Abstract
To discern the possible spread of the Escherichia coli O25b:H4-ST131 clonal group in poultry and the zoonotic potential of avian strains, we made a retrospective search of our strain collection and compared the findings for those strains with the findings for current strains. Thus, we have characterized a collection of 19 avian O25b:H4-ST131 E. coli strains isolated from 1995 to 2010 which, interestingly, harbored the ibeA gene. Using this virulence gene as a criterion for selection, we compared those 19 avian strains with 33 human O25b:H4-ST131 ibeA-positive E. coli strains obtained from patients with extraintestinal infections (1993 to 2009). All 52 O25b:H4-ST131 ibeA-positive E. coli strains shared the fimH, kpsMII, malX, and usp genes but showed statistically significant differences in nine virulence factors, namely, papGIII, cdtB, sat, and kpsMII K5, which were associated with human strains, and iroN, kpsMII K1, cvaC, iss, and tsh, which were associated with strains of avian origin. The XbaI macrorestriction profiles of the 52 E. coli O25b:H4-ST131 ibeA-positive strains revealed 11 clusters (clusters I to XI) of >85% similarity, with four clusters including strains of human and avian origin. Cluster VII (90.9% similarity) grouped 10 strains (7 avian and 3 human strains) that mostly produced CTX-M-9 and that also shared the same virulence profile. Finally, we compared the macrorestriction profiles of the 12 CTX-M-9-producing O25b:H4-ST131 ibeA strains (7 avian and 5 human strains) identified among the 52 strains with those of 15 human O25b:H4-ST131 CTX-M-14-, CTX-M-15-, and CTX-M-32-producing strains that proved to be negative for ibeA and showed that they clearly differed in the level of similarity from the CTX-M-9-producing strains. In conclusion, E. coli clonal group O25b:H4-ST131 ibeA has recently emerged among avian isolates with the new acquisition of the K1 capsule antigen and includes CTX-M-9-producing strains. This clonal group represents a real zoonotic risk that has crossed the barrier between human and avian hosts.
Strains of the extensively antimicrobial-resistant Escherichia coli clonal group of sequence type (ST) 131 (ST131) belonging to serotype O25b:H4 have recently been recognized to be important human pathogens worldwide (9, 33). Although it is commonly associated with the dissemination of CTX-M-15 extended-spectrum cephalosporin resistance, E. coli O25b:H4-ST131 also occurs as a fluoroquinolone (FQ)-resistant but cephalosporin-susceptible pathogen (5, 22, 26, 27). Currently, it is assumed that O25b:H4-ST131 strains circulate not only among humans but also among animal hosts (13, 21, 37), which would contribute to the ongoing global emergence of O25b:H4-ST131, in the case of regular transmission between animals and humans. Even though CTX-M-15 is the most widely distributed extended-spectrum beta-lactamase (ESBL) linked to this clonal group, other, different variants of CTX-M have recently been reported, such as CTX-M-9, CTX-M-14, and CTX-M-32 (4, 34, 36, 39). Noteworthy was the detection, for the first time on poultry farms, of this clonal group producing CTX-M-9 that had macrorestriction profiles and virulence genes very similar to those observed in clinical human isolates (10).
Extraintestinal pathogenic E. coli (ExPEC) strains, which include avian pathogenic E. coli (APEC) and human uropathogenic E. coli (UPEC), septicemic E. coli, and newborn meningitis-causing E. coli (NMEC) strains, exhibit considerable genome diversity and have a wide range of virulence-associated factors (12, 18). While infections caused by APEC strains initially start as a respiratory tract disease which evolves to a systemic infection of the internal organs and, finally, to sepsis, the most frequent origin of human sepsis is urinary tract infection (UTI), especially pyelonephritis (2, 3, 11). However, APEC strains have been recognized to share common traits with human isolates (29, 30, 31), including the K1 capsule antigen (23, 24, 29) and the ibeA gene (14). In addition, retail chicken products have been found to carry nalidixic-resistant ExPEC strains (17, 19), and although it is drug susceptible, an E. coli strain belonging to the O25b:H4-ST131 clonal group has even recently been detected in retail chicken (41), supporting the urgent necessity for the implementation of food control measures.
The aim of the present study was to discern the possible spread of the O25b:H4-ST131 clonal group, especially CTX-M-9-producing strains, in poultry and the zoonotic potential of avian isolates. For this purpose, we made a retrospective search of our human and avian strain collections and compared the findings for those strains with the findings for current strains. Identification of this emerging clone among avian sources and comparison of the clone with clinical human isolates will shed new light on the epidemiology of the O25b:H4-ST131 clonal group.
MATERIALS AND METHODS
E. coli strains.
In the present study, we have characterized a total of 67 avian and human E. coli strains (19 avian and 48 human) belonging to clonal group O25b:H4-B2-ST131.
The 19 poultry O25b:H4-ST131 strains were distributed as follows: 8 strains were isolated from 7 retail chicken samples from among 100 samples obtained from September 2009 to February 2010 in the city of Lugo (northwest Spain) (prevalence, 7%); 10 strains were isolated from birds with different pathologies, with 7 of those 10 strains being obtained from among 463 chicken E. coli strains obtained from 2007 to 2009 in Spain (prevalence, 1.5%) and the remaining 3 (2 from turkeys and 1 from a chicken) being obtained from among a collection of 1,601 avian (974 chicken, 408 turkey, and 159 duck) E. coli strains isolated from 1991 to 2001 in Spain, France, and Belgium (prevalence, 0.2%) (38); and finally, 1 strain was isolated from among 57 chicken E. coli strains obtained from poultry feces in 2003 in Catalonia (northeast Spain) (prevalence, 1.8%) (10).
