Abstract
Legionella pneumophila proliferates in aquatic habitats within free-living protozoa, 17 species of which have been identified as hosts by using in vitro experiments. The present study aimed at identifying protozoan hosts for L. pneumophila by using a biofilm batch test (BBT). Samples (600 ml) collected from 21 engineered freshwater systems, with added polyethylene cylinders to promote biofilm formation, were inoculated with L. pneumophila and subsequently incubated at 37°C for 20 days. Growth of L. pneumophila was observed in 16 of 18 water types when the host protozoan Hartmannella vermiformis was added. Twelve of the tested water types supported growth of L. pneumophila or indigenous Legionella anisa without added H. vermiformis. In 12 of 19 BBT flasks H. vermiformis was indicated as a host, based on the ratio between maximum concentrations of L. pneumophila and H. vermiformis, determined with quantitative PCR (Q-PCR), and the composition of clone libraries of partial 18S rRNA gene fragments. Analyses of 609 eukaryotic clones from the BBTs revealed that 68 operational taxonomic units (OTUs) showed the highest similarity to free-living protozoa. Forty percent of the sequences clustering with protozoa showed ≥99.5% similarity to H. vermiformis. None of the other protozoa serving as hosts in in vitro studies were detected in the BBTs. In several tests with growth of L. pneumophila, the protozoa Diphylleia rotans, Echinamoeba thermarum, and Neoparamoeba sp. were identified as candidate hosts. In vitro studies are needed to confirm their role as hosts for L. pneumophila. Unidentified protozoa were implicated as hosts for uncultured Legionella spp. grown in BBT flasks at 15°C.
Legionella pneumophila, the causative agent of Legionnaires' disease, is a common inhabitant of natural freshwater environments and human-made water systems, including cooling towers, whirlpools, air-conditioning systems, and installations for warm tap water (14). In the aquatic environment L. pneumophila proliferates within certain free-living protozoa, which serve as its hosts (15, 30, 59). Environmental factors favoring the growth and survival of L. pneumophila in freshwater systems include a water temperature between 20°C and 45°C (41, 60) and the presence of biofilms and sediments on which the protozoan hosts can graze (30, 41, 56).
Rowbotham (44) was the first to report the growth of L. pneumophila within free-living amoebae, which belonged to the genera Acanthamoeba and Naegleria. In vitro studies with cocultures have revealed that 14 species of amoebae, viz., Acanthamoeba spp. (1, 35, 44, 53), Balamuthia mandrillaris (47), Echinamoeba exundans (15), Hartmannella spp. (43), Naegleria spp. (38, 44, 53), and Vahlkampfia jugosa (43); the slime mold Dictyostelium discoideum (20, 48); and two species of the ciliate genus Tetrahymena (15, 26) can serve as hosts for L. pneumophila. Recently, it has been reported that L. pneumophila can also replicate within the intestinal tract of the microbiovorous nematode Caenorhabditis elegans (3).
A number of the free-living protozoa mentioned above and others, e.g., Vannella spp. and Saccamoeba spp., have been observed in aquatic environments from which L. pneumophila was cultivated or in which it was detected with PCR (4, 42, 51, 52). However, it remains unknown which of these protozoa actually serve as hosts for L. pneumophila in the aquatic environment, including human-made water systems. Moreover, it cannot be excluded that free-living protozoa other than those tested in vitro can serve as hosts for L. pneumophila as well. Information is also lacking about protozoan hosts for Legionella anisa (13, 49), which is frequently present in water installations in temperate regions (11, 62). Furthermore, it is unknown which free-living protozoa serve as hosts for uncultured Legionella bacteria that can grow at temperatures of about 15°C (61; B. A. Wullings, G. Bakker, and D. van der Kooij, submitted for publication).
L. pneumophila can proliferate in samples of surface water, effluent of wastewater treatment plants, potable water, and water from cooling towers incubated at 25°C, 35°C, or 37°C (28, 45, 56). Consequently, incubation of freshwater samples can be used to amplify protozoan hosts for L. pneumophila and other Legionella spp. In this study, different human-made water types were investigated using a biofilm batch test (BBT) system to (i) amplify and subsequently identify predominating, known, and yet-undescribed hosts for L. pneumophila and (ii) identify potential protozoan hosts for Legionella bacteria that can grow at 15°C.
MATERIALS AND METHODS
Experimental setup of the BBT system.
A biofilm batch test (BBT) was used to amplify and subsequently identify protozoan hosts for L. pneumophila. The BBT consists of well-cleaned, heat-sterilized (4 h at 150°C) Pyrex glass Schott flasks (1 liter) with 600 ml of test water and polyethylene cylinders that were incubated to develop a biofilm of indigenous and inoculated microorganisms. Initial tests were incubated with 12 cylinders of cross-linked polyethylene (PE-Xa) (diameter, 16 mm; each with surface area of about 9 cm2). In subsequent tests each BBT flask contained six gamma-irradiated (Isotron, Ede, Netherlands) polyethylene (PE-80) cylinders (diameter, 20 mm; each with a surface area of about 10 cm2). Nitrate and phosphate were added to each BBT flask at final concentrations of 72.5 μM and 13.5 μM, respectively, to prevent growth limitation by these nutrients.
L. pneumophila was inoculated at a concentration between 4.2 × 103 and 5.0 × 104 mip gene copies liter−1 (6) to ensure the presence of this organism. Each test was carried out in duplicate flasks. The BBT flasks were incubated at 37°C (±1°C) in the dark for at least 20 days without shaking. The concentration and diversity of Legionella and eukaryotic communities in the planktonic phase and biofilm were monitored during the incubation period. Control BBTs in which flasks were inoculated with L. pneumophila and the protozoan host Hartmannella vermiformis were performed to verify if growth of L. pneumophila occurs under the test conditions in the investigated water type. H. vermiformis was inoculated at a concentration between 3.3 × 105 and 4.8 × 105 cells liter−1. Control tests were done in single flasks.
A number of BBT flasks were additionally incubated at 15°C with tap water and with filtered tap water (3.0-μm-pore-size and 47-mm-diameter TSTP Isopore membrane [Millipore, Molsheim, France]) from groundwater supply C to test if growth of indigenous Legionella spp. occurred in the presence and the absence of free-living protozoa (Table 1). Furthermore, BBT flasks inoculated with biomass from the filter beds of groundwater supplies A and B were incubated at 15°C to determine whether growth of indigenous Legionella bacteria occurred in the presence and the absence of inoculated H. vermiformis.
TABLE 1.
