Abstract
The intestinal flora of mammals contains lactic acid bacteria (LAB) that may provide positive health effects for the host. Such bacteria are referred to as probiotic bacteria. From a pig, we have isolated a Lactobacillus reuteri strain that produces an antimicrobial peptide (AMP). The peptide was purified and characterized, and it was unequivocally shown that the AMP was a well-defined degradation product obtained from the mucus adhesion-promoting protein (MapA); it was therefore termed AP48-MapA. This finding demonstrates how large proteins might inherit unexpected pleiotropic functions by conferring antimicrobial capacities on the producer. The MapA/AP48-MapA system is the first example where a large protein of an intestinal LAB is shown to give rise to such an AMP. It is also of particular interest that the protein that provides this AMP is associated with the binding of the bacterium producing it to the surface/lining of the gut. This finding gives us new perspective on how some probiotic bacteria may successfully compete in this environment and thereby contribute to a healthy microbiota.
Mammals have a microbiota in their digestive tract that contains lactic acid bacteria (LAB). It has been increasingly evident that some of these lactic acid bacteria produce antimicrobial peptides that may contribute to the positive effect on their host. Such bacteria are often referred to as probiotics, and one of their important beneficial effects is their ability to produce antimicrobial compounds that prevent or interfere with the growth of pathogenic bacteria in the host.
It is known that the fecal microflora of pigs/piglets is large and diverse and develops rapidly after birth. Lactobacillus reuteri is among the very first lactic acid bacteria that colonize the intestine of new-born piglets, and their numbers gradually increase until they become the most dominant LAB in pigs (5, 17, 28). Other lactobacilli that are also part of the gut microbiota of pigs include L. amylovorus, L. acidophilus, L. salivarius, and L. casei (4, 8). Probiotic isolates have been identified within all these species, and many of them are today used as food/feed supplements to support good health (4, 11, 27). An important part of the antimicrobial arsenal produced by lactic acid bacteria (LAB) is a group of peptides called bacteriocins, which are ribosomally synthesized antibiotic-like peptides (antimicrobial peptides [AMPs]) (3, 7, 19). The bacteriocins constitute a wide range of structurally different peptides that are divided into different classes and subclasses. Some are modified (the lantibiotics, or class I), while others are basically unmodified (class II) (3, 6, 19).
Most bacteriocins are derived from prepeptides, each containing a short leader sequence (14 to 30 amino acids [aa]) which is cleaved off during the secretion of the mature peptide (19). In recent years, a new group of AMPs have been recognized (18); these are different from regular bacteriocins in that they are derived from larger proteins through specific degradations, leading to a defined peptide possessing antimicrobial activity. Such antimicrobial peptides have been known for a long time in mammalian systems. For instance, lactoferrin, a protein in milk, is readily degraded to a specific antimicrobial peptide through heat, acid treatment, or pepsin digestion (14, 24, 26). Defined histone fragments with antimicrobial properties have been isolated from different eukaryotic species (1, 2, 15, 21, 23), and a few antimicrobial peptides derived from larger proteins have been isolated in bacteria, including Helicobacter pylori (22), propionic acid bacteria (9, 10), and Clostridium beijerinckii (13). Such antimicrobial peptides are most likely formed by proteolytic degradation during cell proliferation or death.
Isolation of antimicrobial-producing LAB from piglets.
Fecal samples were collected from 4 different litters of piglets (2 to 4 weeks old), and LAB were isolated by plating serial dilutions of samples onto MRS agar plates which were incubated anaerobically at 37°C. Altogether, 37 LAB were isolated. They were dominated by L. reuteri (13 isolates), but Lactobacillus kitasatonis, L. amylovorus, L. salivarius, Lactobacillus pontis, Lactobacillus mucosae, and Lactobacillus vaginalis were found, in addition to Streptococcus bovis and enterococci. The typing was performed by 16S RNA DNA sequencing. The DNA sequence was based on 1,182 nucleotides, and BLAST analysis showed 99% identity only to L. reuteri or some uncultured bacteria obtained from fecal pig or human gut microflora.
