Abstract
AbiV is an abortive infection protein that inhibits the lytic cycle of several virulent phages infecting Lactococcus lactis, while a mutation in the phage gene sav confers insensitivity to AbiV. In this study, we have further characterized the effects of the bacterial AbiV and its interaction with the phage p2 protein SaV. First, we showed that during phage infection of lactococcal AbiV+ cells, AbiV rapidly inhibited protein synthesis. Among early phage transcripts, sav gene transcription was slightly inhibited while the SaV protein could not be detected. Analyses of other phage p2 mRNAs and proteins suggested that AbiV blocks the activation of late gene transcription, probably by a general inhibition of translation. Using size exclusion chromatography coupled with on-line static light scattering and refractometry, as well as fluorescence quenching experiments, we also demonstrated that both AbiV and SaV formed homodimers and that they strongly and specifically interact with each other to form a stable protein complex.
Bacteriophages infecting Lactococcus lactis strains during milk fermentation are a persisting problem in the dairy industry (1, 47, 49). When growing in large industrial milk fermentation vats, host cells can succumb to attacks from members of many genetically distinct lactococcal phage groups (15, 18). L. lactis strains have evolved numerous natural antiphage barriers to protect themselves against a diverse population of virulent phages (42). These defense mechanisms act at different steps of the phage lytic cycle, such as blocking phage adsorption, DNA entry, DNA replication, or assembly (26, 38, 47). Abortive infection mechanism (Abi) is a broad term used to identify antiphage systems that inhibit phage multiplication after DNA entry but act before the release of phage progeny (32). Abi systems also cause premature bacterial cell death upon phage infection (32). Restriction-modification (1) and CRISPR/Cas (2) systems, which inhibit phage infection by cleaving incoming nucleic acids, are excluded from the Abi group.
At least 23 distinct Abi mechanisms have been identified in L. lactis (13, 23, 34). They form a heterogeneous group usually encoded by a single gene, though some Abi systems are encoded by two genes (7, 16, 17, 28, 52) or more (64). Lactococcal Abi genes have a lower G+C content than other host genes, and they share limited amino acid sequence similarity (13). They also vary significantly in activity against various lactococcal phage groups (10, 13, 61).
Though Abi systems are considered the most efficient group of antiphage systems, their industrial use has led to the emergence of Abi-insensitive phage mutants (47). From an industrial point of view, there is therefore a recurrent need to either discover new phage resistance mechanisms (59) or improve the efficacy of current systems. In order to improve or expand the efficacy of the known mechanisms, one needs to understand their mode of action.
Homologues of lactococcal Abi proteins exist in the databases and are found in an impressive variety of microbes. Unfortunately, no homology with proteins of known detailed function (other than phage resistance) has been found, preventing any prediction about their mode of action from being made (4, 6, 13). For most Abi systems, our knowledge is limited to their overall effect on the phage lytic cycle (13, 22, 61). For example, AbiA, -F, -K, -P, and -T were shown to interfere with DNA replication (7, 22, 25, 29, 33), while AbiB, -G, and -U affected RNA transcription (14, 16, 17, 28, 52). AbiC was shown to cause limited major capsid protein production (50), whereas AbiE, -I, and -Q affected phage packaging (24, 28, 60). With this level of information, it is difficult to separate the primary target of Abi from subsequent effects (4, 6, 13). Comparative genome analyses of Abi-insensitive phage mutants have led to the identification of phage genes involved in the Abi phenotype (4, 5, 7-9, 19-21, 31, 41, 48). Again, many of these phage genes code for proteins of unknown function, thereby complicating prediction of the Abi system's mode of action.
Combined genetic and biochemical studies have provided insight into the mechanism of some lactococcal Abi systems (4, 6, 13). AbiB was demonstrated to either induce or function as an RNase (53). AbiD1 was found to be induced by a phage protein and to act on an essential phage RuvC-like endonuclease (4, 6, 13). AbiZ causes premature cell lysis possibly by interacting with phage holin (23). AbiK was found to possess a key reverse transcriptase motif (27), and a phage single-strand annealing protein is involved in its antiviral activity (54). A deeper understanding of the molecular interactions between Abi mechanisms and phage components will certainly provide valuable information on the mode of action of Abi systems.
Recently, we isolated the chromosomally encoded abortive infection mechanism AbiV (30) and reported that a phage protein (SaV) with antimicrobial properties is involved in AbiV sensitivity (31). Here, we demonstrate that AbiV and SaV proteins interact directly and show that AbiV prevents phage protein synthesis and late gene transcription.
MATERIALS AND METHODS
Bacterial strains, phages, and growth conditions.
Bacterial strains and phages used in this study are listed in Table 1. Escherichia coli was grown at 37°C in LB medium (56) or Turbo broth (Athena Enzyme Systems, Baltimore, MD) for protein expression. L. lactis was grown at 30°C in M17 (62) supplemented with 0.5% glucose (GM17 medium). In experiments with incorporation of radioactive uridine or methionine, L. lactis was grown in SA medium supplemented with 0.5% glucose (GSA medium) (36) and with a reduced methionine concentration (5 μg ml−1) (39). During phage infection experiments, bacterial growth and cell lysis were determined by measuring cell density (optical density at 450 nm [OD450] in GSA medium and OD600 in GM17 medium) using a BioScreen C apparatus (Oy Growth Curves Ab Ltd.). In phage infection experiments, 10 mM CaCl2 was added to the medium. Propagation of phages (25) and phage titration (35) were performed as described previously. When needed, antibiotics were added as follows: for E. coli, 100 μg/ml of ampicillin, 34 μg/ml of chloramphenicol, and 25 μg/ml of kanamycin; for L. lactis, 5 μg/ml of chloramphenicol.
TABLE 1.
List of bacterial strains and phages used in the study
| Bacterial strain or phage | Relevant characteristicsa | Reference |
|---|---|---|
| Bacterial strains | ||
| JH-20 | L. lactis subsp. cremoris MB112(pJH2), Camr AbiV+ | 30 |
| JH-54 | L. lactis subsp. cremoris MB112(pLC5), Camr AbiV− | 30 |
| JH-62 | E. coli M15(pQE70::abiV), Ampr Kmr | 30 |
| JH-65 | E. coli M15(pQE70::sav), Ampr Kmr | 31 |
| Phages | ||
| p2 | Small isometric head, 936 group | 51 |
| sk1 | Small isometric head, 936 group | 12 |
Ampr, ampicillin resistance; Camr, chloramphenicol resistance; Kmr, kanamycin resistance; AbiV+, phage resistance phenotype (abiV is constitutively expressed from pHJ2); AbiV−, phage sensitivity phenotype (empty expression vector).
