Abstract
Recent research in microbe-insect symbiosis has shown that acetic acid bacteria (AAB) establish symbiotic relationships with several insects of the orders Diptera, Hymenoptera, Hemiptera, and Homoptera, all relying on sugar-based diets, such as nectars, fruit sugars, or phloem sap. To date, the fruit flies Drosophila melanogaster and Bactrocera oleae, mosquitoes of the genera Anopheles and Aedes, the honey bee Apis mellifera, the leafhopper Scaphoideus titanus, and the mealybug Saccharicoccus sacchari have been found to be associated with the bacterial genera Acetobacter, Gluconacetobacter, Gluconobacter, Asaia, and Saccharibacter and the novel genus Commensalibacter. AAB establish symbiotic associations with the insect midgut, a niche characterized by the availability of diet-derived carbohydrates and oxygen and by an acidic pH, selective factors that support AAB growth. AAB have been shown to actively colonize different insect tissues and organs, such as the epithelia of male and female reproductive organs, the Malpighian tubules, and the salivary glands. This complex topology of the symbiosis indicates that AAB possess the keys for passing through body barriers, allowing them to migrate to different organs of the host. Recently, AAB involvement in the regulation of innate immune system homeostasis of Drosophila has been shown, indicating a functional role in host survival. All of these lines of evidence indicate that AAB can play different roles in insect biology, not being restricted to the feeding habit of the host. The close association of AAB and their insect hosts has been confirmed by the demonstration of multiple modes of transmission between individuals and to their progeny that include vertical and horizontal transmission routes, comprising a venereal one. Taken together, the data indicate that AAB represent novel secondary symbionts of insects.
Acetic acid bacteria (AAB), especially members of the genera Acetobacter and Gluconacetobacter, have a significant historical role in human activities, having been used over millennia for the production of vinegar for consumption and for medicinal purposes. AAB of the family Acetobacteraceae are ubiquitous and are known to be adapted to various sugar- and ethanol-rich environments. AAB are obligate aerobes, and most of them are unable to oxidize ethanol, sugars, and polyalcohols completely, accumulating large amounts of the corresponding oxidation products in their culture medium. Their oxidative capacity is commercially exploited not only for vinegar production but also in the manufacture of foods and chemical compounds, i.e., kombucha tea, cocoa, sorbose, gluconic acid, etc. (40).
AAB can be isolated from a variety of substrates and natural environments like plants, flowers, herbs, fruits, and fermented foods and beverages. Great attention has recently been directed toward symbiotic associations between AAB and insect hosts, which are emerging as a novel niche for AAB isolation. Interestingly, these prokaryotes have been reported to be associated with insects that rely on sugar-based diets, in particular those belonging to the orders Diptera, Hymenoptera, and Hemiptera (13).
Symbiotic associations can profoundly affect the evolutionary history, lifestyle, and physiology of organisms. Consequently, in recent years, the interactions between insects and microorganisms have received considerable attention (53). The remarkable adaptability of insects to very diverse terrestrial habitats, comprising those that are nutritionally limited or unbalanced, has contributed to their evolutionary success in different ecological environments. In many cases, microbial partners allow their insect hosts to specialize in nutrient-deficient food resources, giving them competitive advantages (24). For example, insects that feed exclusively on nutritionally poor diets such as plant sap, vertebrate blood, or woody material possess microbial endosymbionts. Arthropod-associated microorganisms play numerous different roles besides the nutritional aspects, influencing development, reproduction and speciation, defense against natural enemies, and immunity (14). For example, the aphid facultative endosymbiont Hamiltonella defensa increases the protection of its host from parasitoid wasps (56).
In this minireview, a description of the currently available information about AAB recovered from insects is presented. The main insect sources so far for AAB are bees, mosquitoes, fruit flies, and sugarcane mealybugs; it is, however, likely that the number of insect species found to harbor AAB will significantly increase in the near future. Since the beginning of the 20th century, it was well known that gluconobacters, environmental sugar-loving microbes, represent a significant component of the honeybee microflora (65), while only more recently AAB were found to also inhabit mosquitoes, fruit flies, and leafhoppers.
The family Acetobacteraceae comprises a large variety of species. Since the description of Acetobacter Beijerinck (7) and Gluconobacter Asai (1), the taxonomy of AAB has undergone many changes, especially in the last 30 years. These changes are due to the new species ascribed to the family, the reclassification of strains (67), and the development and introduction of novel technologies for microbial taxonomy. Early classification systems were mainly based on the analyses of morphological and biochemical characteristics. Nowadays, taxonomic classification is based on what is called “polyphasic taxonomy,” in which independent approaches such as phenotypic, chemotaxonomic, and genotyping analyses are combined (10). According to this classification approach, the AAB of the family Acetobacteraceae are classified into 13 genera: Acetobacter, Gluconobacter, Gluconacetobacter, Acidomonas, Asaia, Kozakia (40), Swaminathania (45), Saccharibacter (37), Neoasaia (69), Granulibacter (33, 34), Commensalibacter (60), Tanticharoenia (68), and Ameyamaea (70).
WHICH INSECT HOSTS DO AAB COLONIZE?
Bees were the first insects from which AAB were recovered (65). Bees and the products of beekeeping have been extensively studied over the years, taking into account the microbiological perspective (31). Among the great variety of microorganisms that were identified and isolated, Gluconobacter, Gluconacetobacter, and Acetobacter are some of the predominant bacterial groups of the bee microbiota (4, 36, 50, 51). Another hymenopteran, the endoparasitoid wasp Asobara tabida, hosts several members of the Acetobacteraceae family, represented by Acetobacter (A.) pasteurianus and Acidomonas (Ac.) methanolica, as reported by Zouache et al. (71).
Drosophila melanogaster, the most widely used insect model organism, hosts a rich AAB microbiome (11, 12, 58, 61). Analyzing the bacterial community associated with laboratory-reared and wild-captured D. melanogaster by establishing 16S rRNA gene libraries, Cox and Gilmore (12) showed that Acetobacter represented one of the most abundant genera, with 29% of the clones analyzed. The following species were identified: A. aceti, A. cerevisiae, A. pasteurianus, A. pomorum, and A. peroxydans, together with several species of Gluconobacter and Gluconacetobacter. In the olive fruit fly Bactrocera oleae, which is phylogenetically close to Drosophila, Acetobacter symbionts also dominate, with the single species A. tropicalis (42). Combining cultivation-dependent and -independent techniques with ultrastructural and microscopic analyses, the authors detected the symbionts in all laboratory and field-collected individuals originating from different locations in Greece. The investigation revealed that several A. tropicalis strains can coexist in a single fly.