Forty-eight human O25b:H4-ST131 E. coli strains were selected for comparison with those of avian origin: (i) a group of 28 non-ESBL-producing ibeA-positive O25b:H4-ST131 strains (prevalence, 1.1%) obtained from among 83 strains belonging to the clonal group (prevalence, 3.4%) recovered from 2,464 E. coli-containing cultures of blood from patients admitted to Xeral-Calde Hospital in Lugo from 1993 to 2009 and (ii) an additional group of 20 O25b:H4-ST131 strains producing different CTX-M types obtained from among 761 ESBL-producing E. coli strains (143 [18.8%] belonging to the clonal group) from 2006 to 2009 from patients with extraintestinal infections (urinary tract infections or sepsis) admitted to five hospitals in Galicia (northwest Spain).
Identification of O25b:H4-B2-ST131 strains: serotyping, phylogenetic grouping, and MLST.
The strains in the source collections were first identified by means of PCR by the detection of the specific O25b molecular subtype (4, 8). Afterwards, the strains were confirmed to be O25:H4 by serotyping, and their molecular characterization was completed. The determination of the O25 and H4 antigens was carried out using the method previously described by Guinée et al. (15) with specific antisera obtained from the Laboratorio de Referencia de E. coli, Universidade de Santiago de Compostela. The phylogenetic group of the E. coli strains (group B2) was determined by the multiplex PCR-based method of Clermont et al. (7). Multilocus sequence typing (MLST) (ST131) was performed as described previously by gene amplification and sequencing of the seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) by use of the protocol and primers specified at the E. coli MLST website (http://mlst.ucc.ie/mlst/dbs/Ecoli) (28). The allelic profiles (STs) of the seven gene sequences were obtained via the electronic database at the E. coli MLST website.
Antibiotic susceptibility testing and ESBL typing.
The strains were screened for ESBL production by testing for cephalosporin resistance and performing the double-disk synergy test described by Jarlier et al. (16). MICs were determined by use of the MicroScan WalkAway automated system (Siemens, Spain), according to the manufacturer's instructions. Resistance was interpreted on the basis of the recommended breakpoints of the CLSI (formerly the NCCLS) (32). To determine the genotypes of the ESBLs, PCR was performed using the TEM-, SHV-, CTX-M-1, and CTX-M-9 group-specific primers, as reported previously (25). Sequencing was also performed with the same PCR primers and under the same conditions. The sequences obtained were then compared with those of the corresponding genes available in GenBank.
Virulence genotyping.
The strains were analyzed for the presence of virulence genes, as documented elsewhere (4, 29), using primers specific for genes and operons that encode extraintestinal virulence factors characteristic of ExPEC: fimH, fimAvMT78, papEF (strains with positive results for papEF were tested for the papGI, papGII, and papGIII alleles), sfa and focDE (analyses for which were performed together, and strains with positive results were tested for sfaS and focG), afa and draBC, bmaE, gafD, cnf1, cdtB, sat, hlyA, iucD, iroN, kpsMII (establishing neuC K1, K2, and K5 variants), kpsMIII, cvaC, iss, traT, ibeA, malX, usp, and tsh.
The extraintestinal pathogenic status of the strains was analyzed according to the definition of Johnson et al. (17). A strain satisfied the criteria for being extraintestinal pathogenic if it carried two or more of the following genes: pap (P fimbriae), sfa and focDE (S/F1C fimbriae), afa and draBC (Dr binding adhesins), iucD (aerobactin receptor), and kpsMII (group 2 capsule synthesis).
PFGE.
Pulsed-field gel electrophoresis (PFGE) analysis with XbaI digestion was performed as described previously (29). The PFGE profiles were analyzed with the BioNumerics fingerprinting software (Applied Maths, St-Martens-Latem, Belgium). Cluster analysis of the Dice similarity indices based on the unweighted-pair group method using arithmetic linkages (UPGMA) was done to generate a dendrogram describing the relationship among the PFGE profiles. Isolates were considered related if their Dice similarity index was >85%, according to criteria of Tenover et al. (a difference of six bands or less) (40).
Statistical analysis.
For the avian and human O25b:H4-ST131 populations, Fisher's exact test was used to test the null hypothesis that the gene prevalence rates across the two populations studied were equal. For each comparison, a P value of <0.05 was considered to denote significant differences.
RESULTS
In the present study, we have detected an increasing presence of the clonal group O25b:H4-ST131 not only in association with avian pathology (0.2% from 1991 to 2001 to 1.5% from 2007 to 2009; P = 0.002) but also in retail chicken (7% of samples obtained in 2009 and 2010). The 19 avian O25b:H4-ST131 E. coli strains isolated were compared with 48 human strains belonging to the same clonal group.
ESBL production and associated resistance.
Twenty-seven of the 67 O25b:H4-ST131 E. coli isolates included in the present study were positive for CTX-M-production: 7 of the 19 avian strains were CTX-M-9 producers, and 20 of the 48 human strains produced different CTX-M-types (6 strains produced CTX-M-15, 5 produced CTX-M-14, 5 produced CTX-M-9, 3 produced CTX-M-32, and 1 produced both types CTX-M-14/15).
Table 1 summarizes the antibiotic resistance of the 19 avian strains. Only four strains were susceptible to all antibiotics tested; and high MICs for gentamicin, tobramycin, and ciprofloxacin were detected in all CTX-M-9-producing avian strains.
TABLE 1.