Determination of total RNA and protein synthesis in L. lactis cells.
Exponentially growing cultures (OD600 of 0.5) of L. lactis JH-20 (AbiV+) and L. lactis JH-54 (AbiV−) were infected with phage p2 at a multiplicity of infection (MOI) of 5, while a noninfected L. lactis JH-20 culture served as a control. Then, for labeling of RNA, 2.3 ml of culture was mixed with 2 μl of [14C]uridine (50 μCi ml−1) and 6 μl of 10 mM uridine to a final uridine concentration of 55 μM. Labeling of proteins was done by adding 5 μl of [35S]methionine (15 mCi ml−1) to 1.7 ml of culture (the concentration of methionine in the SA medium was reduced to 5 μg ml−1). Samples (200 μl for RNA and 150 μl for protein) were taken from the labeled cultures at 5-min intervals, transferred to a tube with 3 ml of cold 5% trichloroacetic acid (TCA), and put on ice for 1 to 1.5 h. The precipitated macromolecules were collected on a membrane filter (0.45-μm pore size; Schleicher & Schuell), washed twice with cold 5% TCA and once with boiling water, and left to air dry. The radioactivity on the filters was counted using a Packard instant imager (37).
Effect of AbiV on phage mRNA.
Another set of exponentially growing cultures (OD600 of 0.5) of L. lactis JH-20 (AbiV+) and L. lactis JH-54 (AbiV−) was concentrated 10-fold by centrifugation and resuspended in fresh medium. Then, the cells were infected with phage p2 at an MOI of 5. Two-milliliter samples were taken at 5-min intervals, quickly centrifuged, and snap-frozen in −80°C liquid ethanol. Infected-cell pellets were resuspended and incubated (37°C, 15 min) in 100 μl 0.5 M sucrose with 60 mg ml−1 lysozyme before being mixed with 1 ml TRIzol reagent (Invitrogen). Total RNA was isolated according to the manufacturer's instructions, and the samples were treated with a DNase-based Turbo DNA-free kit (Applied Biosystems) before being stored at −80°C. Immediately before use, the RNA samples were thawed, and 0.5 μg RNA was added to 0.5 ml of a denaturing solution containing 10 mM NaOH and 1 mM EDTA. The RNA samples were blotted onto Zeta-probe nylon membranes (Bio-Rad) by use of a Bio-Dot SF slot blot apparatus (Bio-Rad). After a brief rinse in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) (56) plus 0.1% sodium dodecyl sulfate (SDS) for 1 min at room temperature, the membrane was air dried for 10 min and fixed by exposure to UV light for 2 min on each side. Membranes were prehybridized for a minimum of 2 h in UltraHyb-Oligo hybridization buffer (Ambion) before 32P-labeled probe was added. After hybridization overnight at 42°C, the membranes were washed three times at 42°C in 2× SSC with 0.5% SDS for 30 min before being air dried. Radioactivity was measured by overnight exposure of Storage Phosphor Screens (Amersham) and subsequent detection with a Storm 860 scanner in storage phosphor acquisition mode. Quantification of the radioactive signal was performed using the software ImageQuant TL v.2003.03.
Primers used as probes for the detection of mRNA transcripts are listed in Table 2. The primers covered the following genes of lactococcal phage p2 (with gene products in parentheses): orf2 (terminase large-subunit protein), orf11 (major tail protein [MTP]), orf16 (baseplate protein), orf25 (protein of unknown function), orf26 (SaV), orf27 (protein of unknown function), and orf48 (Holliday junction endonuclease). These oligonucleotides were labeled with [32P]ATP (Easytides; Perkin Elmer) using polynucleotide kinase (Roche) and subsequently purified with NucAway spin columns (Ambion). Labeling efficiency was determined by quantification of 5 μl labeled probe with a Packard instant imager.
TABLE 2.
List of primers used in the phage transcription analyses
| Primer target | Primer sequence (5′-3′) |
|---|---|
| orf2 (terL) | GCCACTTAGGGACACTGCCAATAAGAGGTAAAGCC |
| orf11 (mtp) | GCGATAACCGTCGACAAATTCCCCTGTAACT |
| orf16 | GCTGATGAAGTGTAAAGATAGCCTGTTTCCCACAG |
| orf25 | GACAGGTCTACCATTATCAAGCCACCTGATGACTG |
| orf26 (sav) | GAATTAACTTTAGACCTCTTCAATAAATTCCAAGTATC |
| orf27 | GTTAGATGTTACCCCCAATCCATGTAATAAGCAACG |
| orf48 | GCCTGCAATTGAACCGACTACATACTCATTTGTCAA |
Intracellular detection of phage proteins during infection.
First, anti-ORF26 (SaV) antibodies were produced by PickCell Laboratories BV, while anti-ORF11 (major tail protein) and anti-ORF16 (baseplate protein) antibodies were made by Davids Biotechnologie GmbH. Then, similarly to phage mRNA analyses, exponentially growing cultures (OD600 of 0.5) of L. lactis JH-20 (AbiV+) and L. lactis JH-54 (AbiV−) were concentrated 10-fold and infected with phage p2 at an MOI of 5. Two-milliliter samples were taken at 5-min intervals, flash-frozen (−80°C), and analyzed for intracellular production of phage proteins by Western blotting. Cell pellets were resuspended in 400 μl 10 mM Tris-Cl, pH 8.0, 1 mM EDTA, 0.3% SDS and lysed. Twenty microliters of the solution was mixed with 20 μl sample loading buffer, and proteins were separated by SDS-PAGE (43) using 11% gels for detection of ORF11 and ORF16 and 9% gels for detection of SaV. The proteins were electroblotted (200 mA for 1 h) onto a polyvinylidene difluoride (PVDF) membrane (Hybond P) using a 20% ethanol solution containing 25 mM Tris and 192 mM glycine, pH 8.3, as transfer buffer in a Trans-Blot SD apparatus (Bio-Rad). The membranes were subsequently treated with blocking buffer (5% nonfat dry milk in phosphate-buffered saline supplemented with 0.1% Tween 20 [PBS-T]) for 1 h on an orbital shaker and then treated (1 h, shaking) with primary antibody diluted in blocking buffer. For the SaV, ORF11, and ORF16 antibodies, the dilutions were 1:75,000, 1:100,000, and 1:25,000, respectively. After three washes with PBS-T, the membrane was incubated (1 h, shaking) with secondary antibody (anti-rabbit IgG alkaline phosphatase; Amersham) diluted 1:100,000 in blocking buffer. This was followed by three washes with PBS-T and a 10-min equilibration in PBS before the membrane was treated with ECF substrate (Amersham) according to the manufacturer's instructions. The protein bands were visualized using a Storm 860 scanner in the blue excitation (450 nm) fluorescence acquisition mode. Quantification of the fluorescent signal was performed using the software ImageQuant TL v.2003.03 (Amersham).