Characteristics of the water types tested and results for the controls in the BBT incubated at 37°C
| Origin of sample | Temp (°C)a | Concn of ATP (ng liter−1) ± SDa,d | Concn of H. vermiformis (cells liter−1) ± SDa,d | Growth of L. pneumophila in control flask of BBTb | L. pneumophila/H. vermiformis ratio (log) in control flask of BBTc |
|---|---|---|---|---|---|
| Drinking water supplies | |||||
| Groundwater supply A | |||||
| Treated water | 11 | <1 | <0.5 | NDf | NAg |
| Biomass from filter bed (limestone) | 11 | 4.6 ± 0.04e | <2e | + | 2.1; 2.5 |
| Installation A1 | 39 | 1.9 ± 0.3 | 51 ± 14 | + | 3.6 |
| Installation A2 | 37 | 3.3 ± 0.2 | 8.2 ± 2.2 | + | 2.9 |
| Groundwater supply B | |||||
| Treated water | 11 | 6.7 ± 0.3 | <0.5 | ND | NA |
| Biomass from filter bed (sand) | 11 | 52.6 ± 0.7e | 11.4 ± 7.6e | + | 2.4; 3.5 |
| Installation B1 | 35 | 4.1 ± 0.2 | 6.8 ± 2.5 | + | 2.4 |
| Installation B2 | 37 | 7.8 ± 0.9 | 1,530 ± 290 | + | 3.3 |
| Flushed tap water | 12 | 16.0 ± 3.3 | 805 ± 74 | + | 3.0 |
| Groundwater supply C; tap water | 18 | 3.3 ± 0.4 | <4 | + | 3.1 |
| Surface water supply D | |||||
| Biomass from filter bed (granular activated carbon) | 5 | 22.1 ± 1.3e | 5.0 ± 1.8e | + | 2.6 |
| Biomass from filter bed (sand) | 5 | 55.5 ± 4.3e | 12.3 ± 1.7e | + | 2.0 |
| Surface water | |||||
| Storage reservoir for surface water supply D | 5 | 14.2 ± 0.5 | <6.7 | + | 3.5 |
| Water from Rhine River (autumn) | 10 | 83.2 ± 5.4 | <10 | ND | NA |
| Water from Rhine River (winter) | 4 | 61.1 ± 2.4 | <10 | + | 3.0 |
| Treated sewage | 15 | 705.8 ± 4.9 | 28.0 ± 10.8 | + | 1.9 |
| Water from cooling tower | |||||
| Cooling tower 1 (pH 7.3) | 27 | 101.8 ± 1.5 | <6.6 | + | 2.6 |
| Cooling tower 2 (pH 8.5) | 9 | 35.7 ± 0.3 | <2 | + | 3.2 |
| Cooling tower 3 (pH 7.8) | 24 | 19.0 ± 0.1 | <2 | − | − |
| Cooling tower 4 (pH 6.6) | 19 | 14.7 ± 0.6 | <2 | − | − |
| Cooling tower 5 (pH 8.5) | 47 | 55.8 ± 4.9 | 19 ± 1.6 | + | 2.9 |
At time of sampling.
+, significant growth (P < 0.05); −, no growth.
Log-transformed ratio between the maximum concentrations of L. pneumophila and H. vermiformis.
Standard deviations of the analysis are also shown.
Concentration per gram (wet weight) of filter bed material.
ND, not determined.
NA, not applicable.
Preparation of inoculum for L. pneumophila and H. vermiformis.
A suspension of L. pneumophila serogroup 1, grown for 7 days on buffered charcoal-yeast extract (BCYE) agar plates (12) at 37°C, was diluted in autoclaved tap water and used for inoculation (600 μl per flask). H. vermiformis (ATCC 50237) was axenically cultivated in modified PYNFH medium (ATCC 1034) for 2 weeks at 30°C (16) and was used as inoculum (600 μl per flask). Total direct cell counts of the inoculum suspensions of L. pneumophila and H. vermiformis and suspensions of protozoa for calibration curves were determined using acridine orange and epifluorescence microscopy (21).
Sample collection and preparation.
Samples included biomass from four filter beds and treated water from three different drinking water supplies (A, B, and D), six tap water types from three supply areas (A, B, and C), two surface water types, the effluent of a wastewater treatment plant, and five cooling tower samples (Table 1; see Fig. S1 in the supplemental material for details of the treatment systems for the four drinking water supplies). The samples were collected in containers of sterile glass or polyethylene, stored at 4°C, and processed within 24 h.
In supply A, aerobic groundwater is aerated to remove CO2, followed by limestone filtration to increase the pH and the hardness of the water (see Fig. S1 in the supplemental material for details). In supply B, anaerobic groundwater is treated by intensive aeration, rapid sand filtration, caustic dosage followed by pellet softening, aeration, and a second stage of rapid sand filtration (54). In supply C, anaerobic groundwater is aerated, followed by rapid sand filtration. In supply D, seepage water is treated with iron(III) chloride, storage in a lake, rapid sand filtration, ozonation, pellet softening, granular activated carbon filtration, and slow sand filtration. All four drinking water types are treated and distributed without chemical disinfection (57).
The biomass from the filter beds of supply A (limestone filter), supply B (slow sand filter), and supply D (granular activated carbon filter and slow sand filter) was collected by adding 45 g (wet weight) of filter bed material to 900 ml of the associated treated water or autoclaved tap water, followed by low-energy sonication as previously described (33). Subsequently, the biomass suspension was diluted to obtain an initial ATP concentration in the BBT flask of 10 times the ATP concentration in the related treated water.
Biomass in samples of warm tap water (about 40 liters) was concentrated to about 5 liters with ultrafiltration using the hemoflow method (58). The effluent from a sewage plant was diluted 5-fold with autoclaved tap water, and the sample of flush water was diluted 3-fold before incubation in the BBT flask. All other samples were directly incubated in the BBT flask.
Microbiological analyses.
The attached microorganisms were removed from the polyethylene cylinders and suspended in autoclaved tap water by using low-energy sonication as described elsewhere (30). Total ATP concentrations in the planktonic phase and in the biomass suspension, representing the active biomass, were determined as previously described (33).
Direct plating on BCYE medium and incubation at 37°C for detection of culturable Legionella bacteria (12, 37) were used in the initial BBT flasks inoculated with biomass from the filter beds of supplies A and B.
For DNA isolation, volumes of 50 to 200 ml of the planktonic samples and 50 to 100 ml of biofilm suspensions were filtered through a 0.22-μm-pore-size and 55-mm-diameter polycarbonate Track-Etch membrane (Sortorius, Goettingen, Germany) to isolate the microorganisms. Subsequently, DNA was isolated according to previously described procedures (54). L. pneumophila bacteria were quantified at days 0, 3, 5, 10, and 20 of the incubation by quantitative PCR (Q-PCR) by applying the primers LpneuF and LpneuR and the specific TaqMan probe LpneuP as described earlier (Wullings et al., submitted). Legionella spp. were quantified with Q-PCR on the same sample days as was L. pneumophila with the primers LEG-225 and LEG-858 as described earlier (36). L. anisa was detected with the primers LaF (5′-CAATGTCTACTGTAATGGCAGC-3′) and LaR (5′-AACCGCTTGGAGTACCGT-3′) and the specifc TaqMan probe LaP (5′-AGACGGAATGTCTGGTGCCCAATTGA-3′) targeting the mip gene. The thermal cycling conditions were similar to the conditions of Q-PCR for L. pneumophila. All primers and probes were produced at Biolegio (Malden, Netherlands), and all Q-PCR assays were performed in 96-well plates in an I-cycler real-time PCR detection system (Bio-Rad, Veenendaal, Netherlands). Quantification was based on plasmid-based calibration curves. The concentrations of Legionella spp. are expressed in genome units (GU) liter−1 or GU cm−2. The concentrations of L. pneumophila and L. anisa are measured in mip gene copies liter−1 or mip gene copies cm−2.
Q-PCR assays for detection of Acanthamoeba spp. were performed with the primers AcantF900 and AcantR1100 (40) at days 0 and 10 after incubation and for H. vermiformis were performed with the primers Hv1227F and Hv1728R at all sample days (29). The quantification of Acanthamoeba spp. and H. vermiformis was based on calibration curves which were constructed by preparing 10-fold dilutions of DNA extracted from suspensions with known numbers of cells of Acanthamoeba castellanii (CCAP 1501) and H. vermiformis (ATCC 50237).