Antibiotic resistance of the 19 avian O25b:H4-ST131 ibeA-positive E. coli strains
| Strain code | Yr | Origin | ESBL type | Antibiotic resistancea |
|---|---|---|---|---|
| FV 9211 | 1995 | Avian pathology associatedb | CEF, AMP, PIP, TIC, SXT | |
| FV 9212 | 1995 | Avian pathology associatedb | AMP, PIP, TIC, NAL, SXT | |
| FV 9213 | 1992 | Avian pathology associatedc | CEF, CFZ, AMP, PIP, TIC, NAL, SXT | |
| FV 9807 | 2007 | Avian pathology associatedc | ||
| FV 10608 | 2003 | Poultry fecesc | CTX-M-9 | CEF, CFZ, CXM, CTX, FEP, AMP, PIP, TIC, SXT |
| FV 11686 | 2008 | Avian pathology associatedc | CTX-M-9 | CEF, CFZ, CXM, CTX, FEP, AMP, PIP, TIC |
| FV 11687 | 2008 | Avian pathology associatedc | CTX-M-9 | CEF, CFZ, CXM, CTX, FEP, AMP, PIP, TIC, TOB |
| FV 12593 | 2009 | Avian pathology associatedc | ||
| FV 13455 | 2009 | Avian pathology associatedc | ||
| FV 14067 | 2009 | Avian pathology associatedc | ||
| FV 14087 | 2009 | Avian pathology associatedc | CEF | |
| FV 14287 | 2009 | Avian meatc | CTX-M-9 | CEF, CFZ, CXM, CTX, FEP, AMP, PIP, TIC, NAL, SXT |
| FV 14288 | 2009 | Avian meatc | CTX-M-9 | CEF, CFZ, CXM, CTX, FEP, AMP, PIP, TIC, NAL, SXT |
| FV 14289 | 2009 | Avian meatc | CEF, NAL, SXT | |
| FV 14290 | 2009 | Avian meatc | CTX-M-9 | CEF, CFZ, CXM, CTX, FEP, AMP, PIP, TIC, NAL, GEN, SXT |
| FV 14292 | 2009 | Avian meatc | CEF, NAL, AMP, PIP, TIC, GEN, TOB, SXT | |
| FV 14293 | 2009 | Avian meatc | CTX-M-9 | CEF, CFZ, CXM, CTX, FEP, AMP, PIP, TIC |
| FV 14294 | 2010 | Avian meatc | CEF, CFZ, CXM, FOX, AMC, AMP, TIC | |
| FV 14295 | 2010 | Avian meatc | CEF, NAL, AMP, PIP, TIC, GEN, TOB, SXT |
The antibiotics to which resistance was tested were cephalothin (CEF), cefazolin (CFZ), cefuroxime (CXM), cefotaxime (CTX), cefepime (FEP), cefoxitin (FOX), amoxicillin-clavulanate (AMC), ampicillin (AMP), piperacillin (PIP), ticarcillin (TIC), gentamicin (GEN), tobramycin (TOB), nalidixic acid (NAL), ciprofloxacin (CIP), and trimethoprim-sulfamethoxazole (SXT).
Turkey.
Chicken.
Virulence genotyping.
Interestingly, the 19 avian strains harbored the ibeA gene; for this reason, we compared them with 33 human O25b:H4-ST131 ibeA-positive E. coli strains (28 non-ESBL-producing and 5 CTX-M-9-producing strains) obtained from patients with extraintestinal infections. Table 2 summarizes the virulence genes harbored by the 52 O25b:H4-ST131 ibeA-positive E. coli strains. Apart from the ibeA gene, all 52 strains shared the fimH, kpsMII, malX, and usp genes but showed statistically significant differences in nine virulence factors, namely, papGIII, cdtB, sat, and kpsMII K5, which were associated with human strains, and iroN, kpsMII K1, cvaC, iss, and tsh, which were associated with strains of avian origin.
TABLE 2.
Virulence gene characterization of the 52 O25:H4-ST131 ibeA-positive E. coli strains included in this studya
| Category | Gene(s) | Comment(s) | No. (%) of strains |
P valueb | ||
|---|---|---|---|---|---|---|
| Avian strains (n = 19) | Human ibeA-positive strains (n = 33) | Avian and human strains (n = 52) | ||||
| Adhesins | fimH | d-Mannose-specific adhesin, type 1 fimbriae | 19 (100) | 33 (100) | 52 (100) | 1.000 |
| fimAvMT78 | FimA variant MT78 of type 1 fimbriae | 0 | 0 | 0 | ||
| pap | Pilus associated with pyelonephritis (P fimbriae) | |||||
| papEF | Pilus assembly, central region of pap operon | 1 (8.3) | 12 (36.4) | 13 (25) | 0.011 | |
| papGI | Gal(α1-4) Gal-specific pilus tip adhesin molecule rare | 0 | 0 | 0 | ||
| papGII | Pyelonephritis-associated gene | 1 (8.3) | 0 | 1 (2) | 0.365 | |
| papGIII | Cystitis-associated gene | 0 | 12 (36.4) | 12 (23.1) | 0.002 | |
| sfa and focDE | Central region of sfa and foc operons | 0 | 0 | 0 | ||
