Abstract
Listeria monocytogenes secretes two chitinases and one chitin binding protein. Mutants lacking chiA, chiB, or lmo2467 exhibited normal growth in cultured cells but were defective for growth in the livers and spleens of mice. Mammals lack chitin; thus, L. monocytogenes may have adapted chitinases to recognize alternative substrates to enhance pathogenesis.
Listeria monocytogenes is a Gram-positive bacterium that is found as an inhabitant of soil, water, and decaying vegetation, where the organism is believed to live as a saprophyte (12, 13, 16). Upon consumption of the bacterium by a susceptible host, L. monocytogenes adopts a pathogenic lifestyle that can lead to serious and sometimes fatal infections (1, 3, 15, 22, 32). L. monocytogenes is considered a major food-borne pathogen and has been responsible for some of the largest and most expensive food recalls in U.S. history (6-10, 27, 31). While significant attention has focused on the identification of L. monocytogenes gene products that specifically contribute to bacterial life within host cells, relatively less is known regarding the function of gene products that may be adapted to contribute to bacterial life both inside and outside the infected host.
Chitinases catalyze the hydrolysis of chitin, a linear polymer of N-acetylglucosamine residues linked in β-1,4 glycosidic bonds (5). Microbial chitinases have been associated with nutrient acquisition, as chitin is abundant in the environment and can serve as a source of carbon and nitrogen (17). Recently, a chitinase and a chitin binding protein have been linked to bacterial virulence for two environmental pathogens, Legionella pneumophila (14) and Vibrio cholerae (19, 21). Although chitin is not synthesized by mammals, L. pneumophila chitinase has been implicated in enhancing bacterial colonization of the lungs of infected mice (14), while a chitin binding protein produced by V. cholerae has been implicated in intestinal colonization (19, 21).
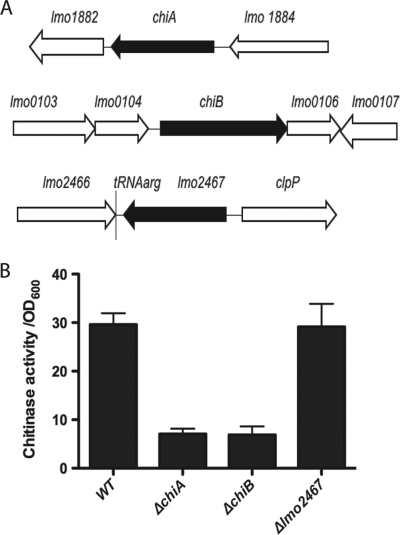
L. monocytogenes produces two chitinases encoded by lmo1883 (chiA) and lmo0105 (chiB) and one chitin binding protein encoded by lmo2467 (Fig. 1 A) (26). chiA and chiB encode proteins containing a glycosyl hydrolase family 18 chitinase domain, and both have been demonstrated to possess chitinolytic activity (25, 26). Interestingly, the expression of chiA has recently been reported to be induced within the cytosol of infected macrophages (11). lmo2467 encodes a hypothetical chitin binding protein that lacks a chitinase domain (26). To investigate whether chiA, chiB, or lmo2467 contributes to host infection, in-frame deletion mutants were constructed in each gene in the L. monocytogenes chromosome using allelic exchange (18). Each of the resulting in-frame deletion mutants was found to resemble wild-type L. monocytogenes with respect to bacterial growth in brain heart infusion (BHI) broth culture (S. Chaudhuri and N. Freitag, data not shown), and the deletion of either chiA or chiB significantly reduced secreted chitinase activity as detected in the culture supernatants of mutant strains following 48 h of growth in LB at 30°C (Fig. 1B).
FIG. 1.
L. monocytogenes genes encoding chitinases and chitin binding proteins. (A) chiA (lmo1883) encodes a chitinase of 352 amino acids, chiB (lmo0105) encodes a chitinase of 756 amino acids, and lmo2467 encodes a predicted chitin binding protein of 478 amino acids (26). Chromosomal deletion mutants were constructed for each gene indicated in black. Surrounding coding regions are represented by white arrows. (B) Chitinase activity detected in supernatants derived from wild-type, ΔchiA, ΔchiB, and Δlmo2467 strains. Bacterial cultures were grown for 48 h at 30°C in LB broth. Bacterial supernatants were assayed for secreted chitinase activity using the substrate 4-nitrophenyl N,N′-diacetyl-β-d-chitobioside (14, 25). Chitinase units are expressed as the absorbance at 405 nm times 100 divided by the culture optical density measured at 600 nm (OD600). Data shown are representative of two independent experiments.