The diversity and composition of the eukaryotic communities in the planktonic and biofilm samples were determined by terminal restriction fragment length polymorphism (T-RFLP) analysis and sequence analysis of 18S rRNA gene fragments (±550 bp) as described earlier (54). T-RFLP analysis was done for all BBT flasks at day 0 and after 20 days of incubation in planktonic samples of 50 to 200 ml and/or in biofilm suspensions of 50 to 100 ml. For each water type, this analysis was performed on the same day to minimize the experimental variation. From the BBT flasks incubated at 37°C and 15°C, a total of 820 eukaryotic clones, varying from 5 to 59 clones per library depending on the complexity of the band pattern of T-RFLP fingerprints, were analyzed. All 18S rRNA gene sequences obtained were grouped in operational taxonomic units (OTUs) with 99% similarity (46). The composition of the Legionella community in the biofilm of the BBT flasks incubated at 15°C was determined by sequence analysis of genus-specific 16S rRNA gene fragments (±650 bp) of clones retrieved as described earlier (61). All 16S rRNA gene sequences obtained were grouped in OTUs with 97% similarity. From each BBT, about 45 16S RNA gene sequences were analyzed.
The sequences obtained were compared to sequences in the National Center for Biotechnology (NCBI) database by BLAST search and imported and aligned into the SSU Ref SILVA94 database released in April 2008 by using the ARB software package as previously described (32, 39). The eukaryotic sequences obtained were organized into taxa based on the classification system of Cavalier-Smith (5) and the structure in the SILVA database (39).
Statistical analysis.
The analysis of variance (ANOVA) F test was used for the comparison of the concentrations of L. pneumophila and Legionella spp. measured with Q-PCR with the concentrations of cultivated Legionella bacteria in the BBT flasks with biomasses of supplies A and B. The statistical significance of the growth of L. pneumophila, L. anisa, and H. vermiformis populations in the BBT flasks between day 0 and day 10 (or day 20 depending on the time point of maximum concentration) was determined with the t test by using log-transformed concentrations. The Shapiro-Wilk normality test was applied to the log10-transformed ratios of the maximum concentrations of L. pneumophila and H. vermiformis (L. pneumophila/H. vermiformis ratio) observed in the BBT flasks. From the normally distributed values for the L. pneumophila/H. vermiformis ratio of the control group results, the upper limit (tolerance limit) of the one-sided 95% confidence interval of the 99th percentiles was calculated.
Nucleotide sequence accession numbers.
The 18S rRNA gene sequences determined in this study have been deposited in GenBank under accession numbers GU970094 to GU970913. The sequences of the Legionella 16S rRNA gene have been deposited in GenBank under accession numbers GU970914 to GU971083.
RESULTS
Development of the biofilm batch test (BBT) system.
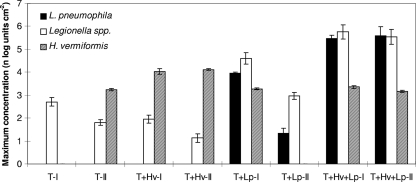
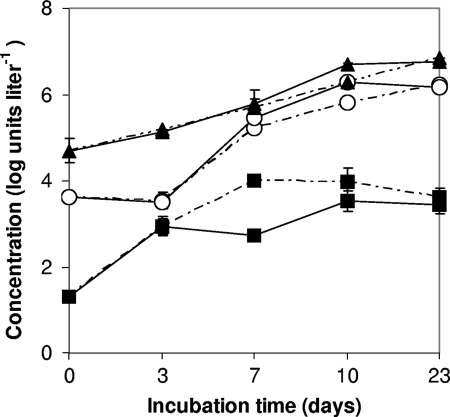
Despite the presence of H. vermiformis, no growth of L. pneumophila was observed in initial BBT flasks incubated at 37°C with biomass from the filter beds of supply A (initial concentration of 7.9 ng ATP liter−1) and supply B (131.7 ng ATP liter−1), without inoculation with L. pneumophila (Fig. 1). Furthermore, inoculated L. pneumophila did not multiply in the BBT flasks with two types of treated water without exogenous addition of H. vermiformis. In contrast, significant growth (P < 0.05) of L. pneumophila was observed in both BBT flasks with water from cooling tower 1 in which H. vermiformis was not detected at day 0 (<20 cells liter−1) (Fig. 2). Within a few days of incubation, a significant growth (P < 0.05) of H. vermiformis also was observed in this water, as was growth of L. pneumophila, which reached its maximum level of growth after about 10 days. Based on these observations, L. pneumophila was added to all BBT flasks to ensure the presence of this organism in the test, and control flasks with L. pneumophila and H. vermiformis were included in each test to verify if the water under investigation supports its growth in the presence of a host protozoan.
FIG. 1.
Maximum concentrations of L. pneumophila (mip gene copies cm−2), Legionella spp. (GU cm−2), and H. vermiformis (cells cm−2) in the biofilm on PE-Xa in the BBT flasks with biomass from a limestone filter bed in treated water of supply A incubated at 37°C for 20 days. Initial concentrations of L. pneumophila (about 2 log units of mip gene copies cm−2) and H. vermiformis (about 3 log units of cells cm−2) are converted from units liter−1 to units cm−2. Abbreviations: T-I and T-II, blank test flasks (no inoculation); T+Hv-I and T+Hv-II, duplicate test flasks inoculated with H. vermiformis; T+Lp-I and T+Lp-II, duplicate test flasks inoculated with L. pneumophila; T+Hv+Lp-I and T+Hv+Lp-II, duplicate test flasks inoculated with L. pneumophila and H. vermiformis (controls). Error bars indicate standard deviations of the analysis.
FIG. 2.
Growth of inoculated L. pneumophila in two biofilm batch test flasks with water from cooling tower 1 during incubation at 37°C for 23 days. Symbols: ○, L. pneumophila (mip gene copies liter−1); ▴, Legionella spp. (genome units [GU] liter−1); ▪, H. vermiformis (cells liter−1). The detection limit for H. vermiformis is 20 cells liter−1. Error bars indicate standard deviations of the analysis.
Significant growth (P < 0.05) of L. pneumophila was observed in 16 of 18 control BBT flasks (Table 1). In these flasks, at least a 2-log-unit increase of the L. pneumophila concentration was observed in the planktonic phase and in the biofilm within 10 days of incubation. Maximum concentrations of colony-forming cells of Legionella and maximum concentrations of Legionella spp. and L. pneumophila as measured by Q-PCR were not significantly different (P < 0.05) in the BBT flasks with biomass from the filter beds of supplies A and B incubated at 37°C. Therefore, Q-PCR was used in subsequent tests to quantify growth of L. pneumophila and Legionella spp. The log-transformed L. pneumophila/H. vermiformis ratios for the controls ranged from 1.9 to 3.6 (average, 2.8 ± 0.5) and were normally distributed according to the Shapiro-Wilk test (P = 0.577). From these data an upper tolerance limit of 4.5 was derived, above which it is most likely that protozoan hosts for growth of L. pneumophila other than H. vermiformis are present in the involved BBT flask.
L. pneumophila did not proliferate in the control BBT flasks with water from cooling towers 3 and 4 (Table 1). Probably, a biocide residual inhibited biofilm development and/or inactivated L. pneumophila because the ATP concentration decreased from 40 ng ATP liter−1 to 12 ng ATP liter−1 during the incubation in the flask with water from cooling tower 3. In the other controls, the maximum ATP concentrations in the water varied between 9.3 × 102 and 3.7 × 103 ng ATP liter−1. In the tests, the maximum ATP concentration in water varied between 5.4 × 101 and 5.1 × 102 ng ATP liter−1 and the maximum concentration of the biofilm on the surface of the PE-80 cylinders ranged from 1.3 to 9.3 ng ATP cm−2.
Growth of Legionella spp. in the BBT flasks at 37°C.