Purification of proteins.
Genes coding for AbiV and SaV were cloned into the pQE-70 vector (Qiagen) to create C-terminal His tags on both proteins in E. coli M15 strains (strains JH-62 and JH-65, respectively). Proteins were purified as follows. After an overnight induction at 25°C with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside), cells were harvested by centrifugation for 10 min at 4,000 × g. The pellet was resuspended in 40 ml of lysis buffer (50 mM Tris, 300 mM NaCl, 10 mM imidazole, pH 8) supplemented with 0.25 mg/ml of lysozyme, 20 μg/ml DNase, 20 mM MgSO4, and EDTA-free antiproteases (Roche) and frozen at −80°C. After thawing and sonication, lysates were cleared by centrifugation (30 min at 12,000 × g). The proteins were purified by nickel affinity chromatography (His-Trap 5-ml column; GE Healthcare) on a fast protein liquid chromatography (FPLC) instrument (Pharmacia AKTA) using a stepwise gradient of imidazole followed by preparative Superdex 200 gel filtration (10 mM Tris, 300 mM NaCl, pH 8). Concentrations of the purified proteins were determined using NanoDrop 1000 (Thermo Scientific). A280 values were corrected for differences in absorption coefficient due to amino acid composition of the protein monomers by using the ProtParam tool (http://www.expasy.org/tools/protparam.html). The phage p2 proteins SSB (single-strand binding protein, ORF34) and Sak3 (ORF35) were purified as described elsewhere (57).
SEC-MALS/UV/RI.
Size exclusion chromatography with on-line multiangle laser light scattering, absorbance, and refractive index detectors (SEC-MALS/UV/RI) was carried out on an Alliance 2695 high-pressure liquid chromatography (HPLC) system (Waters) using a Superose S12 column eluted with buffer (50 mM Tris and 50 mM NaCl, pH 8.0) at a flow of 0.5 ml/min. Detection was performed using a triple-angle light scattering detector (mini-DAWNTM TREOS; Wyatt Technology), a quasi-elastic light scattering instrument (DynaproTM; Wyatt Technology), and a differential refractometer (Optilab_rEX; Wyatt Technology). Molecular weight and hydrodynamic radius (Rh) determination was performed using ASTRA V software (Wyatt Technology), with a dn/dc value of 0.185 ml/g. Proteins were loaded at final concentrations of 0.22 mM and 0.33 mM for AbiV and SaV, respectively.
Fluorescence quenching experiments.
Fluorescence quenching experiments were carried out with a Varian Eclipse spectrofluorimeter using a quartz cuvette in a right-angle configuration as previously described (63). Briefly, the light paths were 1.0 and 0.4 cm for excitation and emission, respectively. The interaction of AbiV with SaV was monitored by recording the quenching of the intrinsic SaV protein fluorescence upon addition of aliquots of AbiV, which does not have an intrinsic fluorescence or absorbance at 285 nm. The excitation wavelength was 285 nm, and the emission spectra were recorded in the range of 320 to 400 nm. The excitation slit was 5 nm and the emission slit was 10 nm for a SaV protein concentration of 0.5 μM. A moving-average smoothing procedure was applied, with a window of 5 nm. Titrations were carried out at room temperature with 0.24 μM quencher protein in 10 mM Tris buffer, 50 mM NaCl, pH 8. No correction of the fluorescence at the maximum level (341 nm) was needed, since the fluorescence and absorbance levels of the buffer and the quencher protein were negligible. The affinity was estimated by plotting the decrease in fluorescence intensity at the emission maximum as 100 − (Ii − Imin)/(I0 − Imin) × 100 against the quencher concentration; I0 is the maximum fluorescence intensity of the protein alone, Ii is the fluorescence intensity after the addition of quencher (i), and Imin is the fluorescence intensity at the saturating concentration of the quencher. The Kd (dissociation constant) values were estimated using Prism 3.02 (GraphPad Software Inc.) by nonlinear regression for a double binding site with the equation Y = Bmax1·X/(Kd1 + X) + Bmax2·X/(Kd2 + X), where Bmax is the maximal binding, Kd1 is the concentration of ligand required to reach half-maximal binding for the first binding site, Kd2 is the required concentration for the second binding site, and X is the concentration of binder added at each step. Additional controls were performed using the same protocol. Because the SSB protein (ORF34) of phage p2 does not possess any tryptophan (Trp) or intrinsic fluorescence or absorbance at 285 nm, it was used in fluorescence quenching experiments with the p2 protein SaV. On the other hand, the Sak3 protein (ORF35) of phage p2, which possesses 5 tryptophan residues, was used in combination with the AbiV protein in fluorescence quenching assays.
RESULTS
AbiV affects total RNA and protein synthesis during phage infection.
We previously demonstrated that the double-stranded DNA (dsDNA) genome of the lactococcal phage p2 replicates in AbiV+ L. lactis cells but that the infection is aborted prior to the packaging of phage DNA (30). To determine the effects of AbiV on synthesis of other macromolecules, we measured the synthesis of RNA and proteins in both AbiV+ (abiV is constitutively expressed) and AbiV− L. lactis cells during infection with the virulent phage p2.