The antimicrobial activity was tested by serial 2-fold dilution of samples and growth inhibition of an indicator organism in microtiter plates as previously described (12). Several of the isolates were found to produce antibacterial activity, and among them, the fecal isolate Lactobacillus reuteri LA92 displayed a fairly broad antimicrobial profile, including inhibition of selected members of the pathogenic bacteria Bacillus cereus, Staphylococcus aureus, and Listeria monocytogenes, as well as some lactic acid bacteria but not Lactobacillus plantarum. Antimicrobial activity was not detected against Escherichia coli or a Salmonella isolate. No activity was lost upon heat treatment (95°C for 10 min), but the activity was sensitive to proteinase K treatment, together suggesting that the antimicrobial compound is heat stable and proteinaceous, characteristics which are typical of most bacteriocins. However, we will not describe this antimicrobial peptide as a bacteriocin since it seems to lack many of the other characteristics typical for bacteriocins (19); we will instead refer it to as an antimicrobial peptide.
Purification of the antimicrobial compound.
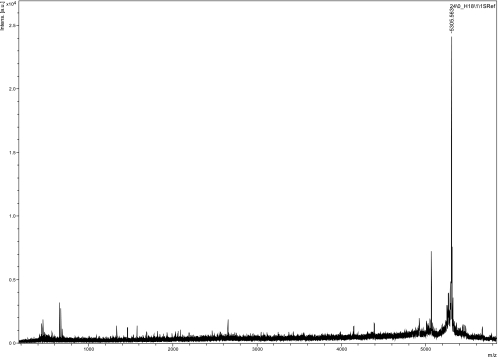
The compound was secreted into the growth medium, from which the activity was purified according to well-established protocols (20, 25). Briefly, the cell-free supernatant from 600 ml of an overnight culture of L. reuteri LA92 was precipitated with 240 g of ammonium sulfate. The protein precipitate was pelleted by centrifugation at 13,000 × g for 20 min at 4°C and dissolved in 60 ml distilled water. This fraction was applied to an ion exchange chromatograph with an SP-XL column. The antimicrobial activity that was bound to the column was washed once with 0.1 M NaCl (15 ml) before being eluted from the column by 1 M NaCl (10 ml). The fractions containing antimicrobial activity were pooled and further subjected to reversed-phase chromatography (RPC), which consisted of one run on a Resource RPC 1-ml column and two runs on a Sephacil peptide C8 column. An isopropanol gradient from 1 to 100% isopropanol in 0.1% trifluoroacetic acid was used to elute the antimicrobial activity. Table 1 summarizes the yields of antimicrobial activity at the different steps of purification. The antimicrobial activity in two fractions (nr 24 and nr 25) (Table 2) eluted at 66.7% isopropanol gave a sharp absorbance (280 nm) peak with some tailing. The two fractions with antimicrobial activity, as well as the two flanking fractions, were analyzed by mass spectrometry (MS). A monoisotopic mass of 5,305.6 Da was identified as the major component in the fraction (nr 24), with 80% of the antimicrobial activity (Fig. 1). The complete mass analyses of all four fractions are presented in Table 2. Several mass peaks were identified, but the 5,306-Da peak was recognized only in the two fractions (nr 24 and nr 25) with antimicrobial activity.
TABLE 1.
Overview of the purification procedure of the antimicrobial peptide from L. reuteri LA92
| Fraction | Volume (ml) | Antimicrobial activity (BU/mla) | Total activity (BU)b | Yield (%) |
|---|---|---|---|---|
| Supernatant | 600 | 20 | 12,000 | |
| Precipitation with (NH4)2SO4 | 60 | 320 | 19,200 | 100 |
| Ion exchange chromatography | 10 | 1,280 | 12,800 | 67 |
| Reversed-phase chromatography (Resource column) | 1 | 500 | 500 | 2.6 |
| Reversed-phase chromatography (C8column) | 0.5 | 4,800 | 2,400 | 12.5 |
| Reversed-phase chromatography (C-8 column) | 0.5 | 400 | 200 | 1.04 |
One bactericidal unit (BU) is the minimum amount of the antimicrobial peptide that inhibits the growth of the indicator by 50%. The indicator organism used in the bacteriocin assay was L. sakei LMGT2313.
Total activity is the product of the volume of the active fraction (ml) and the activity of that fraction (BU/ml).
TABLE 2.
MS analysis and antimicrobial activities of some selected fractions from the last step of purification
| Fraction | MS peak(s) (Da) | Antimicrobial activity (BU/mg)b |
|---|---|---|
| Nr 23 | 3,877 | 0 |
| Nr 24 | 5,065, 5,306a | 22,200 |
| Nr 25 | 3,678, 5,063, 5,306 | 5,000 |
| Nr 26 | 3,678, 5,063 | 0 |
Major peak; see Fig. 1.