| afa and draBC | Dr antigen-specific adhesin operons (AFA, Dr, F1845) | 0 | 5 (15.1) | 5 (9.6) | 0.091 | |
| bmaE | Blood group M-specific adhesin | 0 | 1 (3) | 1 (1.9) | 0.635 | |
| gafD | N-Acetyl-d-glucosamine-specific (G, F17c) fimbria adhesin | 0 | 0 | 0 | ||
| Toxins | cnf1 | Cytotoxic necrotizing factor 1 | 0 | 1 (3) | 1 (1.9) | 0.635 |
| cdtB | Cytolethal distending toxin | 1 (8.3) | 17 (51.5) | 18 (34.6) | 0.001 | |
| sat | Secreted autotransporter toxin | 0 | 7 (21.2) | 7 (13.5) | 0.032 | |
| hlyA | α-Hemolysin | 0 | 1 (3) | 1 (1.9) | 0.635 | |
| Siderophores | iucD | Ferric aerobactin receptor (iron uptake, transport) | 17 (89.5) | 24 (72.7) | 41 (78.8) | 0.142 |
| iroN | Novel catecholate siderophore receptor | 19 (100) | 17 (51.5) | 36 (69.2) | 0.000 | |
| Protectins | kpsMII | Group II capsule | 19 (100) | 33 (100) | 52 (100) | 1.000 |
| kpsMIIK2 | K2 group II capsule | 0 | 0 | 0 | ||
| kpsMIIK5 | K5 group II capsule | 5 (26.3) | 26 (78.8) | 31 (59.6) | 0.000 | |
| neuCK1 | K1 antigen | 14 (73.7) | 7 (21.2) | 21 (40.4) | 0.000 | |
| kpsMII | Group III capsule | 0 | 0 | 0 | ||
| cvaC | ColV; on plasmids with traT, iss, and antibiotic resistance genes | 17 (89.5) | 19 (57.6) | 36 (69.2) | 0.016 | |
| iss | Increased serum survival (outer membrane protein) | 19 (100) | 20 (60.6) | 39 (75) | 0.001 | |
| traT | Surface exclusion, serum survival (outer membrane protein) | 19 (100) | 28 (84.8) | 47 (90.4) | 0.091 | |
| Miscellaneous | malX (PAI) | Pathogenicity-associated island marker | 19 (100) | 33 (100) | 52 (100) | 1.000 |
| usp | Uropathogenic strain-specific protein (bacteriocin) | 19 (100) | 33 (100) | 52 (100) | 1.000 | |
| tsh | Tsh (temperature-sensitive hemagglutinin) serine protease | 15 (78.9) | 5 (15.1) | 20 (38.5) | 0.000 | |
| Mean for virulence genesc | 10.7 | 9.7 | 10.1 | |||
Boldface data indicate statistical significance.
For the avian versus human strains. For each comparison, a P value of <0.05 was considered statistically significant, and a P value of >0.05 was considered not statistically significant.
Range, 6 to 13.
The number of virulence factors harbored by the 52 O25b:H4-ST131 ibeA-positive strains ranged from 6 to 13, and a total of 23 virulence-gene profiles were detected, as shown in Fig. 1. Avian strains showed six different gene profiles, with profile 3 with 11 virulence genes (P3-11) being the most frequently detected (12 strains, 63.2%). Human strains showed 18 different gene profiles, with 1 profile (P3-11, 3 human strains) being shared with avian strains. Forty-six of the 52 O25b:H4-ST131 ibeA-positive E. coli strains (29 human and 17 avian) satisfied the criteria for extraintestinal pathogenic status.
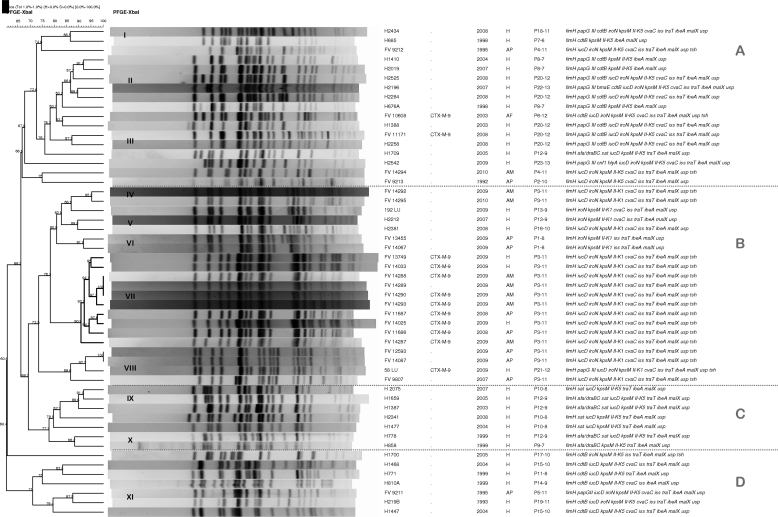
FIG. 1.
Dendrogram of XbaI macrorestriction profiles obtained by PFGE of the 19 avian and 33 human O25b:H4-ST131 ibeA-positive strains characterized in this study. The dendrogram was generated by use of the UPGMA algorithm, based on the Dice similarity coefficient with a 1.0% band position tolerance. The strain code, ESBL type, year and origin (H, human; AP, avian pathology associated; AM, avian meat) of isolation, virulence profile designation-number of virulence factors, and virulence factors are shown on the right-hand side.
In contrast to the 12 avian and human CTX-M-9-producing ibeA-positive strains, none of the remaining 15 human O25b:H4-ST131 E. coli strains producing other CTX-M types (6 producing CTX-M-15, 5 producing CTX-M-14, 3 producing CTX-M-32, and 1 producing CTX-M-14/15) harbored the ibeA gene and showed five gene profiles (profiles P24 to P28) not found among the 23 gene profiles of the 52 human and avian O25b:H4-ST131 ibeA-positive strains (Fig. 2).