A recently studied AAB-insect symbiosis is the association between the cultivable Asaia spp. and the pathogen-transmitting mosquitoes Anopheles (An.) stephensi, An. maculipennis, An. gambiae, and Aedes aegypti (13, 22). Asaia sp. was found as a dominant bacterium within the insect microbial community by the use of several techniques. Asaia sp. was also identified in the bacterial community of the leafhopper Scaphoideus titanus, the vector of flavescence dorèe in grapes (13, 46). Assessed by quantitative PCR, 16S rRNA genes of Asaia sp. constituted 4.9% of the total bacterial 16S rRNA gene copies per leafhopper. Ashbolt and Inkerman (2) detected the presence of AAB in another leafhopper, Perkinsiella saccharidica, for which an AAB community of 5 × 103 cells per individual was estimated.
Other insects in which Asaia sp. symbionts were detected by cultivation-independent techniques are the hymenopteran Marietta leopardina, a hyperparasitoid (47), and the lepidopteron Pieris rapae, the cabbage white butterfly (59).
The pink sugarcane mealybug Saccharicoccus sacchari is a common insect found in sugarcane-growing countries which is able to host several members of the family Acetobacteraceae (2). At least 106 AAB are typically carried by an adult female mealybug actively feeding on aerial storage, whereas fewer than 104 bacteria per insect are maintained in less active adults and individuals from underground mealybug populations. AAB isolates from S. sacchari were identified as A. aceti, Gluconacetobacter (Ga.) diazotrophicus, Ga. liquefaciens, and Ga. sacchari by Franke et al. (26). Further experiments, though, led the authors to the conclusion that AAB are only a relatively small proportion of the microbial community in S. sacchari (25, 27, 28). The presence of AAB in other mealybugs like Planococcus sp. and Dysmicoccus brevis was also demonstrated (2).
A list of AAB that colonize insect hosts is given in Table 1. It should be emphasized that all of these insects rely on sugar-based diets such as nectars, fruit sugars, or phloem sap.
TABLE 1.
AAB inhabit several insect hosts belonging to different orders like Diptera, Hymenopera, Hemiptera, and Homoptera
| AAB and insect host(s) | Reference(s) |
|---|---|
| Acetobacter sp. | |
| D. melanogaster (Diptera: Drosophilidae) | 11, 12, 58, 61 |
| A. mellifera (Hymenoptera: Apidae) | 4, 50 |
| B. oleae (Diptera: Tephritidae) | 42 |
| A. tabida (Hymenoptera: Braconidae) | 71 |
| S. sacchari (Homoptera: Pseudococcidae) | 2 |
| C. intestini | |
| D. melanogaster (Diptera: Drosophilidae) | 60, 61 |
| Gluconobacter sp. | |
| A. mellifera (Hymenoptera: Apidae) | 4, 51 |
| D. melanogaster (Diptera: Drosophilidae) | 11, 12, 58, 60, 61 |
| S. sacchari (Homoptera: Pseudococcidae) | 2 |
| Gluconacetobacter sp. | |
| D. melanogaster (Diptera: Drosophilidae) | 11, 12, 61 |
| A. mellifera (Hymenoptera: Apidae) | 4, 36, 50 |
| S. sacchari (Homoptera: Pseudococcidae) | 2, 25-28 |
| Asaia sp. | |
| Anopheles sp. (Diptera: Culicidae) | 13, 15, 22 |
| A. aegypti (Diptera: Culicidae) | 13 |
| S. titanus (Hemiptera: Cicadellidae) | 13, 46 |
| M. leopardiana (Hymenoptera: Aphelinidae) | 47 |
| P. rapae (Lepidoptera: Pieridae) | 59 |
| Saccharibacter floricola | |
| A. mellifera (Hymenoptera: Apidae) | 51 |
WHICH INSECT ORGANS DO AAB INHABIT?
A major habitat of insect-associated microorganisms is the digestive system, due to the availability of nutrients that are degraded by both host enzymes and microbial activity (17). The microbiota in an insect gut is influenced by structural and physiological factors, as well as by the quality of the ingested food. However, AAB symbionts have been found associated not only with the insect interior but also with the insect surface, as in the case of D. melanogaster (58), underlining their ability to survive in unfavorable environments. Growing on the surface of Drosophila, Acetobacter sp. can have a smaller-than-normal size, as shown by scanning electron microscopy analysis (58). AAB are able to survive other harsh conditions represented, for instance, by starvation or an adverse environment, entering into a viable but not culturable (VBNC) state and reducing the size of the microbial cells (49). Consequently, AAB populations from various substrates can be underestimated when the evaluation is performed only by a culture-based approach. The application of culture-independent techniques such as epifluorescence staining and real-time PCR overcomes this problem (6).
Simple or complex microbial consortia can inhabit the insect gut. For instance, in D. melanogaster, 25 phylotypes with just a few dominant bacterial species, among which are AAB, are associated with the insect gut (12). The anterior hindgut region is the most densely inhabited part of the digestive system, due to the availability of partially digested food coming from the midgut, as well as the products excreted by the Malpighian tubules. It has been estimated that 108 to 109 bacterial cells per g of gut content are present in the honeybee gut (39, 57), while >106 bacteria are typically recovered from an entire old Drosophila fly (58).
The insect gastrointestinal tract (GIT), more structured in the adult stage than in the larval one, is organized similarly in bees, mosquitoes, and fruit flies. The mouth is followed by the esophagus, the ventriculus (stomach), the intestine, the rectum, and the anus. Drosophila flies also possess an acidic crop, together with an alkaline ventriculus and a slightly acidic hindgut, while mosquitoes have three diverticula that arise near the terminal end of the esophagus. The ventral one, also called a crop, is acidic and able to enlarge into the abdomen. A sugar meal such as floral nectar is stored in the diverticula and passes slowly to the midgut for the digestion step (63). Bees possess a honey stomach, an enlargement of the esophagus in which nectar is stored during flight and which ends with the proventriculus. This structure avoids contamination of the nectar with the contents of the stomach, which follows the proventriculus (66). The adult bee gut is normally acidic in comparison to the larval one. The pH is reported to vary from 4.8 to 7.0, depending on the acidity of the ingested pollen. Mohr and Tebbe (50) reported pH values of approximately 4.0 for larvae fed with worker jelly, as well as with nectar and honey.