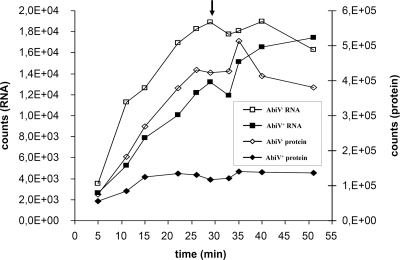
Compared to results for the noninfected cells, the addition of phages (in both AbiV+ and AbiV− cells) caused a rapid decline in RNA and protein synthesis (data not shown). Still, new RNA and proteins were synthesized in phage-infected AbiV− cells, and they both increased until 26 to 28 min (Fig. 1), which coincided with the end of the phage lytic cycle and the release of new p2 virions. In phage-infected AbiV+ cells, new RNA was also produced but at a reduced rate compared to that for the AbiV− culture (Fig. 1). RNA synthesis continued throughout the experiment (50 min), due to the absence of complete cell lysis, and it leveled off between 40 and 50 min. In contrast, protein synthesis was severely inhibited in the phage-infected AbiV+ culture compared to that in the phage-infected AbiV− culture (Fig. 1), and after 15 min, protein synthesis was stopped completely in the AbiV+ culture.
FIG. 1.
Total incorporation of [14C]uridine and [35S]methionine in L. lactis during phage infection. The vertical arrow represents the time of lysis of the sensitive AbiV− culture. Open symbols represent AbiV−, while closed symbols represent AbiV+.
AbiV affects transcription of middle and late phage genes.
Knowing that the AbiV mechanism inhibits protein synthesis, we wanted to address more specifically which part of the phage lytic cycle is targeted by AbiV. In another set of experiments, L. lactis JH-20 (AbiV+) and L. lactis JH-54 (AbiV−) were infected with virulent phage p2, and the synthesis of specific phage transcripts was investigated. Lysis of the sensitive AbiV− culture occurred 29 min after infection and was accompanied by a rise in phage titer corresponding to a burst size of ca. 50 PFU per infected cell. To quantify the transcription levels at different time points during the phage lytic cycle, several radioactively labeled oligonucleotide probes covering early, middle, and late genes of phage p2 were used in a dot blot assay.
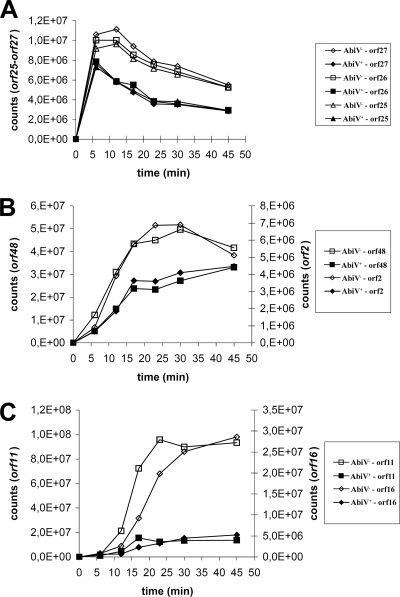
In the early phage region, three genes (orf25, orf26 [sav], and orf27) were analyzed. The sav gene encodes a nonstructural protein located in the early phage region, and SaV is likely the target of AbiV (30, 31). In AbiV− cells, orf25 to orf27 all reached similar levels of transcription, which peaked between 6 and 12 min and then gradually decreased throughout the rest of the experiment (Fig. 2A). In AbiV+ cells, transcription levels were also equal among the three genes. The highest level was reached at 6 min after infection, followed by a gradual decrease in transcription. In AbiV+ cells, the transcription levels of the three analyzed genes (orf25, orf26 [sav], and orf27) were between 60 and 75% of those found in infected AbiV− L. lactis cells.
FIG. 2.
Detection of early (A), middle (B), and late (C) phage p2 transcripts during infection of AbiV+ (closed symbols) and AbiV− (open symbols) L. lactis cells.
Two other phage genes (orf48 and orf2) had almost identical transcription patterns (Fig. 2B), and their burst of expression occurred midway through the phage cycle. In AbiV− cells, both middle gene transcripts increased until 23 min, whereas in the AbiV+ cells they leveled off at 17 min and reached only 50% of the level observed in AbiV− cells.
The late gene transcripts specific to orf11 (encoding the major tail protein [MTP] of phage p2) and orf16 (encoding a baseplate protein of phage p2 [58]) peaked toward the end of the phage cycle in the AbiV− cells (Fig. 2C). The expression of orf16 was slightly delayed compared to that of orf11. Transcription of both genes in the AbiV+ cells ceased at 17 min, concomitant with the middle gene transcripts, and reached only 10% of the level found in the AbiV− cells.
Taken altogether, the above-described transcription data show that phage mRNAs are produced in the presence of AbiV. However, transcription leveled off at 17 min, which affected the levels of early, middle, and late transcripts unequally. Early phage genes were the least affected by AbiV, followed by middle phage transcripts (50% of the wild-type level). The late phage transcripts were almost completely inhibited in the presence of AbiV.
AbiV inhibits translation of phage proteins.
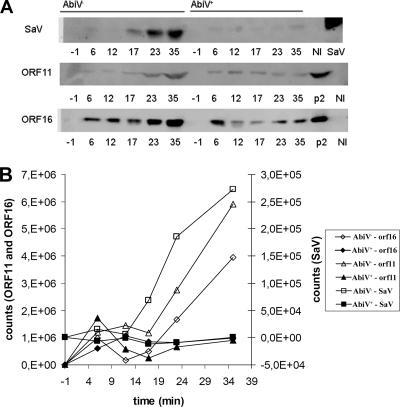
During the above-described phage infection experiments, samples were also taken for phage protein analyses. Using Western blotting and antibodies specific to the phage proteins ORF26 (SaV), ORF11, and ORF16, we followed the production of these proteins during the phage p2 lytic cycle within AbiV− and AbiV+ cells. In the AbiV− cells, SaV production increased throughout the infection until cell lysis (Fig. 3), whereas no significant production of SaV could be detected in the AbiV+ cells during the experiment. A similar pattern was observed for the structural proteins ORF11 and ORF16. Production of the two proteins increased in AbiV− cells starting midway through the lytic cycle and continuing throughout the experiment (Fig. 3). The timing of expression of the three proteins in the AbiV− cells was thus in agreement with mRNA synthesis. In the AbiV+ cells, however, no production of the phage structural proteins ORF11 and ORF16 occurred. A low and constant level of ORF11 and ORF16 was observed, and this was most likely due to the presence of the two structural proteins in the p2 virions used for the infection.
FIG. 3.
Western blotting for detection of ORF26 (SaV), ORF11, and ORF16 during phage infection. (A) Temporal phage protein production in AbiV− (left) and AbiV+ (right) L. lactis cells. Numbers represent minutes in relation to time of infection (time zero). Lanes NI, noninfected cells; lane SaV, purified SaV; lanes p2, CsCl-purified p2 virions. (B) Graphical representation of the expression levels shown in panel A. Open symbols represent AbiV−, while closed symbols represent AbiV+.