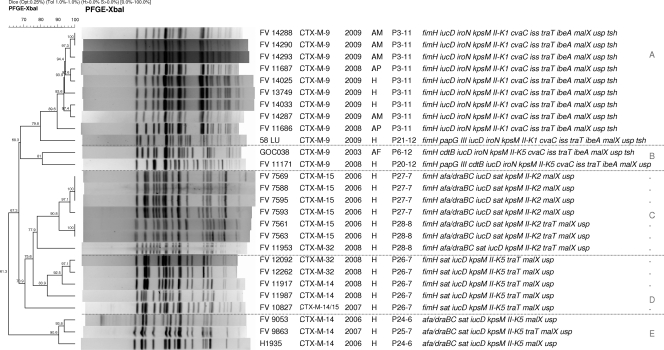
FIG. 2.
Dendrogram of XbaI macrorestriction profiles obtained by PFGE of the 12 CTX-M-9-producing O25b:H4-ST131 strains (7 avian and 5 human strains) characterized in this study by comparison with 15 human O25b:H4-ST131 E. coli strains producing different types of CTX-M enzymes (6 strains producing CTX-M-15, 5 producing CTX-M-14, 3 producing CTX-M-32, and 1 producing CTX-M-14/15). The dendrogram was generated by use of the UPGMA algorithm, based on the Dice similarity coefficient with a 1.0% band position tolerance. The strain code, ESBL type, year and origin (H, human; AP, avian pathology associated; AM, avian meat) of isolation, virulence profile designation-number of virulence factors, and virulence factors are shown on the right-hand side.
Macrorestriction profiles by PFGE.
Figure 1 shows a dendrogram with the XbaI macrorestriction profiles obtained by PFGE of the 52 O25b:H4-ST131 ibeA-positive strains analyzed. The strains formed four groups (groups A, B, C, and D) with similarities of 66.8%, 73.5%, 77.7%, and 71%, respectively. Group B included all 21 K1 strains (14 avian and 7 human strains), while the remaining 31 K5 strains (26 human and 5 avian strains) were divided into groups A (mostly papGIII positive; 11 of 17 strains), C (mostly sat positive; 6 of 7 strains), and D (mostly cdtB positive and papG negative; 6 of 7 strains). Looking at these four large groups, PFGE revealed 11 clusters (clusters I to XI) of >85% similarity, with 4 clusters (clusters IV, VII, VIII, and XI) including strains of human and avian origin. Cluster VII (90.9% similarity) grouped 10 strains (7 avian and 3 human strains) that mostly produced CTX-M-9 (9 strains) and that also shared the same virulence profile (P3-11).
We wanted to compare the macrorestriction profiles of the 12 CTX-M-9-producing O25b:H4-ST131 strains (7 avian and 5 human strains, all of them ibeA positive) characterized in this study with those of 15 human E. coli O25b:H4-ST131 strains (all of them ibeA negative) producing different types of CTX-M (6 producing CTX-M-15, 5 producing CTX-M-14, 3 producing CTX-M-32, and 1 producing CTX-M-14/15). Figure 2 shows the dendrogram of the 27 CTX-M-producing strains that remained distributed according to the type of CTX-M produced and virulence genes in five groups (groups A, B, C, D, and E) with similarities of 79.8%, 81%, 77.9%, 70.9%, and 90.6%, respectively. Group A included 10 CTX-M-9-producing K1 strains with a cluster of 89.6% similarity, including those 9 CTX-M-9-producing strains with virulence profile P3-11. Group B included the remaining two CTX-M-9-producing strains that harbored the K5 capsule. Group C included seven afa and draBC K2 strains (six CTX-M-15-producing strains and one CTX-M-32-producing strain) with a cluster of 90.8% similarity, including those six CTX-M-15-producing strains. Group D included five K5 strains with virulence profile P26-7 producing CTX-M-32, CTX-M-14, and CTX-M-14/15 (both types). Finally, group E included three CTX-M-14-producing K5 strains that were fimH negative and afa and draBC positive.
DISCUSSION
Due to the recent report of clonal group O25b:H4-ST131 among E. coli strains isolated from poultry feces (10), we made a retrospective search for its presence and also obtained a new sample of strains from clinical avian cases and retail chicken in order to better know the epidemiology of this emerging group. In the present study, we have detected an increasing presence of clonal group O25b:H4-ST131 not only in association with avian pathology but also in association with retail chicken. We also report here the detection of O25b:H4-ST131 E. coli strains producing CTX-M-9 from retail chicken as well as from samples associated with avian pathology (7 of the 19 avian strains).
Few data are available on this clonal group from poultry. Recently, Vincent et al. (41) first reported on the presence of one O25b:H4-ST131 isolate from among 250 retail chicken samples (0.4%) with a macrorestriction profile indistinguishable from that of an E. coli strain originating from a human UTI. That O25b:H4-ST131 strain found by Vincent et al. (41) was negative for ESBL production. Before that, Carattoli (6) gathered in her review two reports of CTX-M-9-producing E. coli in poultry but did not detail the serotypes or STs.