In the insect digestive system, AAB find a suitable environment in which they flourish and reproduce, mainly due to the aerobic environment, the acidic pH, and the presence of diet-derived sugars. Moreover, AAB establish a tight association with insect epithelial cells. AAB are known to produce polysaccharidic matrices that are involved in microbial interactions. One of the polysaccharides produced by bacteria is cellulose, which, in animal pathogens, is implicated in biofilm formation, in multicellular behavior, in adhesion to animal cells, or in stress tolerance (5). Several members of the family Acetobacteraceae possess the ability to produce cellulose. Ga. xylinus is the best-known cellulose producer, but other examples can be found, such as Ga. kombuchae, Ga. swingsii, Ga. rhaeticus, Ga. nataicola, and some strains of Ga. hansenii, Ga. europeus, and Ga. oboediens (21, 44). The presence of a cellulose operon has also been reported for a strain of Asaia bogorensis isolated from tropical flowers (NCBI accession no. AB355706).
Investigations by transmission electron microscopy (TEM) of the microbiome associated with the epithelia of mosquitoes and other insects showed an extensive mass of extracellular polysaccharidic matrix around the AAB symbionts (13, 22, 42, 62). This matrix establishes tight contact between the microbial cells and the host epithelium, implying a role in microbial interaction with host surfaces. Crotti et al. (13) also discussed the possibility that this extracellular matrix could protect the bacterial cell from adverse conditions such as alkaline or acidic pH or high osmolarity. Indeed, several members of the family Acetobacteraceae (Tanticharoenia, Acidomonas, Asaia, Saccharibacter, Swaminathania, and Neoasaia spp.) show the ability to grow at high osmolarity (30% glucose), whereas only weak growth under this condition is reported for Kozakia sp. (69, 70).
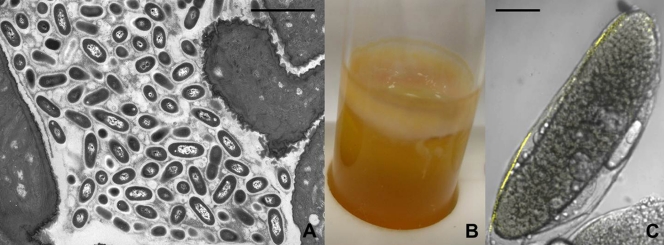
Asaia sp., a dominating bacterium in the microbiota associated with Anopheles sp. and Ae. aegypti mosquitoes and with the leafhopper S. titanus, was analyzed by TEM and in situ hybridization (ISH), proving its ability to produce a gelatinous matrix around the bacterial cells in contact with the host gut epithelium (13, 22, Fig. 1 A). The capability of this strain to colonize and lodge in the gut system was also demonstrated using strains labeled with fluorescent proteins (13, 22). It was shown that the symbiont produces a thick pellicle when grown in glass tubes without shaking (Fig. 1B), a feature typically found in AAB. For instance, when AAB occur as spoilers in bottled wine, a distinctive ring of bacterial biomass is deposited in the neck of the bottle at the interface between the wine and the air headspace (6). Asaia sp. is intimately associated not only with the insect gut epithelium but also with the epithelia of the reproductive systems of both females and males, as well as with the salivary glands, as shown by TEM, ISH, and colonization experiments with Asaia sp. strains tagged with fluorescent proteins. In particular, in S. titanus, Asaia sp. was shown to be harbored in the testicles, intermixed within the spermatic bundles and in the Malpighian tubules associated with brochosomes, while in mosquitoes, the male epithelial genital duct was observed to be lined by microcolonies of Asaia sp. cells (13, 22). In B. oleae, A. tropicalis was found to be tightly entrapped in a brown gelatinous matrix within the peritrophic membrane (42), confirming that the main site of microbial colonization in B. oleae is the gut lumen inside the peritrophic matrix (48). Also, A. tropicalis, when grown in pure culture in a glass tube without shaking, forms a thick pellicle at the liquid-air interface.
FIG. 1.
(A) Asaia sp. colonizes the gut epithelium of Anopheles sp., Ae. aegypti, and S. titanus, establishing a specific association with the insect epithelium mediated by an extracellular polysaccharidic matrix that surrounds Asaia sp. cells. Bar, 3.4 μm. (B) Asaia sp. produces a thick pellicle when cultured in a glass tube without shaking for 10 days. (C) Asaia sp. has been found to be strictly associated with the surface of immature eggs in S. titanus and An. gambiae, as shown by FISH with Asaia sp.-specific fluorescent probes (Asaia sp.-specific probes for S. titanus are shown in yellow), suggesting egg-smearing transmission of Asaia sp. in its hosts. Bar, 400 μm.
TRANSMISSION ROUTES OF AAB SYMBIONTS IN INSECTS
In order to ensure bacterial transmission to different individuals and the next generation, insects have evolved a wide array of mechanisms. Among these are transovarian transmission (i.e., through the mother oocytes) and posthatch mechanisms like “egg smearing” (i.e., eggs are contaminated by the symbionts on the surface, and thus the hatched larvae acquire the symbionts by consuming or probing the eggshell) and “coprophagy” (i.e., preadult stage individuals feed on adult excrement, reacquiring symbionts), although other unique modes of symbiont transmission exist (3, 41).
Most of the knowledge on the transmission routes followed by AAB in their hosts derives from studies on Asaia sp. symbionts (13, 15). Colonization experiments performed by feeding An. stephensi, An. gambiae, Ae. aegypti, and S. titanus with green fluorescent protein- or DsRed-labeled Asaia sp. showed a horizontal transmission route with rapid colonization of the gut, salivary glands, and reproductive organs. Asaia sp. can also be passed horizontally through feeding between insects that belong to phylogenetically distant species. For instance, it was shown that Asaia sp. from Anopheles spp. is able to cross colonize other sugar-feeding insects like Ae. aegypti and the hemipteran S. titanus (13).