Taken altogether, Western blotting showed that translation of both early and late phage proteins was severely inhibited in the presence of AbiV. For SaV, no protein production was observed, while its gene was significantly transcribed (Fig. 2A), thus suggesting that AbiV inhibits translation of phage proteins early in the lytic cycle.
AbiV and SaV interact with each other.
In vitro chemical cross-linking assays indicated a direct protein-protein interaction between AbiV and SaV (data not shown). In order to determine if the proteins do interact directly to confer the antiphage phenotype, we used two different approaches: SEC-MALS/UV/RI and fluorescence quenching experiments.
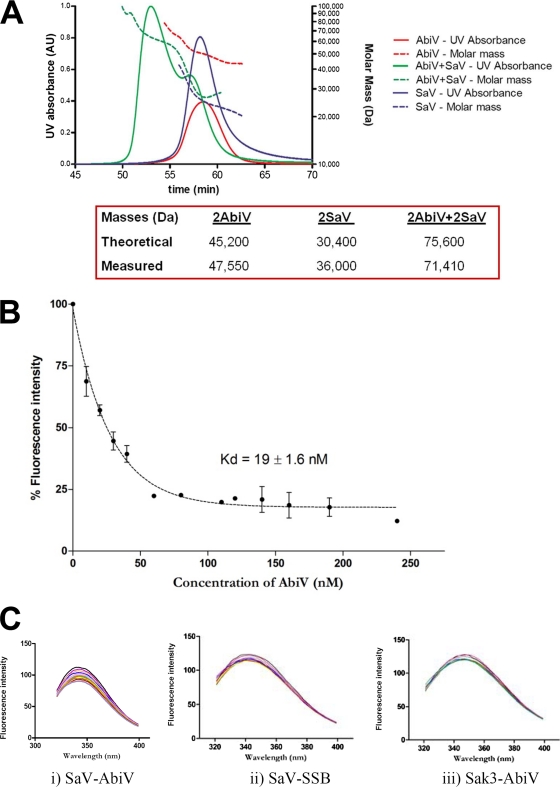
To determine the stoichiometry of the AbiV and SaV homodimers and the size of the AbiV/SaV complex, we used SEC-MALS/UV/RI (Fig. 4A). The MALS/UV/RI analysis gave measured masses of 47,550 Da and 36,000 Da for AbiV and SaV, respectively. Because the theoretical masses of AbiV and SaV are 22.7 kDa and 15.3 kDa, respectively, the measured masses indicate that both proteins form homodimers (Fig. 4A). When both proteins are injected together, the chromatogram shows a single major peak and a second peak corresponding to an excess of unbound SaV. The measured mass (71,410 Da) corresponded to a complex consisting of AbiV2-SaV2. The theoretical mass of such a complex was calculated at 75,600 Da. The above-described data support our previous observations that both AbiV and SaV are probably native dimers (30, 31). The hydrodynamic radius (Rh) values of AbiV and SaV were estimated to be 3.0 nm and 3.2 nm, respectively, while the Rh of the complex is 4.0 nm.
FIG. 4.
SEC-MALS/UV/RI analysis and fluorescence quenching assay. (A) SEC-MALS/UV/RI analysis. The abscissa indicates the time scale for the HPLC injections. AU, absorbance units. (B) Fluorescence quenching assay fitting curve, determined using nonlinear regression with a double binding site equation. The initial concentration of SaV was 0.5 μM, followed by progressive addition of different concentrations of AbiV (calculated for the monomeric forms). Error bars represent standard deviations. (C) Fluorescence spectra obtained for SaV-AbiV, SaV-SSB, and Sak3-AbiV. The Δ values obtained were 17%, 3.9%, and 3.5%, respectively.
Since AbiV has no tryptophan, it was possible to measure the dynamics of the AbiV-SaV association by using SaV tryptophan fluorescence quenching. Addition of AbiV quenched the fluorescence emission of SaV tryptophans when excited at 285 nm. A slightly better fit between the experimental data and the theoretical curve could be obtained assuming two binding sites instead of one: a high-affinity site, with a Kd1 of 19 ± 1.6 nM, indicating a strong interaction between the proteins (Fig. 4B), and a low-affinity site, with a Kd2 of 500 nM, probably reflecting additional nonspecific interactions often observed at high concentrations. The specificity of the high-affinity interaction was assessed by testing SaV and AbiV interaction with the phage p2 proteins SSB (ORF34) and Sak3 (ORF35), respectively. The choice of proteins was made based on availability and composition of tryptophan residues (SSB has no Trp residues, while Sak3 has 5 Trp residues). For both protein couples (SaV-SSB and Sak3-AbiV), the Δ values obtained, which correspond to the percentages of difference between the maximum and minimum fluorescence intensities at the maximum wavelength, were 3.5 to 3.9%. This indicates that there is no interaction between those proteins, thereby confirming the specificity of the SaV-AbiV interaction, which showed a decrease in fluorescence (Δ value) of 17% (Fig. 4C).
DISCUSSION
Recently, we isolated the lactococcal abortive infection mechanism AbiV (30) and identified the phage protein SaV as being necessary for the abortive phenotype (31). Here, we demonstrate a direct protein-protein interaction between the host protein AbiV and the phage protein SaV by SEC-MALS/UV/RI and fluorescence quenching assays (Fig. 4). AbiV and SaV likely form a complex consisting of 2 AbiV and 2 SaV molecules. The strength of the interaction was significant (Kd value of 19 ± 1.6 nM), and this is, to our knowledge, the first demonstration of a direct interaction between an Abi protein and a phage protein. Together with our previous demonstration that a functional SaV is needed for the Abi phenotype (31), this finding suggests that the AbiV2-SaV2 complex is responsible for the Abi phenotype.