According to Johnson et al. (20), it seems that clone ST131 is common among E. coli strains resistant to FQs and does not necessarily have to produce an ESBL. In agreement with this, Peirano and Pitout (35) believe that plasmids carrying CTX-M-15 enzymes were most likely introduced at a later stage and that ST131 was possibly an established successful FQ-resistant clone before it acquired plasmids encoding CTX-M-15. On the basis of our data, it seems that not only plasmids encoding CTX-M-15 but also plasmids encoding other CTX-M types were probably introduced at a later stage. In fact, it has not been until recently that we first detected the CTX-M-9 enzyme in human strains belonging to clonal group O25b:H4-ST131 in our sanitary area (first isolation in 2008). A study of the prevalence of 761 ESBL-producing E. coli isolates recovered from 2006 to 2009 from patients admitted to five hospitals in Galicia (northwest Spain) showed that 143 (18.8%) belonged to the O25b:H4-ST131 clonal group and produced different CTX-M types: 128 of those 143 strains produced CTX-M-15 (89.5%), 6 produced CTX-M-14 (4.2%), 5 produced CTX-M-9 (3.5%), 3 produced CTX-M-32 (2.1%), and 1 produced CTX-M-1 (0.7%) (unpublished data). Those 5 CTX-M-9-producing human strains (all ibeA positive) were compared in the present study with the 19 avian isolates. Interestingly, three of the five CTX-M-9-producing human strains clustered (89.6% similarity) with six avian strains producing the same type of enzyme and showing the same virulence profile (P3-11; Fig. 2). These three human CTX-M-9-producing strains harbored the K1 capsule antigen, a virulence factor statistically linked to strains of avian origin (Table 2). Furthermore, the macrorestriction profiles of the nine P3-11 strains producing CTX-M-9 included in this cluster clearly differed in similarity from the remaining 15 O25b:H4-ST131 human strains producing CTX-M enzymes other than CTX-M-9, none of which was ibeA positive (Fig. 2). The high XbaI macrorestriction similarity shared by the nine CTX-M-9-producing avian and human strains would be indicative of recent emergence and circulation between both hosts, as molecular typing by PFGE shows that small changes happened and quickly accumulated in the genome (local epidemiology). As far as we know, this is the first study that clearly reports on the zoonotic potential of avian strains belonging to clonal group O25b:H4-ST131 and producing CTX-M enzymes.
Peirano and Pitout (35) found that the virulence factors malX, ompT, and usp were more common in ST131 strains than in other successful clonal groups (O15 and clonal group A). These authors suggest that the cited genes might be important in the worldwide dissemination of clonal group ST131. We did not analyze the isolates for the presence of ompT, but all 67 human and avian O25b:H4-ST131 strains included in the present study were positive for usp and malX.
Forty-six of the 52 O25b:H4-ST131 ibeA-positive E. coli strains (29 human and 17 avian strains) satisfied the criteria for extraintestinal pathogenic status. Besides, all 52 ibeA-positive strains showed a high pathogenic potential, according to the number of virulence genes harbored (mean, 10.1; range, 6 to 13). Interestingly, all 19 avian strains of the present study harbored the ibeA gene, which has clearly been linked to the pathogenicity of ExPEC strains and which is positively associated with serogroups O2, O18, and O88 (1, 14).
In conclusion, the O25b:H4-ST131 ibeA-positive clonal group of E. coli has recently emerged among avian isolates. These strains have newly acquired the K1 capsule antigen and include CTX-M-9-producing strains. This clonal group represents a real zoonotic risk that has crossed the barrier between human and avian hosts, since several PFGE clusters (>85% similarity) identified in this study included both human and avian isolates. The most prominent of these clusters was the one with 10 strains showing the same virulence-gene profile, of which 9 were CTX-M-9-producing strains.
Acknowledgments
This work was partially supported by grants REIPI RD06/0008/1016-1018 and PS09/01273 (Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, Fondo de Investigación Sanitaria), AGL-2008-02129 (Ministerio de Ciencia e Innovación), and 09TAL007261PR and 2007/000044-0 (Xunta de Galicia and the European Regional Development Fund [ERDF]). Azucena Mora acknowledges financial support from the Ramón y Cajal program of the Spanish Ministerio de Ciencia e Innovación. Rosalia Mamani acknowledges a grant from the Agencia Española de Cooperación Internacional (AECI) (Ministerio de Asuntos Exteriores y de Cooperación).
We thank Monserrat Lamela and Angeles Espiño for skillful technical assistance.
Footnotes
Published ahead of print on 3 September 2010.
REFERENCES
- 1.Bidet, P., F. Mahjoub-Messai, J. Blanco, J. Blanco, M. Dehem, Y. Aujard, E. Bingen, and S. Bonacorsi. 2007. Combined multilocus sequence typing and O serogrouping distinguishes Escherichia coli subtypes associated with infant urosepsis and/or meningitis. J. Infect. Dis. 196:297-303. [DOI] [PubMed] [Google Scholar]
- 2.Blanco, J., M. P. Alonso, E. A. González, M. Blanco, and J. I. Garabal. 1990. Virulence factors of bacteraemic Escherichia coli with particular reference to production of cytotoxic necrotising factor (CNF) by P-fimbriate strains. J. Med. Microbiol. 31:175-183. [DOI] [PubMed] [Google Scholar]