These investigations showed that several routes occur simultaneously (15). Environmental acquisition appears to be an important way of transmission, aided by the ubiquitous nature of the symbiont, but transmission from the mother to the offspring and from the father to the mother and then to the offspring also occurs (15). An egg-smearing mode of vertical transmission of Asaia sp. in S. titanus was proven by fluorescence ISH (FISH) and colonization experiments with strains labeled with fluorescent proteins (Fig. 1C) (13). During ovarian egg development, an increasing ordered disposition of the superficial bacterial biomass is observable: from a more disperse pattern at the initial developmental stages, the bacterial cells are finally confined to the apical egg regions.
Transfer of Asaia sp. from larvae to pupae and from pupae to adults was also demonstrated (15). It is likely that during insect metamorphosis, Asaia sp. can be transtadially transmitted and escapes the reduction/removal of midgut bacteria that normally occurs during development, perhaps residing at other sites (and thus not being included in the meconium), or being resistant to the antimicrobial exuvial fluid that is ingested as part of the ecdysial process (52).
THE INSECT BODY CAN BE AN APPROPRIATE NICHE FOR AAB
The presence of AAB in the insect digestive system and in other organs reflects the physiochemical properties of these habitats (such as the type of food ingested, redox conditions, and pH) and the metabolic responses and capabilities of these microorganisms (17).
The distribution of arthropod-associated AAB in the different insect species reflects their diverse preference of carbon sources acquired by insect nutrition. Gluconobacter sp., preferring sugars, is generally isolated from honeybees, while ethanol-loving Acetobacter sp. is mainly recovered from Drosophila and Bactrocera flies. Nectar and honey are basically sugar solutions composed of sucrose, glucose, and fructose (9, 64), thus being favorable substrates for Gluconobacter sp. On the other hand, Drosophila flies are attracted by fermenting fruits that are a more suitable environment for acetobacters. Drosophila flies are mostly fungivores, and their association with fruit is indirect in that they primarily eat yeasts that live in rotting fruit. Drosophila flies are also called vinegar flies because they are attracted to the acidic odor of fermentation. Moreover, insects from which AAB are usually recovered have sugar-rich diets that are unbalanced for nitrogen content, so they have to fulfill the nitrogen requirement from other sources, such as the atmosphere (55). Among AAB, several examples of N2-fixing bacteria have been reported, such as Ga. diazotrophicus (32, 66), Ga. johannae, Ga. azotocaptans (30), Ga. kombuchae (21), A. peroxydans (54), A. nitrogenifigens (20), and Swaminathania salitolerans (45). However, further investigations are needed to evaluate if insect-associated AAB could contribute to insect nitrogen metabolism or recycling. The ability of several AAB strains to grow on media devoid of vitamins (16) also suggests the possibility that AAB in insects could synthesize vitamins or cofactors utilized by the host. A. pasteurianus, A. peroxydans, A. estunensis; Ga. liquefaciens, As. bogorensis (43), and Asaia sp. isolated from An. stephensi have been showed to be prototrophic with respect to vitamins. On the other hand, experimental removal of AAB from their insect hosts (as in the case of the Asaia-Anopheles system) did not appear to be detrimental to the host, underlining the secondary symbiont status of these bacteria.
The GIT and circulatory system of AAB-associated insects lack anoxic niches (8, 12). Studies on the gut microbiota associated with Drosophila or Apis spp. confirmed the absence of obligate anaerobic bacteria and the presence of aerobic, facultatively aerobic, or aerotolerant bacteria (12, 50). AAB have a respiratory metabolism that requires oxygen as the final electron acceptor, but they are also able to survive in environments with low oxygen availability (19, 38, 49). Hence, they can find a favorable environment in the insect gut.
Within the insect, AAB, as well as other symbiotic microorganisms, have to face the host innate immune system (35). According to the results obtained by Ryu et al. (61), the AAB microbiome is involved in modulating Drosophila immunity. The authors described a delicate equilibrium between the gut commensals and the fly innate immune system. The normal flora in the fly gut was sufficient to suppress the growth of pathogenic bacteria, maintaining the pathogenic commensal Gluconobacter (G.) morbifer at a low level. When the system was perturbed, an increased number of G. morbifer bacteria led to gut apoptosis. Hence, in healthy individuals, the immune system allows the dominance of two AAB strains (A. pomorum and Commensalibacter intestini), which suppress the proliferation of G. morbifer by competition. Another study gained insight into the interaction between the acetic acid bacterium Asaia sp. and the innate immune system of An. gambiae (18). When the expression of a host gene involved in the innate immune response (AgDscam) gene was silenced, Asaia sp., whose favorable site of colonization is the gut (22), was no longer controlled by the innate immune system and proliferated massively in the host hemolymph (18).
AAB are considered fastidious microorganisms due to their difficult isolation and cultivation on synthetic media, regardless of the great number of media proposed for their growth (6). Moreover, they have been shown to be capable of entering the VBNC state (6, 49). It was not possible to isolate Asaia sp. from S. titanus leafhoppers, in spite of the well-documented presence of the symbiont in the insect as assayed with several cultivation-independent techniques (13). The authors speculated that the failure to isolate Asaia sp. might have been due to the unfavorable environment represented by S. titanus in a not actively feeding state or due to the development of unknown nutritional requirements by the bacteria.
CONCLUDING REMARKS AND PERSPECTIVES
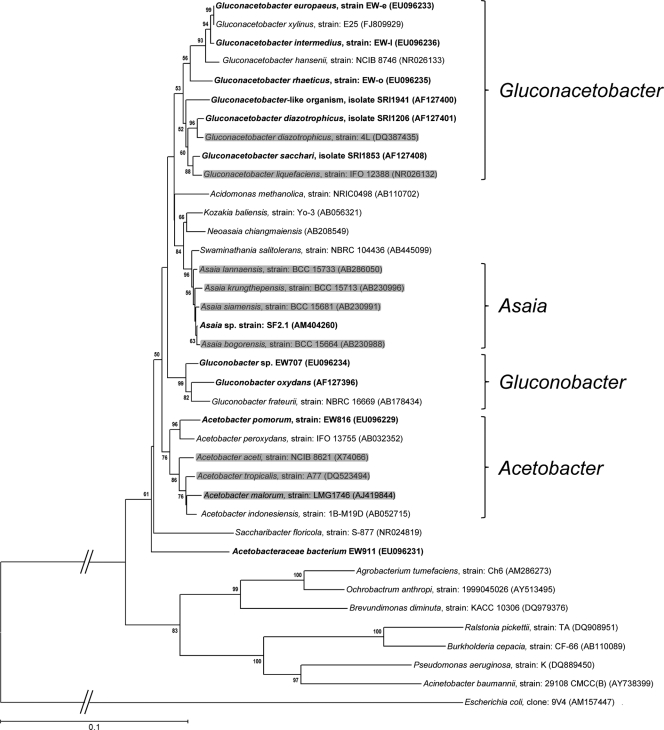
For a long time, AAB have been considered environmental and ubiquitous bacteria. However, an increasing number of reports of stable associations of AAB with insects and the fact that they follow several transmission routes for their propagation in the next insect generation indicate that insect-associated AAB cannot be considered just environmental microorganisms but are also symbionts of the insect body, where they occupy a specific favorable niche. Phylogenetic studies of insect-associated AAB and those recovered from the environment do not show phylogenetic congruence of the insect-associated AAB (Fig. 2). This suggests that AAB have been acquired from the environment by their insect hosts recently (14). Moreover, not being essential for host survival, AAB have to be considered secondary symbionts of insects.