Previous transcription analyses of the lactococcal phage sk1, which shares 96% nucleotide identity with phage p2 (data not shown), have revealed that early transcripts appear 2 to 5 min after the beginning of infection, whereas middle transcripts are observed after 7 to 10 min and late transcripts are observed after 15 min (11). Our phage p2 transcriptional analysis revealed continuous mRNA production in both AbiV+ and AbiV− phage-infected cells (Fig. 2). However, the presence of AbiV reduced the transcription of early (orf25, orf26 [sav], and orf27), middle (orf2 and orf48), and late (orf11 and orf16) genes. The decrease was most evident for the late transcripts, probably due to a general cessation of transcription around 17 min. In the AbiV− cells, early phage p2 transcripts started decreasing after 12 min due to a known switch-off mechanism (11, 22, 53). In the AbiV+ cells, these early phage p2 transcripts decreased as well but earlier, and their overall level was lower, as we observed a 25 to 40% reduction compared to the level in the AbiV− cells. For the middle and late phage genes, the decreases in transcription were approximately 50% and 90%, respectively, in phage-infected AbiV+ cells. It has been demonstrated previously for lactococcal phages that the switch in transcription from early to middle phage genes is mediated by an early translated product activating a middle promoter (5, 12, 40). This activator is probably not fully expressed in AbiV+ cells, thereby causing the observed partial inhibition of middle gene transcription (Fig. 2B). Similarly, it was demonstrated previously that a middle phage gene codes for an activator of late transcription (11, 40). This domino effect of transcription inhibition likely prevented the synthesis of late phage transcripts.
A more profound effect on protein synthesis was observed in phage-infected AbiV+ cells. Total protein synthesis was severely inhibited from the beginning of phage infection and ceased completely after 15 min (Fig. 1). It has been shown previously for the closely related lactococcal phage sk1 that a decrease in early protein production prevented translation of most middle transcripts and all late transcripts (11). This was confirmed by the absence of production of two phage structural proteins, ORF11 (major tail protein) and ORF16 (baseplate protein), in phage-infected AbiV+ cells (Fig. 3). Interestingly, the production of SaV could not be detected in AbiV+ cells (using Western blotting), though sav mRNA was observed (Fig. 2A). A previous study has shown that the SaV protein is necessary for the Abi phenotype (30). Thus, apparently the method used here was not sensitive enough to detect the limited amount of SaV needed to induce the Abi phenotype. Since AbiV caused almost complete inhibition of SaV translation while only minimally affecting its transcription and at the same time severely inhibiting total protein synthesis, we suggest that the AbiV2-SaV2 complex inhibits the cellular translation apparatus.
The exact function of SaV in the phage lytic cycle is still unknown. Structural prediction of SaV carried out using Phyre software (3) revealed homology with the structure of the conserved region 2 of σ70 factor (46). Region 2 is the most conserved in σ factors, and this region is crucial for binding of σ factors to core RNA polymerase (44, 45). A Sav-like protein (E11) was identified previously in the virulent lactococcal phage c2 and was also shown to be involved in AbiV activity (31). Function prediction of E11 carried out using Phyre points toward an anti-sigma factor (FlgM) from Aquifex aeolicus (55). It is thus tempting to speculate that the phage protein SaV, though not essential, is involved in phage transcription.
In conclusion, we have analyzed the interaction between the lactococcal phage resistance mechanism AbiV and the phage protein SaV (31). Our current working hypothesis is that in phage-infected AbiV+ cells, phage DNA replication occurs (30), as does early phage gene expression, including the expression of sav. However, the transcription of middle and in particular late phage genes (coding for, among others, ORF11 and ORF16) is inhibited probably due to the absence of activation. A small amount of SaV is produced early and rapidly interacts with the host AbiV protein to form an active complex that inhibits the translational machinery of the cell and hence also the phage gene-encoded proteins, including activators of the middle and late genes. However, the details of the mechanism used by the AbiV/SaV complex to abort phage infection are still unresolved.
Acknowledgments
We are grateful to Mariella Tegoni for help with the fluorescence assays and to Giuliano Sciara and David Veesler for help with the SEC-MALS/UV/RI assays.
J.H., S.J.L., and J.E.S. received graduate scholarships from the Technical University of Denmark, the Natural Sciences and Engineering Research Council (NSERC) of Canada, and the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT), respectively. This work was funded by a strategic grant from NSERC of Canada (to S.M.) and a grant from the Agence Nationale de la Recherche (ANR-07-BLAN-0095) (to C.C.).
Footnotes
Published ahead of print on 17 September 2010.
REFERENCES
- 1.Allison, G. E., and T. R. Klaenhammer. 1998. Phage resistance mechanisms in lactic acid bacteria. Int. Dairy J. 8:207-226. [Google Scholar]
- 2.Barrangou, R., C. Fremaux, H. Deveau, M. Richards, P. Boyaval, S. Moineau, D. A. Romero, and P. Horvath. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709-1712. [DOI] [PubMed] [Google Scholar]
- 3.Bennett-Lovsey, R. M., A. D. Herbert, M. J. Sternberg, and L. A. Kelley. 2008. Exploring the extremes of sequence/structure space with ensemble fold recognition in the program Phyre. Proteins 70:611-625. [DOI] [PubMed] [Google Scholar]
- 4.Bidnenko, E., M. C. Chopin, S. D. Ehrlich, and J. Anba. 2002. Lactococcus lactis AbiD1 abortive infection efficiency is drastically increased by a phage protein. FEMS Microbiol. Lett. 214:283-287. [DOI] [PubMed] [Google Scholar]
- 5.Bidnenko, E., D. Ehrlich, and M. C. Chopin. 1995. Phage operon involved in sensitivity to the Lactococcus lactis abortive infection mechanism AbiD1. J. Bacteriol. 177:3824-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidnenko, E., S. D. Ehrlich, and M. C. Chopin. 1998. Lactococcus lactis phage operon coding for an endonuclease homologous to RuvC. Mol. Microbiol. 28:823-834. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard, J. D., E. Dion, F. Bissonnette, and S. Moineau. 2002. Characterization of the two-component abortive phage infection mechanism AbiT from Lactococcus lactis. J. Bacteriol. 184:6325-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchard, J. D., and S. Moineau. 2000. Homologous recombination between a lactococcal bacteriophage and the chromosome of its host strain. Virology 270:65-75. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard, J. D., and S. Moineau. 2004. Lactococcal phage genes involved in sensitivity to AbiK and their relation to single-strand annealing proteins. J. Bacteriol. 186:3649-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boucher, I., E. Emond, E. Dion, D. Montpetit, and S. Moineau. 2000. Microbiological and molecular impacts of AbiK on the lytic cycle of Lactococcus lactis phages of the 936 and P335 species. Microbiology 146:445-453. [DOI] [PubMed] [Google Scholar]