One BU (bactericidal unit) is the minimum amount of the antimicrobial peptide that inhibits growth of the indicator by 50%. The protein concentration is based on a value of 1 mg/ml at an optical density at 280 nm of 1.88; the protein concentrations in fractions 24 and 25 were 18 μg/ml and 20 μg/ml, respectively.
FIG. 1.
Mass spectrometry results for the fraction (nr 24) with the highest antimicrobial activity. Intens., intensity; a.u., arbitrary units.
Fraction nr 24, which represented the majority of the activity in the final step, was subjected to peptide sequence analysis by automated Edman degradation as previously described (12). The sequence of the first 19 amino acids was identified (LEGTYSPYSYRKNNKLTGF). In addition to this N-terminal sequencing, the compound was also fragmented by tandem mass spectrometry, and another sequence of 19 amino acids was identified (KNNKLTGFEVDLGKAVAKK); this sequence overlapped with the 8 aa residues from Edman degradation. Altogether, a stretch of 30 amino acids of the purified AMP was identified, as shown in Fig. 2.
FIG. 2.
(A) Amino acid sequence of the MapA protein. (B) Truncated peptides of MapA, whose molecular weights (MW) matched well with the molecular masses of the purified peptides determined by mass spectrometry. MapA (accession no. AJ293860) is a mucus adhesion protein (263 aa) containing a sec leader sequence (underlined). Note that all truncated peptides have a common N terminus, but each has a different C terminus. Bold letters indicate the sequence determined by chemical sequencing of purified peptides.
A BLAST search showed that this 30-aa peptide sequence was identical with an internal sequence (from residue 46 to residue 75) of the 263-aa protein called MapA (mucus adhesion-promoting protein) encoded in the L. reuteri genome (E. Satoh, R. J. Leer, M. Rojas, P. L. Conway, F. J. Tielen, and P. H. Pouwels, unpublished data; GenBank sequence accession number AJ293860) (16). The molecular mass of the purified antimicrobial peptide (5,305.6 Da), now named AP48-MapA, matched with the theoretical mass of an internal sequence of Map A, from residue 46 to residue 93, that gives rise to a sequence of 48 aa with a molecular mass of 5,308.1 Da. MapA contains an N-terminal sec leader (residues 1 to 27) which is probably cleaved upon export of the mature protein, which in turn is processed further from both ends to give rise to AP48-MapA (Fig. 2). The second peak (5,065 Da) in the MS analyses also matched with a truncated peptide sequence (residue 46 to residue 90), of which the theoretical mass was 5,066.8 Da. Another two mass peaks, of 3,678 Da and 3,877 Da, corresponded well with truncated sequences of MapA, one fragment containing residues 46 to 78, with a theoretical mass of 3,679 Da, and the other containing residues 46 to 80, with a theoretical mass of 3,878.5 Da. However, fractions (nr 23 and nr 26) containing these smaller peptide fragments did not show any antimicrobial activity.
In order to provide further evidence that the antimicrobial activity was embedded in the purified peptide of 48 residues, a DNA fragment encoding the peptide was cloned in E. coli by the pET expression system (pET-22b+; Novagen). Positive clones were isolated, but upon induction of the expression of the inserted gene, the host (E. coli) did not survive, and more than 99% of the cells died. The surviving cells did not produce any antimicrobial activity.
The present work has identified an antimicrobial activity produced by L. reuteri isolated from fecal samples of weanling piglets. The peptide appears to be produced in a specific degradation process of the mucus adhesion-promoting protein (MapA) of L. reuteri (E. Satoh et al., unpublished data; GenBank sequence accession number AJ293860) (16). It has occasionally been shown that both bacteria and eukaryotes produce antimicrobial peptides by specific proteolytic degradation of larger proteins. In bacteria, this has been seen in H. pylori when the ribosomal protein L1 is degraded (22). In two different propionic acid bacteria, it has been shown that larger proteins of unknown function are degraded to form antimicrobial peptides. Likewise, histones in eukaryotes have been shown to be degraded to distinct peptides with strong antimicrobial activity (16-20).
L. reuteri is part of the intestinal LAB of pigs, and it is likely that it contributes positively to the gastrointestinal health of the animal. It is certainly tempting to speculate that the AMP derived from MapA is one of the factors that protect the carrier against invading pathogenic bacteria. Since the major function of MapA is probably to attach the bacterium to the lining of the gut, it is also likely that during propagation, bacteria are released into the lumen and degradation of the MapA peptide will take place. Also, degradation of MapA probably occurs among the bacteria when they are attached to the mucus layer.