The concentration of L. pneumophila increased significantly (P < 0.05) by 1 to 3 log units in one or both BBT flasks with 11 of 21 water types inoculated with this organism (Table 2; see also Tables S2.1 to S2.3 in the supplemental material). The maximum concentration of L. pneumophila ranged from 5.3 × 103 to 5.7 × 107 mip gene copies liter−1 and from 4.9 × 102 to 5 × 105 mip gene copies cm−2 in the biofilm depending on the water type. In these BBT flasks the maximum concentration of Legionella spp. was similar to the maximum concentration of L. pneumophila, indicating that the Legionella community was dominated by L. pneumophila. Growth of Legionella spp. was observed, but L. pneumophila did not multiply in two of four BBT flasks with water originating from two tap water installations in the distribution system of supply B. In these water types significant growth (P < 0.05) of indigenous L. anisa to maximum concentrations ranging from 2.1 × 106 to 2.6 × 107 mip gene copies liter−1 was observed (Table 2). No growth of Legionella spp. was observed in 9 of 21 water types.
TABLE 2.
Predominant 18S rRNA gene sequences clustering with free-living protozoa obtained from the biofilm in BBT flasks with growth of L. pneumophila and/or L. anisa after ≥20 days of incubation at 37°C
| Water type | BBT flask | Growth in flaska |
L. pneumophila/H. vermiformis ratiob (log10) | % of clones clustering with: |
Predominant OTU clustering with protozoa |
||||
|---|---|---|---|---|---|---|---|---|---|
| L. pneumophila | H. vermiformis | Protozoa | H. vermiformis | Organism with highest similarity (accession no.) | Similarity (%) | % of clones | |||
| Biomass from limestone filter bed (supply A) | I | + | + | 1.1 | 100 | 100 | Hartmannella vermiformis (AY680840) | 99.5 | 100 |
| Installation A2 (supply A) | II | + | + | 3.0 | 100 | 93 | H. vermiformis (AY680840) | 100 | 93 |
| Biomass from sand filter bed (supply B) | I | + | − | >6.8f | 91 | <2 | Rhinosporidium sp. (AY477020) | 89.3 | 73 |
| II | + | + | 2.0 | 97 | 93 | H. vermiformis (AY680840) | 99.8 | 97 | |
| III | + | − | >6.6f | 16 | <3 | Uncultured cercozoan clone (AY620304) | >97.7 | 80 | |
| Installation B1 (supply B) | II | −c | + | 1.9e | 100 | 27 | Sphaeroeca volvox (Z34900) | 83.6 | 73 |
| Installation B2 (supply B) | I | +c | + | 2.0 (2.3e) | 100 | 100 | H. vermiformis (AY680840) | 100 | 100 |
| II | −c | + | 2.9e | 100 | 100 | H. vermiformis (AY680840) | 100 | 100 | |
| Biomass from granular activated carbon filter bed (supply D) | I | + | + | 3.4 | 100 | <13 | Nuclearia simplex (AF484687) | 100 | 100 |
| Biomass from sand filter bed (supply D) | II | + | + | 1.6 | 92 | 92 | H. vermiformis (AY680840) | 100 | 100 |
| Water from Rhine River (autumn) | I | + | − | >5.6f | 15 | <5 | Neoparamoeba sp. (AF371972) | 95.6 | 67 |
| II | + | − | >5.7f | 87 | <4 | Uncultured eukaryote from treated water of groundwater supply B (EU860860) | 93.9 | 100 | |
| Water from Rhine River | I | + | + | 5.5f | <3 | <3 | No protozoan observed | ||
| (winter) | II | + | + | 2.1 | 61 | 52 | H. vermiformis (AY680840) | 99.8 | 86 |
| Treated sewage | II | + | − | >4.6f | 52 | <4 | Diphylleia rotans (AF420478) | 99.3 | 100 |
| Cooling tower 1 | I | + | + | 2.7 | 42 | 21 | H. vermiformis (AF426157) | 100 | 50 |
| II | + | + | 2.1 | 50 | 17 | H. vermiformis (AF426157) | 99.8 | 33 | |
| Cooling tower 5 | I | + | − | >4.5f | 64 | <4 | Echinamoeba thermarum (AJ489262-AJ489268)g | 96.2-98.7 | 79 |
| II | + | −d | 3.6 | 89 | 7 | E. thermarum (AJ489262-AJ489267-AJ489268)g | 96.2-98.9 | 42 | |
+, significant growth (P < 0.05); −, no growth.
Log-transformed ratio between the maximum concentration of L. pneumophila and the maximum concentration of H. vermiformis.
Significant growth (P < 0.05) of L. anisa in the BBT flask.
H. vermiformis was detected, but no significant growth was observed.
Log-transformed ratio between the maximum concentration of L. anisa and the maximum concentration of H. vermiformis.
Log-transformed L. pneumophila/H. vermiformis ratio exceeds the tolerance limit of L. pneumophila/H. vermiformis ratio for the control group (4.5).
More than one OTU with highest similarity to E. thermarum.
Growth of H. vermiformis and Acanthamoeba spp. in the BBT flasks at 37°C.
H. vermiformis was detected at day 0 in 10 of 21 water types at concentrations ranging from 6.8 to 1,530 cells liter−1 and from 6.2 to 12.3 cells g (wet weight)−1 of filter bed material (Table 1). Significant growth of indigenous H. vermiformis in the BBT flasks was observed in 11 of 21 water types tested, with significant growth of L. pneumophila or L. anisa in 9 of these 11 water types (Table 2). The concentration of H. vermiformis increased by 0.6 to 4 log units in the planktonic phase and in the biofilm depending on the water type and the initial concentration. In four water types, H. vermiformis was not observed at day 0 but appeared within a few days of incubation. Acanthamoeba spp. were detected in water collected from tap water installation A2 and in water from cooling tower 4 at concentrations of 0.3 cells liter−1 and 1.7 cells liter−1, respectively. However, in none of the BBT flasks was growth of Acanthamoeba spp. observed.
The L. pneumophila/H. vermiformis ratios in 12 of 19 BBT flasks with growth of L. pneumophila did not exceed the derived tolerance limit, and in 11 of these 12 BBT flasks significant growth of H. vermiformis was observed (Table 2). In BBT flask II with cooling water 5, no significant growth of H. vermiformis was observed, but this protozoan was detected during the incubation and the L. pneumophila/H. vermiformis ratio did not exceed the derived tolerance limit. These observations indicate that H. vermiformis served as a host for L. pneumophila in these 12 BBT flasks. In seven BBT flasks with growth of L. pneumophila, the L. pneumophila/H. vermiformis ratio did exceed the derived tolerance limit and in six of these seven BBT flasks no significant growth of H. vermiformis or Acanthamoeba spp. was observed (detection limit, <20 cells liter−1) (Table 2). The absence of H. vermiformis and/or the high L. pneumophila/H. vermiformis ratio indicates that free-living protozoa other than H. vermiformis served as hosts for L. pneumophila in these seven BBT flasks.
Identity and diversity of eukaryotes and free-living protozoa predominating in the BBT flasks at 37°C.