- 11.Chandry, P. S., B. E. Davidson, and A. J. Hillier. 1994. Temporal transcription map of the Lactococcus lactis bacteriophage sk1. Microbiology 140:2251-2261. [DOI] [PubMed] [Google Scholar]
- 12.Chandry, P. S., S. C. Moore, J. D. Boyce, B. E. Davidson, and A. J. Hillier. 1997. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol. Microbiol. 26:49-64. [DOI] [PubMed] [Google Scholar]
- 13.Chopin, M. C., A. Chopin, and E. Bidnenko. 2005. Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 8:473-479. [DOI] [PubMed] [Google Scholar]
- 14.Cluzel, P. J., A. Chopin, S. D. Ehrlich, and M. C. Chopin. 1991. Phage abortive infection mechanism from Lactococcus lactis subsp. lactis, expression of which is mediated by an iso-ISS1 element. Appl. Environ. Microbiol. 57:3547-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffey, A., and R. P. Ross. 2002. Bacteriophage-resistance systems in dairy starter strains: molecular analysis to application. Antonie Van Leeuwenhoek 82:303-321. [PubMed] [Google Scholar]
- 16.Dai, G., P. Su, G. E. Allison, B. L. Geller, P. Zhu, W. S. Kim, and N. W. Dunn. 2001. Molecular characterization of a new abortive infection system (AbiU) from Lactococcus lactis LL51-1. Appl. Environ. Microbiol. 67:5225-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng, Y. M., M. L. Harvey, C. Q. Liu, and N. W. Dunn. 1997. A novel plasmid-encoded phage abortive infection system from Lactococcus lactis biovar. diacetylactis. FEMS Microbiol. Lett. 146:149-154. [DOI] [PubMed] [Google Scholar]
- 18.Deveau, H., S. J. Labrie, M. C. Chopin, and S. Moineau. 2006. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 72:4338-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinsmore, P. K., and T. R. Klaenhammer. 1994. Phenotypic consequences of altering the copy number of abiA, a gene responsible for aborting bacteriophage infections in Lactococcus lactis. Appl. Environ. Microbiol. 60:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinsmore, P. K., and T. R. Klaenhammer. 1997. Molecular characterization of a genomic region in a Lactococcus bacteriophage that is involved in its sensitivity to the phage defense mechanism AbiA. J. Bacteriol. 179:2949-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domingues, S., A. Chopin, S. D. Ehrlich, and M. C. Chopin. 2004. A phage protein confers resistance to the lactococcal abortive infection mechanism AbiP. J. Bacteriol. 186:3278-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domingues, S., A. Chopin, S. D. Ehrlich, and M. C. Chopin. 2004. The lactococcal abortive phage infection system AbiP prevents both phage DNA replication and temporal transcription switch. J. Bacteriol. 186:713-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durmaz, E., and T. R. Klaenhammer. 2006. Abortive phage resistance mechanism AbiZ speeds the lysis clock to cause premature lysis of phage-infected Lactococcus lactis. J. Bacteriol. 189:1417-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emond, E., E. Dion, S. A. Walker, E. R. Vedamuthu, J. K. Kondo, and S. Moineau. 1998. AbiQ, an abortive infection mechanism from Lactococcus lactis. Appl. Environ. Microbiol. 64:4748-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emond, E., B. J. Holler, I. Boucher, P. A. Vandenbergh, E. R. Vedamuthu, J. K. Kondo, and S. Moineau. 1997. Phenotypic and genetic characterization of the bacteriophage abortive infection mechanism AbiK from Lactococcus lactis. Appl. Environ. Microbiol. 63:1274-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forde, A., and G. F. Fitzgerald. 1999. Bacteriophage defence systems in lactic acid bacteria. Antonie Van Leeuwenhoek 76:89-113. [PubMed] [Google Scholar]
- 27.Fortier, L. C., J. D. Bouchard, and S. Moineau. 2005. Expression and site-directed mutagenesis of the lactococcal abortive phage infection protein AbiK. J. Bacteriol. 187:3721-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garvey, P., G. F. Fitzgerald, and C. Hill. 1995. Cloning and DNA sequence analysis of two abortive infection phage resistance determinants from the lactococcal plasmid pNP40. Appl. Environ. Microbiol. 61:4321-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garvey, P., D. van Sinderen, D. P. Twomey, C. Hill, and G. F. Fitzgerald. 1995. Molecular genetics of bacteriophage and natural phage defence systems in the genus Lactococcus. Int. Dairy J. 5:905-947. [Google Scholar]
- 30.Haaber, J., S. Moineau, L. C. Fortier, and K. Hammer. 2008. AbiV, a novel antiphage abortive infection mechanism on the chromosome of Lactococcus lactis subsp. cremoris MG1363. Appl. Environ. Microbiol. 74:6528-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haaber, J., G. M. Rousseau, K. Hammer, and S. Moineau. 2009. Identification and characterization of the phage gene sav, involved in sensitivity to the lactococcal abortive infection mechanism AbiV. Appl. Environ. Microbiol. 75:2484-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill, C. 1993. Bacteriophage and bacteriophage resistance in lactic acid bacteria. FEMS Microbiol. Rev. 12:87-108. [Google Scholar]
- 33.Hill, C., L. A. Miller, and T. R. Klaenhammer. 1990. Cloning, expression, and sequence determination of a bacteriophage fragment encoding bacteriophage resistance in Lactococcus lactis. J. Bacteriol. 172:6419-6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill, C., L. A. Miller, and T. R. Klaenhammer. 1990. Nucleotide sequence and distribution of the pTR2030 resistance determinant (hsp) which aborts bacteriophage infection in lactococci. Appl. Environ. Microbiol. 56:2255-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarvis, A. W. 1978. Serological studies of a host range mutant of a lactic streptococcal bacteriophage. Appl. Environ. Microbiol. 36:785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen, P. R., and K. Hammer. 1993. Minimal requirements for exponential growth of Lactococcus lactis. Appl. Environ. Microbiol. 59:4363-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorgensen, C. M., K. Hammer, and J. Martinussen. 2003. CTP limitation increases expression of CTP synthase in Lactococcus lactis. J. Bacteriol. 185:6562-6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Josephsen, J., and H. Neve. 2004. Bacteriophage and antiphage mechanisms of lactic acid bacteria, p. 295-350. In S. Salminen, A. von Wright, and A. Ouwehand (ed.), Lactic acid bacteria: microbiological and functional aspects. CRC Press, London, United Kingdom.
