xylooligosaccharides from an acid hydrolysate of cotton-seed xylan: suitability of cotton-seed xylan as a starting material for the preparation of (1->4)-beta-D-xylooligosaccharides. Carbohydr. Res. 337:657-661. [DOI] [PubMed] [Google Scholar]
- 31.Sunna, A., and G. Antranikian. 1997. Xylanolytic enzymes from fungi and bacteria. Crit. Rev. Biotechnol. 17:39-67. [DOI] [PubMed] [Google Scholar]
- 32.Sunna, A., and P. L. Bergquist. 2003. A gene encoding a novel extremely thermostable 1,4-beta-xylanase isolated directly from an environmental DNA sample. Extremophiles 7:63-70. [DOI] [PubMed] [Google Scholar]
- 33.Sunna, A., M. D. Gibbs, and P. L. Bergquist. 2000. The thermostabilizing domain, XynA, of Caldibacillus cellulovorans xylanase is a xylan binding domain. Biochem. J. 346:583-586. [PMC free article] [PubMed] [Google Scholar]
- 34.Tucker, M. P., A. Mohagheghi, K. Grohmann, and M. E. Himmel. 1989. Ultra-thermostable cellulases from Acidothermus cellulolyticus: comparison of temperature optima with previously reported cellulases. Biotechnology (NY) 7:817-819. [Google Scholar]
- 35.Viikari, L., M. Alapuranen, T. Puranen, J. Vehmaanperä, and M. Siika-Aho. 2007. Thermostable enzymes in lignocellulose hydrolysis. Adv. Biochem. Eng. Biotechnol. 108:121-145. [DOI] [PubMed] [Google Scholar]
- 36.Winterhalter, C., P. Heinrich, A. Candussio, G. Wich, and W. Liebl. 1995. Identification of a novel cellulose-binding domain within the multidomain 120 kDa xylanase of the hyperthermophile bacterium Thermotoga maritima. Mol. Microbiol. 15:431-444. [DOI] [PubMed] [Google Scholar]
- 37.Winterhalter, C., and W. Liebl. 1995. Two extremely thermostable xylanases of the hyperthermophilic bacterium Thermotoga maritima MSB8. Appl. Environ. Microbiol. 61:1810-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhengqiang, J., A. Kobayashi, M. M. Ahsan, L. Lite, M. Kitaoka, and K. Hayashi. 2001. Characterization of a thermostable family 10 endoxylanase (XynB) from Thermotoga maritima that cleaves p-nitrophenyl-beta-D-xyloside. J. Biosci. Bioeng. 92:423-428. [DOI] [PubMed] [Google Scholar]