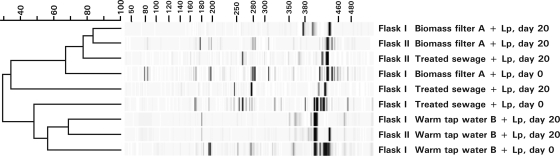
T-RFLP fingerprints revealed complex eukaryotic communities in the water types directly after sampling. During incubation, the eukaryotic diversity in the planktonic phase decreased and different eukaryotic communities developed in duplicate BBT flasks (Fig. 3). Free-living protozoa constituted the largest proportion of the OTUs (>40%) and clones (>56%) in the BBT flasks with water samples from drinking water supplies and cooling towers, but OTUs (64%) and sequences (61%) with the highest similarity to fungi predominated in the BBT flasks with surface water (Table 3). A total of 68 OTUs representing 58% of the clones showed the highest similarity to free-living protozoa (Table 3; see also Tables S2.1 to S2.3 in the supplemental material for details). Almost 40% of these clones represent one OTU, which showed ≥99.5% similarity to H. vermiformis, and were obtained from all three water types. Only three other OTUs were obtained from two water types, and all 64 of the other OTUs were observed only once in a single BBT flask.
FIG. 3.
UPGMA (unweighted-pair group method using average linkages) dendrogram of T-RFLP fingerprints of the BBT flasks with (i) biomass from a limestone filter bed of supply A (biomass filter A), (ii) treated sewage, and (iii) water from warm tap water installation B1 in distribution area of supply B (warm tap water B) at day 0 and after 20 days of incubation at 37°C with L. pneumophila (+ Lp). Sample volumes, 50 to 200 ml of the planktonic sample and 50 to 100 ml of the biofilm suspensions. The numbers above the dendrogram represent percent similarity, and those above the fingerprints represent fragment length (number of nucleotides).
TABLE 3.
Classification of eukaryotic clones retrieved from the biofilm in the BBT flask after ≥20 days of incubation at 37°C with samples from drinking water supplies, surface water, and cooling towers inoculated with L. pneumophila
| Kingdom or subkingdom | Drinking water supplies |
Surface water |
Cooling towers |
All analyzed samples |
||||
|---|---|---|---|---|---|---|---|---|
| No.a of OTUs (%) | % of clone libraries | No. of OTUs (%) | % of clone libraries | No. of OTUs (%) | % of clone libraries | No. of OTUs (%) | % of clone libraries | |
| Free-living protozoa | 35 (40.4) | 66.4 | 7 (25.0) | 34.8 | 30 (56.6) | 57.4 | 68 (41.2) | 57.7 |
| Amoebozoa | 8 (22.2) | 58.2 | 4 (57.1) | 57.4 | 21 (70) | 66.7 | 29 (42.6) | 59.7 |
| Cercozoa | 8 (22.2) | 8.4 | 3 (42.9) | 42.6 | 5 (16.7) | 15.2 | 17 (25) | 14.2 |
| Choanozoa | 11 (30.6) | 25.9 | 0 (0.0) | 0 | 3 (10) | 16.7 | 14 (20.6) | 20.7 |
| Euglenozoa | 2 (5.6) | 5.0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 2 (2.9) | 3.4 |
| Myzozoa | 5 (13.9) | 2.1 | 0 (0.0) | 0 | 0 (0.0) | 0 | 5 (7.4) | 1.4 |
| Stramenopiles | 1 (2.8) | 0.4 | 0 (0.0) | 0 | 1 (3.3) | 1.5 | 2 (2.9) | 0.6 |
| Fungi | 25 (28.1) | 15.8 | 18 (64.3) | 60.7 | 17 (32.1) | 31.3 | 60 (36.4) | 28.7 |
| Metazoa | 14 (15.7) | 10.8 | 0 (0) | 0 | 2 (3.8) | 4.3 | 14 (8.5) | 7.2 |
| Protophyta and plants | 3 (3.4) | 1.1 | 3 (10.7) | 4.4 | 2 (3.8) | 3.5 | 8 (4.8) | 2.3 |
| Organisms with <75% similarity | 12 (13.5) | 5.8 | 0 (0) | 0 | 2 (3.8) | 3.5 | 15 (9.1) | 4.1 |
| Total | 89 (100) | 100 | 28 (100) | 100 | 53 (100) | 100.0 | 165 (100) | 100 |
OTUs obtained from more than one sample type are included only once.
Sequences clustering with free-living protozoa predominated in 16 of 19 clone libraries of the BBT flasks with growth of L. pneumophila or L. anisa (Table 2; see also Tables S2.1 to S2.3 in the supplemental material for details). H. vermiformis constituted more than 50% of the clones clustering with free-living protozoa in 8 of the 19 clone libraries of BBT flasks with growth of L. pneumophila and/or L. anisa. The L. pneumophila/H. vermiformis ratios in these BBT flasks varied between 1.1 and 3.0 and did not exceed the derived tolerance limit (Table 2). The L. pneumophila/H. vermiformis ratio also did not exceed the tolerance limit in four other BBT flasks, viz., with water from tap water installation B1, with biomass from the granular activated carbon filter bed of supply D, and with water from cooling towers 1 and 5. Obviously, H. vermiformis served as host for L. pneumophila without predominating in these clone libraries.
The predominating OTUs in the clone libraries of the seven BBT flasks with L. pneumophila/H. vermiformis ratios exceeding the tolerance limit showed the highest similarity to Diphylleia rotans (99.3%), an uncultured cercozoan clone (97.7%), Echinamoeba thermarum (>96%), Neoparamoeba sp. (95.6%), an uncultured eukaryote from treated water of supply B (93.9%), and Rhinosporidium sp. (89.3%) (Table 2). These free-living protozoa may have served as hosts for L. pneumophila in these BBT flasks. No sequences related to free-living protozoa were obtained from the BBT flask with water from the Rhine River (winter) in which growth of L. pneumophila was observed.
Sequences related to Sphaeroeca volvox (>83.6% similarity) predominated in the clone libraries of two BBT flasks with water from tap water installation B1, in one of which growth of L. anisa and H. vermiformis was observed (Table 2; also see Table S2.1 in the supplemental material for details). This observation suggests that the free-living protozoan related to S. volvox did not serve as a host for L. anisa or for L. pneumophila. Most clone libraries of the BBT flasks without growth of L. pneumophila and Legionella spp. were dominated by OTUs clustering with fungi and metazoa. Predominating OTUs of the protozoan community in these BBT flasks had the highest similarities to Ichthyophonus irregularis (89.2%), Platyamoeba stenopodia (93.9%), an uncultured cercozoan (>98.9%), and an uncultured eukaryote retrieved from treated water from supply A in an earlier study (54) (see Tables S2.1 to S2.3 in the supplemental material for details). Obviously, these free-living protozoa did not serve as hosts for L. pneumophila under the test conditions.
Transfer of one PE cylinder with biofilm and 60 ml of the planktonic phase from BBT flasks with the different water types which had been incubated for at least 50 days to freshly prepared BBT flasks with autoclaved tap water and five PE cylinders, followed by incubation at 37°C, did not induce growth of L. pneumophila in most flasks. Growth of L. pneumophila in freshly prepared BBT flasks was observed only in the presence of H. vermiformis, e.g., in the flasks inoculated from the BBT flask with treated sewage and with biomass from the granular activated carbon filter bed of supply D. The L. pneumophila/H. vermiformis ratios in these BBT flasks indicated that H. vermiformis served as a host for L. pneumophila.
Growth of Legionella spp. and eukaryotes in the BBT system at 15°C.
Growth of indigenous Legionella spp. was observed in one of the BBT flasks with tap water of supply C and in the BBT flask inoculated with H. vermiformis but not in the membrane-filtered (3.0-μm) sample. At least a 2-log-unit increase of the Legionella community was observed in all flasks inoculated with biomass from filter beds of supplies A and B, but no colonies were observed on the BCYE medium. The inoculated L. pneumophila did not multiply in the BBT flasks during incubation at 15°C. The Legionella community (maximum concentration, 3.1 × 105 GU cm−2) grown in the BBT flasks with biomass from the limestone filter bed of supply A with and without addition of H. vermiformis was dominated (>92% of the clone library) by OTUs clustering with sequences obtained from treated water and raw water from supply A in an earlier study (Wullings et al., submitted). These sequences differ from the species described (data not shown). The Legionella community (maximum concentration, 3.0 × 104 GU cm−2) in the BBT flasks with biomass from a sand filter bed of supply B was dominated by one OTU (84% of the clone libraries), which differs from reported sequences.