Several degradation products from MapA were identified, but only one dominating peptide seems to be responsible for the antimicrobial activity, namely, AP48-MapA, which is a small (48-aa), heat-stable peptide with a high pI (9.63), physicochemical properties that are typical of bacteriocins. The processing/degradation required to produce AP48-MapA is not known, but all the peptides identified from the various fractions of the last reversed-phase column appeared to have a common N-terminal processing site at aa residue 48, while the C-terminal degradation site varied according to the MS data. Since the production of AP48-MapA was found in the supernatant of a monoculture, it seems likely that the bacterium itself can carry out this specific processing in the gut without depending on other enzymatic activity.
Acknowledgments
We thank K. Sletten at the University of Oslo for N-terminal peptide sequencing and M. Skaugen at Norwegian University of Life Sciences for performing the MS analysis.
This work is supported by the Norwegian Research Council.
Footnotes
Published ahead of print on 10 September 2010.
REFERENCES
- 1.Birkemo, G. A., T. Luders, O. Andersen, I. F. Nes, and J. Nissen-Meyer. 2003. Hipposin, a histone-derived antimicrobial peptide in Atlantic halibut (Hippoglossus hippoglossus L.). Biochim. Biophys. Acta 1646:207-215. [DOI] [PubMed] [Google Scholar]
- 2.Cho, J. H., B. H. Sung, and S. C. Kim. 2009. Buforins: histone H2A-derived antimicrobial peptides from toad stomach. Biochim. Biophys. Acta 1788:1564-1569. [DOI] [PubMed] [Google Scholar]
- 3.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 4.De Angelis, M., S. Siragusa, M. Berloco, L. Caputo, L. Settanni, G. Alfonsi, M. Amerio, A. Grandi, A. Ragni, and M. Gobbetti. 2006. Selection of potential probiotic lactobacilli from pig feces to be used as additives in pelleted feeding. Res. Microbiol. 157:792-801. [DOI] [PubMed] [Google Scholar]
- 5.De Angelis, M., S. Siragusa, L. Caputo, A. Ragni, R. Burzigotti, and M. Gobbetti. 2007. Survival and persistence of Lactobacillus plantarum 4.1 and Lactobacillus reuteri 3S7 in the gastrointestinal tract of pigs. Vet. Microbiol. 123:133-344. [DOI] [PubMed] [Google Scholar]
- 6.de Vos, W. M., O. P. Kuipers, J. R. van der Meer, and R. J. Siezen. 1995. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by gram-positive bacteria. Mol. Microbiol. 17:427-437. [DOI] [PubMed] [Google Scholar]
- 7.Diep, D. B., and I. F. Nes. 2002. Ribosomally synthesized antibacterial peptides in Gram positive bacteria. Current Drug Targets 3:107-122. [DOI] [PubMed] [Google Scholar]
- 8.Du Toit, M., L. M. Dicks, and W. H. Holzapfel. 2003. Identification of heterofermentative lactobacilli isolated from pig faeces by numerical analysis of total soluble cell protein patterns and RAPD-PCR. Lett. Appl. Microbiol. 37:12-16. [DOI] [PubMed] [Google Scholar]
- 9.Faye, T., D. A. Brede, T. Langsrud, I. F. Nes, and H. Holo. 2002. An antimicrobial peptide is produced by extracellular processing of a protein from Propionibacterium jensenii. J. Bacteriol. 184:3649-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faye, T., D. A. Brede, T. Langsrud, I. F. Nes, and H. Holo. 2004. Prevalence of the genes encoding propionicin T1 and protease-activated antimicrobial peptide and their expression in classical propionibacteria. Appl. Environ. Microbiol. 70:2240-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacin, B., I. Rogelj, and B. B. Matijasic. 2008. Lactobacillus isolates from weaned piglets' mucosa with inhibitory activity against common porcine pathogens. Folia Microbiol. 53:569-576. [DOI] [PubMed] [Google Scholar]