FIG. 2.
Phylogenetic positions of insect-associated AAB among the most representative members of family Acetobacteraceae. The tree shown is based on bacterial 16S rRNA gene sequences. AAB recovered from insects are in bold. Shaded species belong to clusters including environmental AAB, as well as those recovered from insects. Values at nodes are percentages of bootstrap replications calculated from 2,000 replicate trees. Accession numbers of reference sequences are in parentheses. The species used as the outgroup belongs to the Gammaproteobacteria taxon. The scale bar represents 10% sequence divergence.
Clarification of the function(s) exerted by the bacteria in and for their hosts will be a major step toward understanding the bacterium-insect association. AAB are probably involved in many aspects of insect biology, such as (i) the host metabolism by supplying nutrients or by oxidizing certain substrates, (ii) defense against harmful microorganisms by decreasing the gut pH or by competitive exclusion, (iii) contributing to the maintenance of host gut homeostasis (61), (iv) interference with cell-to-cell communication through the production of volatile compounds, and (v) maintaining an equilibrium of the microbial consortia by supplying metabolites to other microbes beneficial to the host. Genome sequencing of the insect-associated AAB will be a particularly valuable tool in this context.
Not only are further studies needed to clarify the role of insect-associated AAB, but efforts should also be directed toward understanding AAB biological diversity and behavior in the host. An interesting topic could be the investigation of body invasion mechanisms adopted by AAB, whether they follow tissue tropism, as shown for the alphaproteobacterial symbiont Wolbachia sp. (29).
Symbiont biology receives increasing attention since insect symbionts can potentially be used to control vector-borne diseases or suppress insect pests. AAB could be used as agents for natural or paratransgenic symbiotic control. Bacteria like Asaia spp. or A. tropicalis possess features interesting in this context, like easy cultivability, preservability, transformability, and dominance and prevalence in the insect microbiome. Moreover, vertical and horizontal transmission routes and cross-colonizing capability could ensure the spread of these biological agents through host populations (23).
Acknowledgments
We thank for financial support the European Union in the ambit of project BIODESERT (European Community's Seventh Framework Programme CSA-SA REGPOT-2008-2 under grant agreement 245746) and the Italian Ministry for Research (MIUR) in the ambit of project PRIN 2007 Caratterizzazione del microbiota associato a Scaphoideus titanus e Hyalesthes obsoletus, cicaline vettrici di fitoplasmi nella vite ed isolamento e studio della localizzazione di batteri acetici simbionti. K.B. is grateful to the European Union (European Community's Seventh Framework Programme CSA-SA_REGPROT-2007-1 under grant agreement 203590), the International Atomic Energy Agency, and the University of Ioannina, which have supported the research in his laboratory. C.B., K.B., A.C., E.C., and D.D. benefited from travel grants from Cost Action FA0701, Arthropod Symbiosis: from Fundamental Studies to Pest and Disease Management.
We thank Stefan Oehler for his comments.
Footnotes
Published ahead of print on 17 September 2010.
REFERENCES
- 1.Asai, T. 1935. Taxonomic studies on acetic acid bacteria and allied oxidative bacteria isolated from fruits. A new classification of the oxidative bacteria. J. Agric. Chem. Soc. Jpn. 11:674-708. (In Japanese.) [Google Scholar]
- 2.Ashbolt, N. J., and P. A. Inkerman. 1990. Acetic acid bacteria biota of the pink sugar cane mealybug, Saccharococcus sacchari, and its environs. Appl. Environ. Microbiol. 56:707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attardo, G. M., C. Lohs, A. Heddi, U. H. Alam, S. Yildirim, and S. Aksoy. 2008. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J. Insect Physiol. 54:1236-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babendreier, D., D. Joller, J. Romeis, F. Bigler, and F. Widmer. 2007. Bacterial community structures in honeybee intestines and their response to two insecticidal proteins. FEMS Microbiol. Ecol. 59:600-610. [DOI] [PubMed] [Google Scholar]
- 5.Barak, J. D., C. E. Jahn, D. L. Gibson, and A. O. Charkowsky. 2007. The role of cellulose and O-antigen capsule in the colonization of plants by Salmonella enterica. Mol. Plant Microbe Interact. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 6.Bartowsky, E. J., and P. A. Henschke. 2008. Acetic acid bacteria spoilage of bottled red wine—a review. Int. J. Food Microbiol. 125:60-70. [DOI] [PubMed] [Google Scholar]
- 7.Beijerinck, M. W. 1898. Über die Arten der Essigbakterien. Zentralbl. Bakteriol. Naturwiss. 4:209-216. [Google Scholar]
- 8.Bodenstein, D., K. W. Cooper, G. F. Ferris, A. Miller, D. F. Poulson, and B. P. Sonnenblick. 1950. Biology of Drosophila. John Wiley & Sons, Inc., New York, NY.