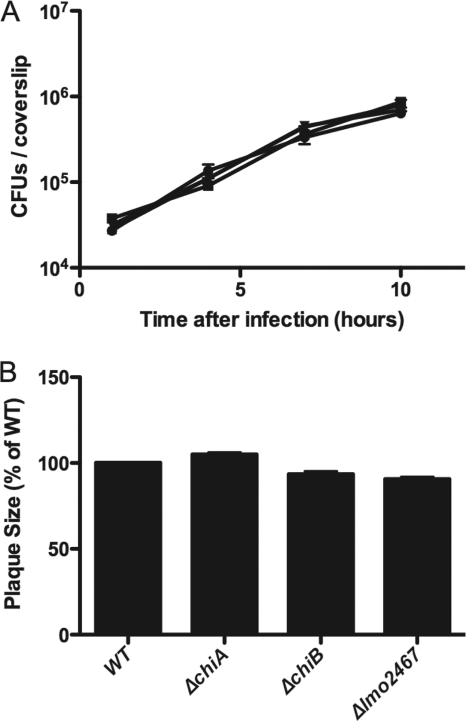
The ability of L. monocytogenes chitinase and chitin binding protein mutants to invade host cells, escape from the phagosome, and replicate in the cytosol was investigated via bacterial infection of the human intestinal Caco-2 cell line (Fig. 2 A). Monolayers of Caco-2 cells were grown on glass coverslips and infected with L. monocytogenes at a multiplicity of infection (MOI) of 10 bacteria per cell. At 1 h postinfection, monolayers were washed and medium containing 10 μg/ml gentamicin was added to kill extracellular bacteria. Intracellular bacterial replication was monitored by removing the coverslips at the indicated time points postinfection, lysing the infected host cells by vortexing the coverslips in water, and plating for viable CFU on LB agar media (29). Bacterial invasion and intracellular growth of each mutant strain in Caco-2 cells were found to be similar to those of wild-type L. monocytogenes (Fig. 2A). Identical patterns of bacterial replication were also observed in the murine-derived J774 macrophage-like cell line using a similar approach (S. Chaudhuri and N. Freitag, data not shown).
FIG. 2.
Chitinase and chitin binding protein deletion mutants exhibit normal patterns of intracellular growth and cell-to-cell spread in tissue culture cells. (A) Monolayers of Caco-2 intestinal epithelial cells grown on glass coverslips were infected with L. monocytogenes wild-type (•), ΔchiA (▪), ΔchiB (▴), and Δlmo2467 (▾) strains at an MOI of 10:1. After 1 h, monolayers were washed with phosphate-buffered saline (PBS) and gentamicin was added to kill any remaining extracellular bacteria. Coverslips were removed at the indicated time points, followed by host cell lysis and enumeration of bacterial CFU on agar media. Data are representative of three independent experiments. (B) Monolayers of L2 fibroblast cells were infected with wild-type, ΔchiA, ΔchiB, and Δlmo2467 bacteria as previously described (30). Bacterial intracellular growth and cell-to-cell spread were monitored by measuring the diameter of the zones of clearance (plaques) in the infected monolayers. Relative plaque diameters are indicated as a percentage of those of the wild type (WT), which was set to 100%. Data shown are the average plaque diameter ± standard error from results from three independent experiments.
The ability of L. monocytogenes to spread to adjacent cells during tissue culture infection can be rapidly assessed by measuring the ability of bacteria to form small zones of cell clearing or plaques during the infection of mouse fibroblast cell monolayers (30). Mouse L2 fibroblast cells were grown in 3.5-cm wells and infected with L. monocytogenes as previously described (30). One hour postinfection, the monolayers were washed and gentamicin was added along with a Dulbecco modified Eagle medium (DMEM)-0.7% agar overlay. Plaque formation was assessed at 3 days postinfection by staining viable L2 cells with neutral red. The chitinase and chitin binding protein mutants formed plaques in L2 cell monolayers with the same efficiency and of the same diameter as those formed by wild-type L. monocytogenes (Fig. 2B), indicating that each of the mutant strains was fully capable of intracellular bacterial replication and cell-to-cell spread.