Acanthamoeba spp. were not observed in the BBT flasks incubated at 15°C, and H. vermiformis was observed only in the BBT flasks inoculated with this protozoan. Changes in the T-RFLP fingerprints of the eukaryotic communities in the biofilm during incubation at 15°C indicated that growth of indigenous eukaryotes occurred (results not shown). Sequences clustering with free-living protozoa were obtained from all four eukaryotic clone libraries of the BBT flasks with biomass from filter beds of supplies A and B incubated at 15°C. A total of 51 OTUs showed the highest similarity to protozoan phyla, viz., Amoebozoa (7 OTUs), Cercozoa (16 OTUs), Choanozoa (17 OTUs), Ciliophora (1 OTU), Euglenozoa (7 OTUs), Myzozoa (2 OTUs), and Stramenopiles (1 OTU). More than 80% of the clones clustering with free-living protozoa that were retrieved from the BBT flasks inoculated with biomass from the limestone filter bed of supply A showed the highest similarity with the Cercozoa phylum (see Tables S3.1 and S3.2 in the supplemental material for details). Sequences clustering with Choanozoa and Euglenozoa predominated in the clone libraries of the BBT flasks with biomass from a sand filter bed of supply B. Most (57%) of the OTUs that clustered with free-living protozoa showed the highest similarity to sequences which differ from already-described species retrieved in an earlier study from treated water or distribution system biofilms of supplies A and B (54). These observations indicate that yet-undescribed protozoa serve as hosts for yet-uncultured Legionella bacteria.
DISCUSSION
Performance of the biofilm batch test (BBT) system.
Only a few in vivo studies have shown that certain free-living protozoa serve as hosts for L. pneumophila (15, 30, 59), but a large number of free-living protozoa have been identified as hosts for L. pneumophila by using in vitro studies (17, 26, 35, 38, 43, 44, 47, 53). Random in vitro testing of free-living protozoa to determine whether they serve as hosts for L. pneumophila is time-consuming, and only a minor fraction of the free-living protozoa is available in culture collections. Furthermore, it is unknown if free-living protozoa which serve as hosts when tested in vitro with a pure culture of L. pneumophila serve as hosts in aquatic environments. Therefore, a batch test with biofilm growth in combination with molecular techniques for detection and identification was used to identify potential protozoan hosts for L. pneumophila in different freshwater types (Table 1).
The present study shows that Legionella spp. and free-living protozoa multiplied in the BBT flasks at 37°C and also at 15°C. Therefore, this approach can be used to amplify and subsequently identify free-living protozoa that can serve as hosts for L. pneumophila and other Legionella spp. in freshwater types of different origins. The maximum concentrations of active biomass on the surface of the PE-80 cylinders varied between 1.3 and 9.3 ng ATP cm−2 and are in the same range as biofilm concentrations measured in tap water installations (55). Therefore, the BBT design represents the environment in water installations. L. pneumophila serogroup 1 was added to ensure the presence of this organism in the BBT flasks (Fig. 1). Growth of indigenous L. anisa was observed in two water types despite the inoculation with a relatively high number of L. pneumophila bacteria. L. anisa is a common inhabitant of engineered water systems (11, 62) and also needs free-living protozoa for growth in aquatic environments (13, 49).
Growth of H. vermiformis in the BBT flasks was followed by growth of L. pneumophila (Fig. 2). The L. pneumophila/H. vermiformis ratio in the test in combination with the tolerance limit for this ratio derived from the controls can be used as an indicator for the role of H. vermiformis as protozoan host in the BBT flasks. The obtained L. pneumophila/H. vermiformis ratios, which ranged from 1.9 to 3.6 log units, are consistent with the average ratios of 2.5 to 2.6 log units obtained in a biofilm batch model system with autoclaved tap water inoculated with pure cultures of L. pneumophila and H. vermiformis (28) (Table 1). The Legionella-to-host ratio may be different for other protozoan hosts, e.g., Acanthamoeba spp., depending on their cell size.
In more than 50% of the tested water types, growth of L. pneumophila or L. anisa was observed only in one of two BBT flasks incubated at 37°C. Different protozoan communities can develop in duplicate samples (Fig. 3 and Table 2), indicating that slight differences in environmental conditions result in predominance of other free-living protozoa. This observation is consistent with the observation of highly diverse free-living protozoan communities at different locations within drinking water distribution systems (54).
The procedure for the identification of the free-living protozoa in clone libraries, as applied in this study, has some limitations. First of all, 18S rRNA gene sequences of a few genera within the phyla Amoebozoa, Euglenozoa, and Percolozoa were not amplified with the primers used. These organisms include Naegleria spp. (38, 44) and Vahlkampfia jugosa (43), which have been identified as hosts for L. pneumophila by using in vitro tests. Furthermore, the composition of the clone libraries does not exactly reflect the composition of the involved eukaryotic communities, because 18S rRNA genes are present in different copy numbers in each eukaryotic species (31). Moreover, although one OTU related to free-living protozoa predominated in the clone libraries of most BBT flasks with growth of L. pneumophila (Table 2), it cannot be excluded that more than one protozoan type served as hosts for L. pneumophila in one BBT flask. Despite these limitations, a number of candidate hosts for L. pneumophila were detected.
Identity of protozoan hosts for L. pneumophila and L. anisa.
The observation of H. vermiformis in 14 of the 21 water types tested (before or during incubation) is consistent with the ubiquitous presence of this organism in drinking water supplies (34, 42, 51, 52, 54), cooling towers (28), and surface water (29, 34, 52) (Tables 1 and 2). In 12 of the 19 flasks with growth of L. pneumophila or L. anisa, growth of H. vermiformis also was observed. The L. pneumophila/H. vermiformis ratios in 11 of these 12 BBT flasks did not exceed the derived tolerance limit. OTUs with the highest similarity to H. vermiformis predominated in 8 of the associated clone libraries, thus confirming that H. vermiformis served as host for L. pneumophila or L. anisa in these BBT flasks.
Significant growth of H. vermiformis was observed in two water types without growth of L. pneumophila and other Legionella spp., and also no growth of L. pneumophila was observed in the control for one of these water types. All sequences with the highest similarity to H. vermiformis showed a minimum of 99.5% similarity to each other and to the H. vermiformis strain (ATCC 50237) used in the controls. The absence of growth of Legionella spp. in the presence of H. vermiformis remains unexplained.
Acanthamoeba spp. were observed at low initial concentrations in 2 of the 21 water types, indicating that this organism is much less common in freshwater in temperate regions than is H. vermiformis. Still, Acanthamoeba spp. are frequently used to study proliferation and growth of L. pneumophila within free-living protozoa and numbers between >1.0 × 102 and 4.5 × 104 CFU of L. pneumophila per amoeba or vesicle have been reported (18, 22, 24, 43, 44). Growth of Acanthamoeba spp. was not observed in the BBT flasks, although most species of this genus can multiply at 37°C (9, 19). Of the free-living protozoa, the slime mold, and the metazoan which have been identified as hosts for L. pneumophila by using in vitro studies, only H. vermiformis was identified as a host for L. pneumophila or L. anisa in the present study. The prominent position of H. vermiformis as a host for L. pneumophila in aquatic environments is consistent with results of other in vivo experiments (15, 30, 59). However, the potential of the other listed organisms to serve as hosts for L. pneumophila in the aquatic environment needs confirmation using in vivo tests.