- 9.Chalcoff, V. R., M. A. Aizen, and L. Galetto. 2006. Nectar concentration and composition of 26 species from the temperate forest of South America. Ann. Bot. 97:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleenwerck, I., and P. De Vos. 2008. Polyphasic taxonomy of acetic acid bacteria: an overview of the currently applied methodology. Int. J. Food Microbiol. 125:2-14. [DOI] [PubMed] [Google Scholar]
- 11.Corby-Harris, V., A. C. Pontaroli, L. J. Shimkets, J. L. Bennetzen, K. E. Habel, and D. E. Promislow. 2007. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl. Environ. Microbiol. 73:3470-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox, C., and M. Gilmore. 2007. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect. Immun. 75:1565-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotti, E., C. Damiani, M. Pajoro, E. Gonella, A. Rizzi, I. Ricci, I. Negri, P. Scuppa, P. Rossi, P. Ballarini, N. Raddadi, M. Marzorati, L. Sacchi, E. Clementi, M. Genchi, M. Mandrioli, C. Bandi, G. Favia, A. Alma, and D. Daffonchio. 2009. Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically-distant genera and orders. Environ. Microbiol. 11:3252-3264. [DOI] [PubMed] [Google Scholar]
- 14.Dale, C., and N. Moran. 2006. Molecular interactions between bacterial symbionts and their hosts. Cell 126:453-465. [DOI] [PubMed] [Google Scholar]
- 15.Damiani, C., I. Ricci, E. Crotti, P. Rossi, A. Rizzi, P. Scuppa, F. Esposito, C. Bandi, D. Daffonchio, and G. Favia. 2008. Paternal transmission of symbiotic bacteria in malaria vectors. Curr. Biol. 18:R1087-R1088. [DOI] [PubMed] [Google Scholar]
- 16.De Ley, J., and J. Frateur. 1974. Genus Acetobacter Beijerinck, 1898, p. 276-278. In R. E. Buchanan and N. E. Gibbons (ed.), Bergey's manual of determinative bacteriology, 8th ed. The Williams & Wilkins Co., Baltimore, MD.
- 17.Dillon, R. J., and V. M. Dillon. 2004. The gut bacteria of insects: non-pathogenic interactions. Annu. Rev. Entomol. 49:71-92. [DOI] [PubMed] [Google Scholar]
- 18.Dong, Y., H. E. Taylor, and G. Dimopoulos. 2006. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol. 4:1137-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Toit, W. J., and I. S. Pretorius. 2002. The occurrence, control and esoteric effect of acetic acid bacteria in winemaking. Ann. Microbiol. 52:155-179. [Google Scholar]
- 20.Dutta, D., and R. Gachhui. 2006. Novel nitrogen-fixing Acetobacter nitrogenifigens sp. nov., isolated from Kombucha tea. Int. J. Syst. Evol. Microbiol. 56:1899-1903. [DOI] [PubMed] [Google Scholar]
- 21.Dutta, D., and R. Gachhui. 2007. Nitrogen-fixing and cellulose-producing Gluconacetobacter kombuchae sp. nov., isolated from Kombucha tea. Int. J. Syst. Evol. Microbiol. 57:353-357. [DOI] [PubMed] [Google Scholar]
- 22.Favia, G., I. Ricci, C. Damiani, N. Raddadi, E. Crotti, M. Marzorati, A. Rizzi, R. Urso, L. Brusetti, S. Borin, D. Mora, P. Scuppa, L. Pasqualini, E. Clementi, M. Genchi, S. Corona, I. Negri, G. Grandi, A. Alma, L. Kramer, F. Esposito, C. Bandi, L. Sacchi, and D. Daffonchio. 2007. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl. Acad. Sci. U. S. A. 104:9047-9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favia, G., I. Ricci, M. Marzorati, I. Negri, A. Alma, L. Sacchi, C. Bandi, and D. Daffonchio. 2008. Bacteria of the genus Asaia: a potential paratransgenic weapon against malaria. Adv. Exp. Med. Biol. 627:49-59. [DOI] [PubMed] [Google Scholar]
- 24.Feldhaar, H., and R. Gross. 2009. Insects as hosts for mutualistic bacteria. Int. J. Med. Microbiol. 299:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke, I. H., M. Fegan, C. Hayward, G. Leonard, and L. I. Sly. 2000. Molecular detection of Gluconacetobacter sacchari associated with the pink sugarcane mealybug Saccharicoccus sacchari (Cockerell) and the sugarcane leaf sheath microenvironment by FISH and PCR. FEMS Microbiol. Ecol. 31:61-71. [DOI] [PubMed] [Google Scholar]
- 26.Franke, I. H., M. Fegan, C. Hayward, G. Leonard, E. Stackebrandt, and L. I. Sly. 1999. Description of Gluconacetobacter sacchari sp. nov., a new species of acetic acid bacterium isolated from the leaf sheath of sugar cane and from the pink sugar-cane mealy bug. Int. J. Syst. Bacteriol. 49:1681-1693. [DOI] [PubMed] [Google Scholar]
- 27.Franke-Whittle, I. H., M. G. O'Shea, G. J. Leonard, and L. I. Sly. 2004. Molecular investigation of the microbial populations of the pink sugarcane mealybug, Saccharicoccus sacchari. Ann. Microbiol. 54:455-470. [Google Scholar]
- 28.Franke-Whittle, I. H., M. G. O'Shea, G. J. Leonard, and L. I. Sly. 2005. Design, development, and use of molecular primers and probes for the detection of Gluconacetobacter species in the pink sugarcane mealybug. Microb. Ecol. 50:128-139. [DOI] [PubMed] [Google Scholar]
- 29.Frydman, H. M., J. M. Li, D. N. Robson, and E. Wieschaus. 2006. Somatic stem cell niche tropism in Wolbachia. Nature 441:509-512. [DOI] [PubMed] [Google Scholar]
- 30.Fuentes-Ramírez, L. E., R. Bustillos-Cristales, A. Tapia-Hernandez, T. Jimenez-Salgado, E. T. Wang, E. Martinez-Romero, and J. Caballero-Mellado. 2001. Novel nitrogen-fixing acetic acid bacteria, Gluconacetobacter johannae sp. nov. and Gluconacetobacter azotocaptans sp. nov., associated with coffee plants. Int. J. Syst. Evol. Microbiol. 51:1305-1314. [DOI] [PubMed] [Google Scholar]