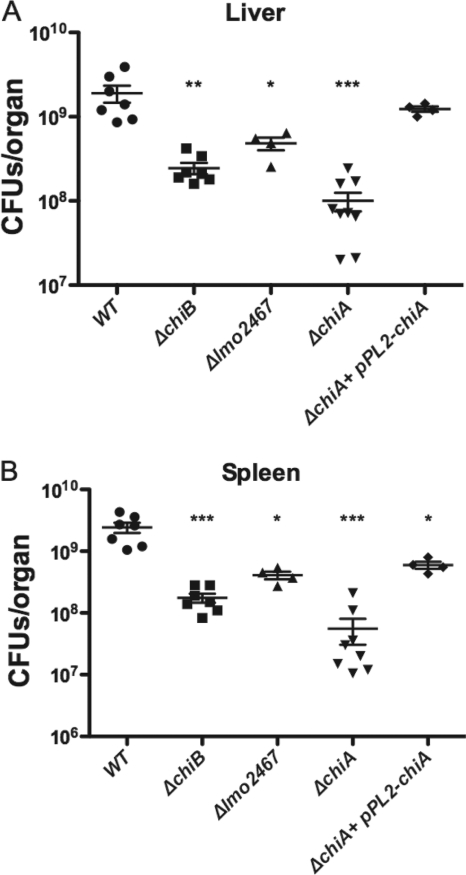
The chitinase and chitin binding protein mutants were then tested for virulence in a mouse model of infection. ND4 Swiss Webster mice were infected via tail vein injection (2) with the ΔchiA and ΔchiB chitinase deletion mutants as well as with the Δlmo2467 mutant and wild-type L. monocytogenes (Fig. 3). Each mouse was inoculated with 2 × 104 CFU, and at 3 days postinfection, the animals were sacrificed and the numbers of bacterial CFU were determined from homogenates of isolated livers and spleens. Each of the mutants exhibited a significant defect in bacterial replication within infected mice (Fig. 3). The Δlmo2467 mutant exhibited the most modest level of attenuation, with approximately 4-fold fewer bacteria recovered from the livers and 6-fold fewer from the spleens of infected animals than from those infected with wild-type L. monocytogenes (Fig. 3). The ΔchiB mutant exhibited more significant levels of attenuation, with approximately 8-fold and 14-fold fewer bacteria recovered from the livers and spleens, respectively (Fig. 3). Attempts to complement the defect of the ΔchiB mutant by providing a wild-type copy of the gene in trans using plasmid vector pPL2 (24) were not successful, as it was not possible to recover stable L. monocytogenes plasmid integrants for reasons that are not apparent (S. Chaudhuri and N. Freitag, data not shown). The most dramatic virulence defect was observed for strains lacking chiA (Fig. 3). The absence of chiA reduced bacterial colonization of the liver and spleen more than 19-fold and 45-fold, respectively, and the defect could be fully complemented in the liver and almost fully complemented in the spleen by providing a PCR-amplified wild-type copy of chiA in trans on the integrative plasmid vector pPL2 (24). These data indicate a significant role for the chitinase ChiA in L. monocytogenes virulence and suggest that additional contributions are made by chiB and lmo2467 during mouse infection.
FIG. 3.
L. monocytogenes chitinase mutants are defective for growth in the livers and spleens of infected mice. ND4 Swiss Webster mice were infected via tail injection (2) with 2 × 104 CFU of wild-type, ΔchiA, ΔchiB, Δlmo2467, and ΔchiA bacteria complemented with plasmid-borne chiA (ΔchiA+pPL2-chiA). After 72 h of infection, the animals were euthanized and the bacterial burdens were measured in the livers (A) and spleens (B). A minimum of 5 mice were inoculated per strain tested, and the mean and standard deviation are shown. Statistical significance was determined using one-way analysis of variance with Tukey's multiple-comparison test (*, P < 0.01; **, P < 0.001; ***, P < 0.0001).