- 39.Koch, B., M. Kilstrup, F. K. Vogensen, and K. Hammer. 1998. Induced levels of heat shock proteins in a dnaK mutant of Lactococcus lactis. J. Bacteriol. 180:3873-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar, A., R. A. Malloch, N. Fujita, D. A. Smillie, A. Ishihama, and R. S. Hayward. 1993. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J. Mol. Biol. 232:406-418. [DOI] [PubMed] [Google Scholar]
- 41.Labrie, S. J., and S. Moineau. 2007. Abortive infection mechanisms and prophage sequences significantly influence the genetic makeup of emerging lytic lactococcal phages. J. Bacteriol. 189:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labrie, S. J., J. E. Samson, and S. Moineau. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8:317-327. [DOI] [PubMed] [Google Scholar]
- 43.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 44.Lesley, S. A., M. A. Brow, and R. R. Burgess. 1991. Use of in vitro protein synthesis from polymerase chain reaction-generated templates to study interaction of Escherichia coli transcription factors with core RNA polymerase and for epitope mapping of monoclonal antibodies. J. Biol. Chem. 266:2632-2638. [PubMed] [Google Scholar]
- 45.Lesley, S. A., and R. R. Burgess. 1989. Characterization of the Escherichia coli transcription factor sigma 70: localization of a region involved in the interaction with core RNA polymerase. Biochemistry 28:7728-7734. [DOI] [PubMed] [Google Scholar]
- 46.Malhotra, A., E. Severinova, and S. A. Darst. 1996. Crystal structure of a sigma 70 subunit fragment from E. coli RNA polymerase. Cell 87:127-136. [DOI] [PubMed] [Google Scholar]
- 47.Moineau, S. 1999. Applications of phage resistance in lactic acid bacteria. Antonie Van Leeuwenhoek 76:377-382. [PubMed] [Google Scholar]
- 48.Moineau, S., S. Pandian, and T. Klaenhammer. 1994. Evolution of a lytic bacteriophage via DNA acquisition from the Lactococcus lactis chromosome. Appl. Environ. Microbiol. 60:1832-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moineau, S., D. Tremblay, and S. Labrie. 2002. Phages of lactic acid bacteria: from genomics to industrial applications. ASM News 68:388-393. [Google Scholar]
- 50.Moineau, S., E. Durmaz, S. Pandian, and T. R. Klaenhammer. 1993. Differentiation of two abortive mechanisms by using monoclonal antibodies directed toward lactococcal bacteriophage capsid proteins. Appl. Environ. Microbiol. 59:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moineau, S., S. A. Walker, E. R. Vedamuthu, and P. A. Vandenbergh. 1995. Cloning and sequencing of LlaDCHI [corrected] restriction/modification genes from Lactococcus lactis and relatedness of this system to the Streptococcus pneumoniae DpnII system. Appl. Environ. Microbiol. 61:2193-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Connor, L., A. Coffey, C. Daly, and G. F. Fitzgerald. 1996. AbiG, a genotypically novel abortive infection mechanism encoded by plasmid pCI750 of Lactococcus lactis subsp. cremoris UC653. Appl. Environ. Microbiol. 62:3075-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parreira, R., S. D. Ehrlich, and M. C. Chopin. 1996. Dramatic decay of phage transcripts in lactococcal cells carrying the abortive infection determinant AbiB. Mol. Microbiol. 19:221-230. [DOI] [PubMed] [Google Scholar]
- 54.Ploquin, M., A. Bransi, E. R. Paquet, A. Z. Stasiak, A. Stasiak, X. Yu, A. M. Cieslinska, E. H. Egelman, S. Moineau, and J. Y. Masson. 2008. Functional and structural basis for a bacteriophage homolog of human RAD52. Curr. Biol. 18:1142-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pons, T., B. Gonzalez, F. Ceciliani, and A. Galizzi. 2006. FlgM anti-sigma factors: identification of novel members of the family, evolutionary analysis, homology modeling, and analysis of sequence-structure-function relationships. J. Mol. Model. 12:973-983. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 57.Scaltriti, E., M. Tegoni, C. Rivetti, H. Launay, J. Y. Masson, A. H. Magadan, D. Tremblay, S. Moineau, R. Ramoni, J. Lichiere, V. Campanacci, C. Cambillau, and M. Ortiz-Lombardia. 2009. Structure and function of phage p2 ORF34(p2), a new type of single-stranded DNA binding protein. Mol. Microbiol. 73:1156-1170. [DOI] [PubMed] [Google Scholar]
- 58.Sciara, G., C. Bebeacua, P. Bron, D. Tremblay, M. Ortiz-Lombardia, J. Lichiere, M. van Heel, V. Campanacci, S. Moineau, and C. Cambillau. 2010. Structure of lactococcal phage p2 baseplate and its mechanism of activation. Proc. Natl. Acad. Sci. U. S. A. 107:6852-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sturino, J. M., and T. R. Klaenhammer. 2006. Engineered bacteriophage-defence systems in bioprocessing. Nat. Rev. Microbiol. 4:395-404. [DOI] [PubMed] [Google Scholar]
- 60.Su, P., M. Harvey, H. J. Im, and N. W. Dunn. 1997. Isolation, cloning and characterisation of the abiI gene from Lactococcus lactis subsp. lactis M138 encoding abortive phage infection. J. Biotechnol. 54:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tangney, M., and G. F. Fitzgerald. 2002. Effectiveness of the lactococcal abortive infection systems AbiA, AbiE, AbiF and AbiG against P335 type phages. FEMS Microbiol. Lett. 210:67-72. [DOI] [PubMed] [Google Scholar]
- 62.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tremblay, D. M., M. Tegoni, S. Spinelli, V. Campanacci, S. Blangy, C. Huyghe, A. Desmyter, S. Labrie, S. Moineau, and C. Cambillau. 2006. Receptor-binding protein of Lactococcus lactis phages: identification and characterization of the saccharide receptor-binding site. J. Bacteriol. 188:2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Twomey, D. P., P. J. De Urraza, L. L. McKay, and D. J. O'Sullivan. 2000. Characterization of AbiR, a novel multicomponent abortive infection mechanism encoded by plasmid pKR223 of Lactococcus lactis subsp. lactis KR2. Appl. Environ. Microbiol. 66:2647-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]