- 31.Gilliam, M. 1997. Identification and roles of non-pathogenic microflora associated with honey bees. FEMS Microbiol. Lett. 155:1-10. [Google Scholar]
- 32.Gillis, M., K. Kersters, B. Hoste, D. Janssens, R. M. Kroppenstedt, M. P. Stephan, K. R. S. Teixeira, J. Dobereiner, and J. De Ley. 1989. Acetobactev diazotrophicus sp. nov., a nitrogen-fixing acetic acid bacterium associated with sugarcane. Int. J. Syst. Bacteriol. 39:361-364. [Google Scholar]
- 33.Greenberg, D. E., L. Ding, A. M. Zelazny, F. Stock, A. Wong, V. L. Anderson, G. Miller, D. E. Kleiner, A. R. Tenorio, L. Brinster, D. W. Dorward, P. R. Murray, and S. M. Holland. 2006. A novel bacterium associated with lymphadenitis in a patient with chronic granulomatous disease. PLoS Pathog. 2:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberg, D. E., S. F. Porcella, F. Stock, A. Wong, P. S. Conville, P. R. Murray, S. M. Holland, and A. M. Zelazny. 2006. Granulibacter bethesdensis gen. nov., sp. nov., a distinctive pathogenic acetic acid bacterium in the family Acetobacteraceae. Int. J. Syst. Evol. Microbiol. 56:2609-2616. [DOI] [PubMed] [Google Scholar]
- 35.Gross, R., F. Vavre, A. Heddi, G. D. Hurst, E. Zchori-Fein, and K. Bourtzis. 2009. Immunity and symbiosis. Mol. Microbiol. 73:751-759. [DOI] [PubMed] [Google Scholar]
- 36.Jeyaprakash, A., M. A. Hoy, and M. H. Allsopp. 2003. Bacterial diversity in worker adults of Apis mellifera capensis and Apis mellifera scutellata (Insecta: Hymenoptera) assessed using 16S rRNA sequences. J. Invertebr. Pathol. 84:96-103. [DOI] [PubMed] [Google Scholar]
- 37.Jojima, Y., Y. Mihara, S. Suzuki, K. Yokozeki, S. Yamanaka, and R. Fudou. 2004. Saccharibacter floricola gen. nov., sp. nov., a novel osmophilic acetic acid bacterium isolated from pollen. Int. J. Syst. Evol. Microbiol. 54:2263-2267. [DOI] [PubMed] [Google Scholar]
- 38.Joyeux, A., S. Lafon-Lafourcade, and P. Ribereau-Gayon. 1984. Evolution of acetic acid bacteria during fermentation and storage of wine. Appl. Environ. Microbiol. 48:153-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kacaniova, M., R. Chlebo, M. Kopernicky, and A. Trakovicka. 2004. Microflora of the honeybee gastrointestinal tract. Folia Microbiol. 49:169-171. [DOI] [PubMed] [Google Scholar]
- 40.Kersters, K., P. Lisdiyanti, K. Komagata, and J. Swings. 2006. The family Acetobacteraceae: the genera Acetobacter, Acidomonas, Asaia, Gluconacetobacter, Gluconobacter, and Kozakia, p. 163-200. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, 3rd ed., vol. 5. Springer, New York, NY. [Google Scholar]
- 41.Kikuchi, Y., and T. Fukatsu. 2009. Insect-bacterium mutualism without vertical transmission, p. 143-161. In K. Bourtzis and T. A. Miller (ed.), symbiosis, vol. 3. CRC Press, Boca Raton, FL. [Google Scholar]
- 42.Kounatidis, I., E. Crotti, P. Sapountzis, L. Sacchi, A. Rizzi, B. Chouaia, C. Bandi, A. Alma, D. Daffonchio, P. Mavragani-Tsipidou, and K. Bourtzis. 2009. Acetobacter tropicalis is a major symbiont in the olive fruit fly Bactrocera oleae. Appl. Environ. Microbiol. 75:3281-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lisdiyanti, P., H. Kawasaki, T. Seki, Y. Yamada, T. Uchimura, and K. Komagata. 2000. Systematic study of the genus Acetobacter with descriptions of Acetobacter indonesiensis sp. nov., Acetobacter tropicalis sp. nov., Acetobacter orleanensis (Henneberg 1906) comb. nov., Acetobacter lovaniensis (Frateur 1950) comb. nov., and Acetobacter estunensis (Carr 1958) comb. nov. J. Gen. Appl. Microbiol. 46:147-165. [DOI] [PubMed] [Google Scholar]
- 44.Lisdiyanti, P., R. R. Navarro, T. Uchimura, and K. Komagata. 2006. Reclassification of Gluconacetobacter hansenii strains and proposals of Gluconacetobacter saccharivorans sp. nov. and Gluconacetobacter nataicola sp. nov. Int. J. Syst. Evol. Microbiol. 56:2101-2111. [DOI] [PubMed] [Google Scholar]
- 45.Loganathan, P., and S. Nair. 2004. Swaminathania salitolerans gen. nov., sp. nov., a salt-tolerant, nitrogen-fixing and phosphate-solubilizing bacterium from wild rice (Porteresia coarctata Tateoka). Int. J. Syst. Evol. Microbiol. 54:1185-1190. [DOI] [PubMed] [Google Scholar]
- 46.Marzorati, M., A. Alma, L. Sacchi, M. Pajoro, S. Palermo, L. Brusetti, N. Raddadi, A. Balloi, R. Tedeschi, E. Clementi, S. Corona, F. Quaglino, P. A. Bianco, T. Beninati, C. Bandi, and D. Daffonchio. 2006. A novel Bacteroidetes symbiont is localized in Scaphoideus titanus, the insect vector of flavescence dorée in Vitis vinifera. Appl. Environ. Microbiol. 72:1467-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matalon, Y., N. Katzir, Y. Gottlieb, V. Portnoy, and E. Zchori-Fein. 2007. Cardinium in Plagiomerus diaspidis (Hymenoptera: Encyrtidae). J. Invertebr. Pathol. 96:106-108. [DOI] [PubMed] [Google Scholar]
- 48.Mazzon, L., A. Piscedda, M. Simonato, I. Martinez-Sañudo, A. Squartini, and V. Girolami. 2008. Presence of specific symbiotic bacteria in flies of the subfamily Tephritinae (Diptera Tephritidae) and their phylogenetic relationships: proposal of ‘Candidatus Stammerula tephritidis’. Int. J. Syst. Evol. Microbiol. 58:1277-1287. [DOI] [PubMed] [Google Scholar]
- 49.Millet, V., and A. Lonvaud-Funel. 2000. The viable but non-culturable state of wine micro-organisms during storage. Lett. Appl. Microbiol. 30:136-141. [DOI] [PubMed] [Google Scholar]