Given the absence of chitin in mammals, what are the potential roles for chitinases and chitin binding proteins in L. monocytogenes virulence? For the secreted V. cholerae chitin binding protein GbpA, it has been reported that the protein serves to enhance bacterial colonization of the intestine through the binding of mucin (4, 21), which consists of glycoproteins rich in carbohydrates that include N-acetylglucosamine (28). Kawada et al. have recently reported that a chitin binding protein expressed by Serratia marcescens increased bacterial adhesion to colonic epithelial cells via binding to the chitinase 3-like 1 (CHI3L1) protein expressed on the host cell surface (20). The L. pneumophila chitinase has been reported to contribute to bacterial colonization of mouse lung; however, the mechanism by which the enzyme enhances bacterial colonization of lung epithelium has not yet been established (14). For L. monocytogenes, neither ChiA, ChiB, nor Lmo2467 appeared to have any influence on bacterial invasion or replication within tissue culture cell lines (Fig. 2), although the proteins clearly contributed to virulence via bloodstream infection of mice (Fig. 3). It remains possible that one or more of the secreted proteins recognize glycoproteins or carbohydrate moieties present on host cells (but not expressed by the tissue culture cell lines examined in this study) and that this recognition somehow serves to enhance bacterial uptake. Alternatively, the expression of chiA has been reported to be induced within infected macrophages (11) and also by the L. monocytogenes central virulence regulator PrfA (23), raising the possibility that ChiA and/or the other chitin binding proteins may serve to modify host immune responses by binding to glycoproteins or other carbohydrate moieties that contribute to immune signaling. Elucidation of the role of chitinases and chitin binding proteins in L. monocytogenes pathogenesis should provide insight into how bacterial factors that contribute to survival in the outside environment can be exploited for additional roles within an infected mammalian host.
Acknowledgments
This work was supported by Public Health Service grants AI076693 (N.P.C. and N.E.F.) and AI41816 (N.E.F.) from NIAID.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding sources.
Footnotes
Published ahead of print on 3 September 2010.
REFERENCES
- 1.Allerberger, F., and M. Wagner. 2010. Listeriosis: a resurgent foodborne infection. Clin. Microbiol. Infect. 16:16-23. [DOI] [PubMed] [Google Scholar]
- 2.Alonzo, F., III, G. C. Port, M. Cao, and N. E. Freitag. 2009. The posttranslocation chaperone PrsA2 contributes to multiple facets of Listeria monocytogenes pathogenesis. Infect. Immun. 77:2612-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbuddhe, S. B., and T. Chakraborty. 2009. Listeria as an enteroinvasive gastrointestinal pathogen. Curr. Top. Microbiol. Immunol. 337:173-195. [DOI] [PubMed] [Google Scholar]
- 4.Bhowmick, R., A. Ghosal, B. Das, H. Koley, D. R. Saha, S. Ganguly, R. K. Nandy, R. K. Bhadra, and N. S. Chatterjee. 2008. Intestinal adherence of Vibrio cholerae involves a coordinated interaction between colonization factor GbpA and mucin. Infect. Immun. 76:4968-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauchie, H. M. 2002. Chitin production by arthropods in the hydrosphere. Hydrobiologia 470:63-96. [Google Scholar]
- 6.CDC. 1998. Multistate outbreak of listeriosis—United States, 1998. MMWR Morb. Mortal. Wkly. Rep. 47:1085-1086. [PubMed] [Google Scholar]
- 7.CDC. 2009. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, 2008. MMWR Morb. Mortal. Wkly. Rep. 58:333-337. [PubMed] [Google Scholar]
- 8.CDC. 2004. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—selected sites, United States, 2003. MMWR Morb. Mortal. Wkly. Rep. 53:338-343. [PubMed] [Google Scholar]
- 9.CDC. 1999. Update: multistate outbreak of listeriosis—United States, 1998-1999. MMWR Morb. Mortal. Wkly. Rep. 47:1117-1118. [PubMed] [Google Scholar]
- 10.Chan, Y. C., and M. Wiedmann. 2009. Physiology and genetics of Listeria monocytogenes survival and growth at cold temperatures. Crit. Rev. Food Sci. Nutr. 49:237-253. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee, S. S., H. Hossain, S. Otten, C. Kuenne, K. Kuchmina, S. Machata, E. Domann, T. Chakraborty, and T. Hain. 2006. Intracellular gene expression profile of Listeria monocytogenes. Infect. Immun. 74:1323-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturongakul, S., S. Raengpradub, M. Wiedmann, and K. J. Boor. 2008. Modulation of stress and virulence in Listeria monocytogenes. Trends Microbiol. 16:388-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czuprynski, C. J. 2005. Listeria monocytogenes: silage, sandwiches and science. Anim. Health Res. Rev. 6:211-217. [DOI] [PubMed] [Google Scholar]
- 14.DebRoy, S., J. Dao, M. Soderberg, O. Rossier, and N. P. Cianciotto. 2006. Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc. Natl. Acad. Sci. U. S. A. 103:19146-19151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drevets, D. A., and M. S. Bronze. 2008. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol. Med. Microbiol. 53:151-165. [DOI] [PubMed] [Google Scholar]
- 16.Freitag, N. E., G. C. Port, and M. D. Miner. 2009. Listeria monocytogenes—from saprophyte to intracellular pathogen. Nat. Rev. Microbiol. 7:623-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooday, G. W. 1990. The ecology of chitin degradation, p. 387-430. In K. C. Marshall (ed.), Advances in microbial ecology, vol. 11. Plenum Press, New York, NY. [Google Scholar]
- 18.Higgins, D. E., C. Buchrieser, and N. E. Freitag. 2006. Genetic tools for use with Listeria monocytogenes, p. 620-633. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens, 2nd ed. ASM Press, Washington, DC.