- 3.Blanco, J. E., M. Blanco, A. Mora, W. H. Jansen, V. García, M. L. Vázquez, and J. Blanco. 1998. Serotypes of Escherichia coli isolated from septicaemic chickens in Galicia (northwest Spain). Vet. Microbiol. 61:229-235. [DOI] [PubMed] [Google Scholar]
- 4.Blanco, M., M. P. Alonso, M. H. Nicolas-Chanoine, G. Dahbi, A. Mora, J. E. Blanco, C. López, P. Cortés, M. Llagostera, V. Leflon-Guibout, B. Puentes, R. Mamani, A. Herrera, M. A. Coira, F. García-Garrote, J. M. Pita, and J. Blanco. 2009. Molecular epidemiology of Escherichia coli producing extended-spectrum β-lactamases in Lugo (Spain): dissemination of clone O25b:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 63:1135-1141. [DOI] [PubMed] [Google Scholar]
- 5.Cagnacci, S., L. Gualco, E. Debbia, G. C. Schito, and A. Marchese. 2008. European emergence of ciprofloxacin-resistant Escherichia coli clonal groups O25:H4-ST 131 and O15:K52:H1 causing community-acquired uncomplicated cystitis. J. Clin. Microbiol. 46:2605-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carattoli, A. 2008. Animal reservoirs for extended spectrum beta-lactamase producers. Clin. Microbiol. Infect. 14(Suppl. 1):117-123. [DOI] [PubMed] [Google Scholar]
- 7.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clermont, O., H. Dhanji, M. Upton, T. Gibreel, A. Fox, D. Boyd, M. R. Mulvey, P. Nordmann, E. Ruppé, J. L. Sarthou, T. Frank, S. Vimont, G. Arlet, C. Branger, N. Woodford, and E. Denamur. 2009. Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J. Antimicrob. Chemother. 64:274-277. [DOI] [PubMed] [Google Scholar]
- 9.Coque, T. M., A. Novais, A. Carattoli, L. Poirel, J. Pitout, L. Peixe, F. Baquero, R. Cantón, and P. Nordmann. 2008. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum β-lactamase CTX-M-15. Emerg. Infect. Dis. 14:195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortés, P., V. Blanc, A. Mora, G. Dahbi, J. E. Blanco, M. Blanco, C. López, A. Andreu, F. Navarro, M. P. Alonso, G. Bou, J. Blanco, and M. Llagostera. 2010. Isolation and characterization of potentially pathogenic antimicrobial-resistant Escherichia coli strains from chicken and pig farms in Spain. Appl. Environ. Microbiol. 76:2799-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dho-Moulin, M., and J. M. Fairbrother. 1999. Avian pathogenic Escherichia coli (APEC). Vet. Res. 30:299-316. [PubMed] [Google Scholar]
- 12.Ewers, C., G. Li, H. Wilking, S. Kiessling, K. Alt, E. M. Antáo, C. Laturnus, I. Diehl, S. Glodde, T. Homeier, U. Böhnke, H. Steinrück, H. C. Philipp, and L. H. Wieler. 2007. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: how closely related are they? Int. J. Med. Microbiol. 297:163-176. [DOI] [PubMed] [Google Scholar]
- 13.Ewers, C., M. Grobbel, I. Stamm, P. A. Kopp, I. Diehl, T. Semmler, A. Fruth, J. Beutlich, B. Guerra, L. H. Wieler, and S. Guenther. 2010. Emergence of human pandemic O25:H4-ST131 CTX-M-15 extended-spectrum-beta-lactamase-producing Escherichia coli among companion animals. J. Antimicrob. Chemother. 65:651-660. [DOI] [PubMed] [Google Scholar]
- 14.Germon, P., Y. H. Chen, L. He, J. E. Blanco, A. Brée, C. Schouler, S. H. Huang, and M. Moulin-Schouleur. 2005. ibeA, a virulence factor of avian pathogenic Escherichia coli. Microbiology 51:1179-1186. [DOI] [PubMed] [Google Scholar]
- 15.Guinée, P. A. M., W. H. Jansen, T. Wadström, and R. Sellwood. 1981. Escherichia coli associated with neonatal diarrhoea in piglets and calves, p. 126-162. In P. W. Leeww and P. A. M. Guinée (ed.), Laboratory diagnosis in neonatal calf and pig diarrhoea. Current topics in veterinary and animal science. Martinus-Nijhoff, The Hague, Netherlands.
- 16.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, J. R., A. C. Murray, A. Gajewski, M. Sullivan, P. Snippes, M. A. Kuskowski, and K. E. Smith. 2003. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob. Agents Chemother. 47:2161-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, J. R., and T. A. Russo. 2005. Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int. J. Med. Microbiol. 295:383-404. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, J. R., J. S. McCabe, D. G. White, B. Johnston, M. A. Kuskowski, and P. McDermott. 2009. Molecular analysis of Escherichia coli from retail meats (2002-2004) from the United States National Antimicrobial Resistance Monitoring System. Clin. Infect. Dis. 49:195-201. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, J. R., M. Menard, B. Johnston, M. A. Kuskowski, K. Nichol, and G. G. Zhanel. 2009. Epidemic clonal groups of Escherichia coli as a cause of antimicrobial-resistant urinary tract infections in Canada, 2002 to 2004. Antimicrob. Agents Chemother. 53:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, J. R., S. Miller, B. Johnston, C. Clabots, and C. Debroy. 2009. Sharing of Escherichia coli sequence type ST131 and other multidrug-resistant and urovirulent E. coli strains among dogs and cats within a household. J. Clin. Microbiol. 47:3721-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, J. R., B. Johnston, C. Clabots, M. A. Kuskowski, S. Pendyala, C. Debroy, B. Nowicki, and J. Rice. 2010. Escherichia coli sequence type ST131 as an emerging fluoroquinolone-resistant uropathogen among renal transplant recipients. Antimicrob. Agents Chemother. 54:546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, K. S. 2002. Strategy of Escherichia coli for crossing the blood-brain barrier, J. Infect. Dis. 186(Suppl. 2):S220-S224. [DOI] [PubMed] [Google Scholar]