L. pneumophila did not proliferate in the absence of H. vermiformis in freshly prepared BBT flasks inoculated with the microbiota grown in BBT flasks with selected water types, and no attempts were made to culture free-living protozoa from these BBT flasks. Consequently, not yet-recognized protozoan hosts for L. pneumophila were not isolated in this study. The composition of a number of eukaryotic clone libraries and the values of the L. pneumophila/H. vermiformis ratio suggest that testing of certain protozoa in coculture with L. pneumophila may lead to the identification of novel protozoan hosts for L. pneumophila (Table 2). The involved organisms include (i) Diphylleia rotans (99.3% similarity), an algivorous heterotrophic flagellate that feeds on cyanobacteria (27); (ii) Echinamoeba thermarum (>96%), an extremely thermophilic protozoan (2) related to E. exundans, which is described as a host for L. pneumophila (15); and (iii) Neoparamoeba sp. (95.6% similarity) of the class Flabelinea with the genera Platyamoeba and Vannella, which are affiliated with Legionella spp. (50). These candidate hosts were observed in BBT flasks with surface water, water from a cooling tower, and biomass from a sand filter bed of a groundwater supply, but not from tap water (Table 2).
Free-living protozoa growing at 37°C were dominated by OTUs clustering within Amoebozoa, while the clone libraries of the BBT flasks at 15°C were dominated by OTUs with the highest similarity to cercozoan and choanozoan types. Also in drinking water supplies (<20°C), only a minor fraction of OTUs of free-living protozoa clustered with Amoebozoa (54). OTUs related to free-living protozoa with pathogenic properties, viz., Acanthamoeba spp. (7, 25) and Balamuthia mandrillaris (23, 47), were observed in several water types (see Tables S2.1 to S2.3 in the supplemental material for details). However, it is unclear whether these sequences represent pathogenic organisms. In most BBT flasks without growth of Legionella spp., OTUs that showed the highest similarity to fungi and metazoa predominated. The free-living protozoa related to Sphaeroeca volvox, Ichthyophonus irregularis, and Platyamoeba stenopodia and an uncultured cercozoan did not serve as hosts for L. pneumophila under the test conditions (see Tables S2.1 to S2.3 in the supplemental material for details).
Identity and diversity of Legionella spp. and free-living protozoa in the BBT system at 15°C.
Legionella bacteria, including several yet-uncultured species, are ubiquitously present in freshwater (8, 10, 61; Wullings et al., submitted). Growth of Legionella spp. at 15°C was observed in the BBT system but not with membrane-filtered water (3.0 μm), confirming that these bacteria require free-living protozoa for growth. The yet-undescribed Legionella (sequence) types predominating in the BBT flasks with biomass from a limestone filter bed of supply A clustered with several sequence types previously obtained from raw and treated water of the associated supply (Wullings et al., submitted). The sequences of the protozoa predominating in the BBT flasks with Legionella spp. growing at 15°C clustered with sequences related to cercozoan types retrieved from the treated water of supplies A and B in an earlier study (54). However, inoculated L. pneumophila did not grow at 15°C, nor did it grow in one of the BBT flasks incubated at 37°C with biomass from a limestone filter bed of supply A, despite the predominance of cercozoan types (see Tables S2.1 and S3.1 in the supplemental material for details). Growth of unidentified Legionella spp. at 15°C in the presence of free-living protozoa related to choanozoan and euglenozoan types in the BBT flasks with biomass from a sand filter bed of supply B suggests that also these protozoa can serve as hosts. One choanozoan type (Rhinosporidium sp.) also predominated in the clone library of a BBT flask with growth of L. pneumophila at 37°C (L. pneumophila/H. vermiformis ratio, >6.8), suggesting that this organism also served as a host for L. pneumophila (Table 2).
Most (57%) OTUs of free-living protozoa in the BBT flasks inoculated with biomass from filter beds and incubated at 15°C showed the highest similarity to sequences obtained from treated water or distribution system biofilms of supplies A and B in an earlier study (54). Obviously, either the many free-living protozoa present in the treated water and biofilms in the distribution systems have their origin in the filter beds and/or the environmental conditions in the filter bed resemble those in the biofilm in the distribution systems.
In conclusion, our observations confirm the prominent position of H. vermiformis as a host for L. pneumophila, whereas none of the other protozoa serving as hosts in in vitro studies were observed in the BBT system. A few protozoa, e.g., Diphylleia rotans, Echinamoeba thermarum, Neoparamoeba sp., and Rhinosporidium sp., were identified as candidate hosts for L. pneumophila, but in vitro studies with these organisms are needed for confirmation.
Supplementary Material
Acknowledgments
This study was financed by Delft Cluster project CT06.10 and by the water supply companies in the Netherlands in the framework of the Joint Research Program.
We thank the staff of the water supply companies Vitens and Waternet and Dow Benelux B.V. for facilitating sampling of their installations. Bastiaan Egging, M.Sc. student at Wageningen University, is gratefully acknowledged for his technical assistance. The statistical support of Paul Baggelaar (Icastat) is greatly appreciated. We thank Gerhard Wubbels (Waterlaboratorium Noord, Glimmen), who was involved in developing the Q-PCR for L. anisa. We are grateful to Wim Hoogenboezem (Het Waterlaboratorium, Haarlem), Hauke Smidt (Wageningen University), and Johannes Hackstein (Radboud University Nijmegen) for valuable discussions and the staff of the Laboratory for Microbiology of KWR Watercycle Research Institute for skillful technical assistance.