- 19.Jude, B. A., R. M. Martinez, K. Skorupski, and R. K. Taylor. 2009. Levels of the secreted Vibrio cholerae attachment factor GbpA are modulated by quorum-sensing-induced proteolysis. J. Bacteriol. 191:6911-6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawada, M., C. C. Chen, A. Arihiro, K. Nagatani, T. Watanabe, and E. Mizoguchi. 2008. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab. Invest. 88:883-895. [DOI] [PubMed] [Google Scholar]
- 21.Kirn, T. J., B. A. Jude, and R. K. Taylor. 2005. A colonization factor links Vibrio cholerae environmental survival and human infection. Nature 438:863-866. [DOI] [PubMed] [Google Scholar]
- 22.Kreft, J., and J. A. Vazquez-Boland. 2001. Regulation of virulence genes in Listeria. Int. J. Med. Microbiol. 291:145-157. [DOI] [PubMed] [Google Scholar]
- 23.Larsen, M. H., J. J. Leisner, and H. Ingmer. 30 July 2010. Listeria monocytogenes EGD chitinolytic activity is regulated by carbohydrates but also by the virulence regulator, PrfA. Appl. Environ. Microbiol. doi: 10.1128/AEM.00297-10. [DOI] [PMC free article] [PubMed]
- 24.Lauer, P., M. Y. N. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184:4177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leisner, J. J., M. H. Larsen, H. Ingmer, B. O. Petersen, J. O. Duus, and M. M. Palcic. 2009. Cloning and comparison of phylogenetically related chitinases from Listeria monocytogenes EGD and Enterococcus faecalis V583. J. Appl. Microbiol. 107:2080-2087. [DOI] [PubMed] [Google Scholar]
- 26.Leisner, J. J., M. H. Larsen, R. L. Jørgensen, L. Brøndsted, L. E. Thomsen, and H. Ingmer. 2008. Chitin hydrolysis by Listeria spp., including L. monocytogenes. Appl. Environ. Microbiol. 74:3823-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mead, P. S., E. F. Dunne, L. Graves, M. Wiedmann, M. Patrick, S. Hunter, E. Salehi, F. Mostashari, A. Craig, P. Mshar, T. Bannerman, B. D. Sauders, P. Hayes, W. Dewitt, P. Sparling, P. Griffin, D. Morse, L. Slutsker, and B. Swaminathan. 2006. Nationwide outbreak of listeriosis due to contaminated meat. Epidemiol. Infect. 134:744-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moncada, D. M., S. J. Kammanadiminti, and K. Chadee. 2003. Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol. 19:305-311. [DOI] [PubMed] [Google Scholar]
- 29.Shetron-Rama, L. M., H. Marquis, H. G. A. Bouwer, and N. E. Freitag. 2002. Intracellular induction of Listeria monocytogenes actA expression. Infect. Immun. 70:1087-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun, A. N., A. Camilli, and D. A. Portnoy. 1990. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 58:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swaminathan, B., and P. Gerner-Smidt. 2007. The epidemiology of human listeriosis. Microbes Infect. 9:1236-1243. [DOI] [PubMed] [Google Scholar]
- 32.Vázquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Domínguez-Bernal, W. Goebel, B. González-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]