- 50.Mohr, K. I., and C. C. Tebbe. 2006. Diversity and phylotype consistency of bacteria in the guts of three bee species (Apoidea) at an oilseed rape field. Environ. Microbiol. 8:258-272. [DOI] [PubMed] [Google Scholar]
- 51.Mohr, K. I., and C. C. Tebbe. 2007. Field study results on the probability and risk of a horizontal gene transfer from transgenic herbicide-resistant oilseed rape pollen to gut bacteria of bees. Appl. Microbiol. Biotechnol. 75:573-582. [DOI] [PubMed] [Google Scholar]
- 52.Moll, R. M., W. S. Romoser, M. C. Modrzakowski, A. C. Moncayo, and K. Lerdthusnee. 2001. Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J. Med. Entomol. 38:29-32. [DOI] [PubMed] [Google Scholar]
- 53.Moran, N. A. 2006. Symbiosis. Curr. Biol. 16:R866-R871. [DOI] [PubMed] [Google Scholar]
- 54.Muthukumarasamy, R., I. Cleenwerck, G. Revathi, M. Vadivelu, D. Janssens, B. Hoste, K. U. Gum, K. D. Park, C. Y. Son, T. Sa, and J. Caballero-Mellado. 2005. Natural association of Gluconacetobacter diazotrophicus and diazotrophic Acetobacter peroxydans with wetland rice. Syst. Appl. Microbiol. 28:277-286. [DOI] [PubMed] [Google Scholar]
- 55.Nardi, J. B., R. I. Mackie, and J. O. Dawson. 2002. Could microbial symbionts of arthropod guts contribute significantly to nitrogen fixation in terrestrial ecosystems? J. Insect Physiol. 48:751-763. [DOI] [PubMed] [Google Scholar]
- 56.Oliver, K. M., J. A. Russell, N. A. Moran, and M. S. Hunter. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. U. S. A. 100:1803-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rada, V., M. Machova, J. Huk, M. Marounek, and D. Duskova. 1997. Microflora in the honeybee digestive tract: counts, characteristics and sensitivity to veterinary drugs. Apidologie 28:357-365. [Google Scholar]
- 58.Ren, C., P. Webster, S. E. Finkel, and J. Tower. 2007. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 6:144-152. [DOI] [PubMed] [Google Scholar]
- 59.Robinson, C. J., P. Schloss, Y. Ramon, K. Raffa, and J. Handelsman. 2010. Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb. Ecol. 59:199-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roh, S. W., Y.-D. Nam, H.-W. Chang, K.-H. Kim, M.-S. Kim, J.-H. Ryu, S.-H. Kim, W.-J. Lee, and J.-W. Bae. 2008. Characterization of two novel commensal bacteria involved with innate immune homeostasis in Drosophila melanogaster. Appl. Environ. Microbiol. 74:6171-6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryu, J.-H., S.-H. Kim, H.-Y. Lee, J. Y. Bai, Y.-D. Nam, J.-W. Bae, D. G. Lee, S. C. Shin, E.-M. Ha, and W.-J. Lee. 2008. Innate immune homeostasis by the homeobox gene Caudal and commensal-gut mutualism in Drosophila. Science 319:777-782. [DOI] [PubMed] [Google Scholar]
- 62.Sacchi, L., M. Genchi, E. Clementi, E. Bigliardi, A. M. Avanzati, M. Pajoro, I. Negri, M. Marzorati, E. Gonella, A. Alma, D. Daffonchio, and C. Bandi. 2008. Multiple symbiosis in the leafhopper Scaphoideus titanus (Hemiptera: Cicadellidae): details of transovarial transmission of Cardinium sp. and yeast-like endosymbionts. Tissue Cell 40:231-242. [DOI] [PubMed] [Google Scholar]
- 63.Thompson, M. T. 1905. Alimentary canal of the mosquito. Proc. Boston Soc. Nat. Hist. 32:145-202. [Google Scholar]
- 64.White, J. W., Jr., M. L. Riethof, M. H. Subers, and I. Kushnir. 1962. Composition of American honeys. Technical bulletin 1261. Agricultural Research Service, U.S. Department of Agriculture, Washington, DC.
- 65.White, P. B. 1921. The normal bacterial flora of the bee. J. Pathol. Bacteriol. 24:64-78. [Google Scholar]
- 66.Winston, M. L. 1987. The biology of the honey bee. Harvard University Press, Cambridge, MA.
- 67.Yamada, Y., K.-I. Hoshino, and T. Ishikawa. 1997. The phylogeny of acetic acid bacteria based on the partial sequences of 16S ribosomal RNA: the elevation of the subgenus Gluconoacetobacter to the generic level. Biosci. Biotechnol. Biochem. 61:1244-1251. [DOI] [PubMed] [Google Scholar]
- 68.Yukphan, P., T. Malimas, Y. Muramatsu, M. Takahashi, S. Tanasupawat, Y. Nakagawa, K. W. Suzuki, and Y. Yamada. 2008. Tanticharoenia sakaeratensis gen. nov., sp. nov., a new osmotolerant acetic acid bacterium in the alpha-Proteobacteria. Biosci. Biotechnol. Biochem. 72:672-676. [DOI] [PubMed] [Google Scholar]
- 69.Yukphan, P., T. Malimas, W. Potacharoen, S. Tanasupawat, M. Tanticharoen, and Y. Yamada. 2005. Neoasaia chiangmaiensis gen. nov., sp. nov., a novel osmotolerant acetic acid bacterium in the alpha-Proteobacteria. J. Gen. Appl. Microbiol. 51:301-311. [DOI] [PubMed] [Google Scholar]
- 70.Yukphan, P., T. Malimas, Y. Muramatsu, M. Takahashi, M. Kaneyasu, W. Potacharoen, S. Tanasupawat, Y. Nakagawa, K. Hamana, Y. Tahara, K. Suzuki, M. Tanticharoen, and Y. Yamada. 2009. Ameyamaea chiangmaiensis gen. nov., sp. nov., an acetic acid bacterium in the alpha-Proteobacteria. Biosci. Biotechnol. Biochem. 73:2156-2162. [DOI] [PubMed] [Google Scholar]
- 71.Zouache, K., D. Voronin, V. Tran-Van, and P. Mavingui. 2009. Composition of bacterial communities associated with natural and laboratory populations of Asobara tabida infected with Wolbachia. Appl. Environ. Microbiol. 75:3755-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]