- 12.Holo, H., O. Nilssen, and I. F. Nes. 1991. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J. Bacteriol. 173:3879-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemperman, R., A. Kuipers, H. Karsens, A. Nauta, O. Kuipers, and J. Kok. 2003. Identification and characterization of two novel clostridial bacteriocins, circularin A and closticin 574. Appl. Environ. Microbiol. 69:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liepke, C., H. D. Zucht, W. G. Forssmann, and L. Stèandker. 2001. Purification of novel peptide antibiotics from human milk. J. Chromatogr. B 752:369-377. [DOI] [PubMed] [Google Scholar]
- 15.Luders, T., G. A. Birkemo, J. Nissen-Meyer, O. Andersen, and I. F. Nes. 2005. Proline conformation-dependent antimicrobial activity of a proline-rich histone H1 N-terminal peptide fragment isolated from the skin mucus of Atlantic salmon. Antimicrob. Agents Chemother. 49:2399-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyoshi, Y., S. Okada, T. Uchimura, and E. Satoh. 2006. A mucus adhesion promoting protein, MapA, mediates the adhesion of Lactobacillus reuteri to Caco-2 human intestinal epithelial cells. Biosci. Biotechnol. Biochem. 70:1622-1628. [DOI] [PubMed] [Google Scholar]
- 17.Naito, S., H. Hayashidani, K. Kaneko, M. Ogawa, and Y. Benno. 1995. Development of intestinal lactobacilli in normal piglets. J. Appl. Bacteriol. 79:230-236. [DOI] [PubMed] [Google Scholar]
- 18.Nes, I. F., D. A. Brede, and H. Holo. 2006. The nonlantibiotic heat-stable bacteriocins in Gram-positive bacteria, p. 107-114. In A. Kastin (ed.), Handbook of biologically active peptides. Academic Press, Amsterdam, Netherlands.
- 19.Nes, I. F., D. B. Diep, L. S. Havarstein, M. B. Brurberg, V. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek 70:113-128. [DOI] [PubMed] [Google Scholar]
- 20.Nieto Lozano, J. C., J. N. Meyer, K. Sletten, C. Pelaz, and I. F. Nes. 1992. Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J. Gen. Microbiol. 138:1985-1990. [DOI] [PubMed] [Google Scholar]
- 21.Park, I. Y., C. B. Park, M. S. Kim, and S. C. Kim. 1998. Parasin I, an antimicrobial peptide derived from histone H2A in the catfish, Parasilurus asotus. FEBS Lett. 437:258-262. [DOI] [PubMed] [Google Scholar]
- 22.Putsep, K., C. I. Branden, H. G. Boman, and S. Normark. 1999. Antibacterial peptide from H. pylori. Nature 398:671-672. [DOI] [PubMed] [Google Scholar]
- 23.Richards, R. C., D. B. O'Neil, P. Thibault, and K. V. Ewart. 2001. Histone H1: an antimicrobial protein of Atlantic salmon (Salmo salar). Biochem. Biophys. Res. Commun. 284:549-555. [DOI] [PubMed] [Google Scholar]
- 24.Saito, H., H. Miyakawa, Y. Tamura, S. Shimamura, and M. Tomita. 1991. Potent bactericidal activity of bovine lactoferrin hydrolysate produced by heat treatment at acidic pH. J. Dairy Sci. 74:3724-3730. [DOI] [PubMed] [Google Scholar]
- 25.Tichaczek, P. S., J. Nissen-Meyer, I. F. Nes, R. F. Vogel, and W. P. Hammes. 1992. Characterization of the bacteriocins Curvacin-a from Lactobacillus curvatus Lth1174 and sakacin-P from L. sake Lth673. Syst. Appl. Microbiol. 15:460-468. [Google Scholar]
- 26.Tomita, M., M. Takase, H. Wakabayashi, and W. Bellamy. 1994. Antimicrobial peptides of lactoferrin. Adv. Exp. Med. Biol. 357:209-218. [DOI] [PubMed] [Google Scholar]
- 27.Walsh, M. C., G. E. Gardiner, O. M. Hart, P. G. Lawlor, M. Daly, B. Lynch, B. T. Richert, S. Radcliffe, L. Giblin, C. Hill, G. F. Fitzgerald, C. Stanton, and P. Ross. 2008. Predominance of a bacteriocin-producing Lactobacillus salivarius component of a five-strain probiotic in the porcine ileum and effects on host immune phenotype. FEMS Microbiol. Ecol. 64:317-327. [DOI] [PubMed] [Google Scholar]
- 28.Wang, H. F., W. Y. Zhu, W. Yao, and J. X. Liu. 2007. DGGE and 16S rDNA sequencing analysis of bacterial communities in colon content and feces of pigs fed whole crop rice. Anaerobe 13:127-133. [DOI] [PubMed] [Google Scholar]