- 24.Kim, K. J., S. J. Elliott, F. Di Cello, M. F. Stins, and K. S. Kim. 2003. The K1 capsule modulates trafficking of E. coli-containing vacuoles and enhances intracellular bacterial survival in human brain microvascular endothelial cells. Cell. Microbiol. 5:245-252. [DOI] [PubMed] [Google Scholar]
- 25.Leflon-Guibout, V., C. Jurand, S. Bonacorsi, F. Espinasse, M. C. Guelfi, F. Duportail, B. Heym, E. Bingen, and M. H. Nicolas-Chanoine. 2004. Emergence and spread of three clonally related virulent isolates of CTX-M-15-producing Escherichia coli with variable resistance to aminoglycosides and tetracycline in a French geriatric hospital. Antimicrob. Agents Chemother. 48:3736-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leflon-Guibout, V., J. Blanco, K. Amaqdouf, A. Mora, L. Guize, and M. H. Nicolas-Chanoine. 2008. Absence of CTX-M enzymes but high prevalence of clones, including clone ST131, among fecal Escherichia coli isolates from healthy subjects living in the area of Paris, France. J. Clin. Microbiol. 46:3900-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manges, A. R., H. Tabor, P. Tellis, C. Vincent, and P. P. Tellier. 2008. Endemic and epidemic lineages of Escherichia coli that cause urinary tract infections. Emerg. Infect. Dis. 14:1575-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Medina, M., A. Mora, M. Blanco, C. López, M. P. Alonso, S. Bonacorsi, M. H. Nicolas-Chanoine, A. Darfeuille-Michaud, J. Garcia-Gil, and J. Blanco. 2009. Similarity and divergence among adherent-invasive Escherichia coli and extraintestinal pathogenic E. coli strains. J. Clin. Microbiol. 47:3968-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mora, A., C. López, G. Dabhi, M. Blanco, J. E. Blanco, M. P. Alonso, A. Herrera, R. Mamani, S. Bonacorsi, M. Moulin-Schouleur, and J. Blanco. 2009. Extraintestinal pathogenic Escherichia coli O1:K1:H7/NM from human and avian origin: detection of clonal groups B2 ST95 and D ST59 with different host distribution. BMC Microbiol. 9:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moulin-Schouleur, M., C. Schouler, P. Tailliez, M. R. Kao, A. Brée, P. Germon, E. Oswald, J. Mainil, M. Blanco, and J. Blanco. 2006. Common virulence factors and genetic relationships between O18:K1:H7 Escherichia coli isolates of human and avian origin. J. Clin. Microbiol. 44:3484-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moulin-Schouleur, M., M. Répérant, S. Laurent, A. Brée, S. Mignon-Grasteau, P. Germon, D. Rasschaert, and C. Schouler. 2007. Extraintestinal pathogenic Escherichia coli strains of avian and human origin: link between phylogenetic relationships and common virulence patterns. J. Clin. Microbiol. 45:3366-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NCCLS/CLSI. 2008. Performance standards for antimicrobial disc susceptibility testing: 18th informational supplement. M100-S18. NCCLS/CLSI, Wayne, PA.
- 33.Nicolas-Chanoine, M. H., J. Blanco, V. Leflond-Guibout, R. Demarty, M. P. Alonso, M. M. Caniça, Y. J. Park, J. P. Lavigne, J. Pitout, and J. R. Johnson. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61:273-281. [DOI] [PubMed] [Google Scholar]
- 34.Oteo, J., K. Diestra, C. Juan, V. Bautista, A. Novais, M. Pérez-Vázquez, B. Moyá, E. Miró, T. M. Coque, A. Oliver, R. Cantón, F. Navarro, J. Campos, and the Spanish Network in Infectious Pathology Project (REIPI). 2009. Extended-spectrum beta-lactamase-producing Escherichia coli in Spain belong to a large variety of multilocus sequence typing types, including ST10 complex/A, ST23 complex/A and ST131/B2. Int. J. Antimicrob. Agents 34:173-176. [DOI] [PubMed] [Google Scholar]
- 35.Peirano, G., and J. D. Pitout. 2010. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int. J. Antimicrob. Agents 35:316-321. [DOI] [PubMed] [Google Scholar]
- 36.Pitout, J. D., D. B. Gregson, L. Campbell, and K. B. Laupland. 2009. Molecular characteristics of extended-spectrum-beta-lactamase-producing Escherichia coli isolates causing bacteremia in the Calgary Health Region from 2000 to 2007: emergence of clone ST131 as a cause of community-acquired infections. Antimicrob. Agents Chemother. 53:2846-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pomba, C., J. D. da Fonseca, B. C. Baptista, J. D. Correia, L. Martínez-Martínez. 2009. Detection of the pandemic O25-ST131 human virulent Escherichia coli CTX-M-15-producing clone harboring the qnrB2 and aac(6′)-Ib-cr genes in a dog. Antimicrob. Agents Chemother. 53:327-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stordeur, P., D. Marlier, J. Blanco, E. Oswald, F. Biet, M. Dho-Moulin, and J. Mainil. 2002. Examination of Escherichia coli from poultry for selected adhesin genes important in disease caused by mammalian pathogenic E. coli. Vet. Microbiol. 84:231-241. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki, S., N. Shibata, K. Yamane, J. Wachino, K. Ito, and Y. Arakawa. 2009. Change in the prevalence of extended-spectrum-beta-lactamase-producing Escherichia coli in Japan by clonal spread. J. Antimicrob. Chemother. 63:72-79. [DOI] [PubMed] [Google Scholar]
- 40.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent, C., P. Boerlin, D. Daignault, C. M. Dozois, L. Dutil, C. Galanakis, R. J. Reid-Smith, P. P. Tellier, P. A. Tellis, K. Ziebell, and A. R. Manges. 2010. Food reservoir for Escherichia coli causing urinary tract infections. Emerg. Infect. Dis. 16:88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]