Footnotes
Published ahead of print on 17 September 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Anand, C. M., A. R. Skinner, A. Malic, and J. B. Kurtz. 1983. Interaction of L. pneumophilia and a free living amoeba (Acanthamoeba palestinensis). J. Hyg. (Lond.) 91:167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgartner, M., A. Yapi, R. Grobner-Ferreira, and K. O. Stetter. 2003. Cultivation and properties of Echinamoeba thermarum n. sp., an extremely thermophilic amoeba thriving in hot springs. Extremophiles 7:267-274. [DOI] [PubMed] [Google Scholar]
- 3.Brassinga, A. K., J. M. Kinchen, M. E. Cupp, S. R. Day, P. S. Hoffman, and C. D. Sifri. 2010. Caenorhabditis is a metazoan host for Legionella. Cell. Microbiol. 12:343-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breiman, R. F., B. S. Fields, G. N. Sanden, L. Volmer, A. Meier, and J. S. Spika. 1990. Association of shower use with Legionnaires' disease. Possible role of amoebae. JAMA 263:2924-2926. [PubMed] [Google Scholar]
- 5.Cavalier-Smith, T. 2002. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int. J. Syst. Evol. Microbiol. 52:297-354. [DOI] [PubMed] [Google Scholar]
- 6.Cianciotto, N. P., B. I. Eisenstein, C. H. Mody, G. B. Toews, and N. C. Engleberg. 1989. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect. Immun. 57:1255-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culbertson, C. G. 1961. Pathogenic Acanthamoeba (Hartmanella). Am. J. Clin. Pathol. 35:195-202. [DOI] [PubMed] [Google Scholar]
- 8.Declerck, P., J. Behets, V. van Hoef, and F. Ollevier. 2007. Detection of Legionella spp. and some of their amoeba hosts in floating biofilms from anthropogenic and natural aquatic environments. Water Res. 41:3159-3167. [DOI] [PubMed] [Google Scholar]
- 9.De Jonckheere, J. F. 1980. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl. Environ. Microbiol. 39:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diederen, B. M., C. M. de Jong, I. Aarts, M. F. Peeters, and A. van der Zee. 2007. Molecular evidence for the ubiquitous presence of Legionella species in Dutch tap water installations. J. Water Health 5:375-383. [DOI] [PubMed] [Google Scholar]
- 11.Doleans, A., H. Aurell, M. Reyrolle, G. Lina, J. Freney, F. Vandenesch, J. Etienne, and S. Jarraud. 2004. Clinical and environmental distributions of Legionella strains in France are different. J. Clin. Microbiol. 42:458-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelstein, P. H. 1981. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J. Clin. Microbiol. 14:298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields, B. S., J. M. Barbaree, G. N. Sanden, and W. E. Morrill. 1990. Virulence of a Legionella anisa strain associated with Pontiac fever: an evaluation using protozoan, cell culture, and guinea pig models. Infect. Immun. 58:3139-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields, B. S., G. N. Sanden, J. M. Barbaree, W. E. Morrill, R. M. Wadowsky, E. H. White, and J. C. Feeley. 1989. Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr. Microbiol. 18:131-137. [Google Scholar]
- 16.Fields, B. S., T. A. Nerad, T. K. Sawyer, C. H. King, J. M. Barbaree, W. T. Martin, W. E. Morrill, and G. N. Sanden. 1990. Characterization of an axenic strain of Hartmannella vermiformis obtained from an investigation of nosocomial legionellosis. J. Protozool. 37:581-583. [DOI] [PubMed] [Google Scholar]
- 17.Fields, B. S., E. B. Shotts, Jr., J. C. Feeley, G. W. Gorman, and W. T. Martin. 1984. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl. Environ. Microbiol. 47:467-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greub, G., and D. Raoult. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin, J. L. 1972. Temperature tolerance of pathogenic and nonpathogenic free-living amoebas. Science 178:869-870. [DOI] [PubMed] [Google Scholar]
- 20.Hagele, S., R. Kohler, H. Merkert, M. Schleicher, J. Hacker, and M. Steinert. 2000. Dictyostelium discoideum: a new host model system for intracellular pathogens of the genus Legionella. Cell. Microbiol. 2:165-171. [DOI] [PubMed] [Google Scholar]
- 21.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holden, E. P., H. H. Winkler, D. O. Wood, and E. D. Leinbach. 1984. Intracellular growth of Legionella pneumophila within Acanthamoeba castellanii Neff. Infect. Immun. 45:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, Z. H., A. Ferrante, and R. F. Carter. 1999. Serum antibodies to Balamuthia mandrillaris, a free-living amoeba recently demonstrated to cause granulomatous amoebic encephalitis. J. Infect. Dis. 179:1305-1308. [DOI] [PubMed] [Google Scholar]
- 24.Hwang, M. G., H. Katayama, and S. Ohgaki. 2006. Effect of intracellular resuscitation of Legionella pneumophila in Acanthamoeba polyphage cells on the antimicrobial properties of silver and copper. Environ. Sci. Technol. 40:7434-7439. [DOI] [PubMed] [Google Scholar]
- 25.Jones, D. B., G. S. Visvesvara, and N. M. Robinson. 1975. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans. Ophthalmol. Soc. U. K. 95:221-232. [PubMed] [Google Scholar]
- 26.Kikuhara, H., M. Ogawa, H. Miyamoto, Y. Nikaido, and S. Yoshida. 1994. Intracellular multiplication of Legionella pneumophila in Tetrahymena thermophila. J. UOEH 16:263-275. [DOI] [PubMed] [Google Scholar]
- 27.Kim, B. R., S. Nakano, B. H. Kim, and M. S. Han. 2006. Grazing and growth of the heterotrophic flagellate Diphylleia rotans on the cyanobacterium Microcystis aeruginosa. Aquat. Microb. Ecol. 45:163-170. [Google Scholar]
- 28.Kuiper, M. W. 2006. Occurrence of Legionella pneumophila and Hartmannella vermiformis in fresh water environments and their interactions in biofilms. Ph.D. thesis. Wageningen University, Wageningen, Netherlands.
- 29.Kuiper, M. W., R. M. Valster, B. A. Wullings, H. Boonstra, H. Smidt, and D. Van der Kooij. 2006. Quantitative detection of the free-living amoeba Hartmannella vermiformis in surface water by using real-time PCR. Appl. Environ. Microbiol. 72:5750-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuiper, M. W., B. A. Wullings, A. D. Akkermans, R. R. Beumer, and D. van der Kooij. 2004. Intracellular proliferation of Legionella pneumophila in Hartmannella vermiformis in aquatic biofilms grown on plasticized polyvinyl chloride. Appl. Environ. Microbiol. 70:6826-6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long, E. O., and I. B. Dawid. 1980. Repeated genes in eukaryotes. Annu. Rev. Biochem. 49:727-764. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Förster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. König, T. Liss, R. Lüßmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magic-Knezev, A., and D. van der Kooij. 2004. Optimisation and significance of ATP analysis for measuring active biomass in granular activated carbon filters used in water treatment. Water Res. 38:3971-3979. [DOI] [PubMed] [Google Scholar]
- 34.Michel, R., R. Hoffmann, A. Giese, and K. D. Muller. 1995. Untersuchung von drei Grundwasserwerken auf Vorkommen von Acanthamoeben, Naeglerien und anderen freilebenden Amöben. Acta Hydrochim. Hydrobiol. 23:202-211. [Google Scholar]
- 35.Miyamoto, H., H. Taniguchi, and S. Yoshida. 2003. A simple qualitative assay for intracellular growth of Legionella pneumophila within Acanthamoeba culbertsoni. Kansenshogaku Zasshi 77:343-345. [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto, H., H. Yamamoto, K. Arima, J. Fujii, K. Maruta, K. Izu, T. Shiomori, and S. Yoshida. 1997. Development of a new seminested PCR method for detection of Legionella species and its application to surveillance of legionellae in hospital cooling tower water. Appl. Environ. Microbiol. 63:2489-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nederlands Normalisatie Instituut. 1991. Bacteriological examination of water: examination on the presence and the number of colony forming units (CFU) of Legionella bacteria. NEN6265. Nederlands Normalisatie Instituut, Delft, Netherlands.
- 38.Newsome, A. L., R. L. Baker, R. D. Miller, and R. R. Arnold. 1985. Interactions between Naegleria fowleri and Legionella pneumophila. Infect. Immun. 50:449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prüsse, E., C. Quast, K. Knittel, B. M. Fuchs, W. Ludwig, J. Peplies, and F. O. Glöckner. 2007. SILVA; a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35:7188-7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qvarnstrom, Y., G. S. Visvesvara, R. Sriram, and A. J. da Silva. 2006. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J. Clin. Microbiol. 44:3589-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers, J., A. B. Dowsett, P. J. Dennis, J. V. Lee, and C. W. Keevil. 1994. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl. Environ. Microbiol. 60:1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohr, U., S. Weber, R. Michel, F. Selenka, and M. Wilhelm. 1998. Comparison of free-living amoebae in hot water systems of hospitals with isolates from moist sanitary areas by identifying genera and determining temperature tolerance. Appl. Environ. Microbiol. 64:1822-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowbotham, T. J. 1986. Current views on the relationships between amoebae, legionellae and man. Isr. J. Med. Sci. 22:678-689. [PubMed] [Google Scholar]
- 44.Rowbotham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanden, G. N., W. E. Morrill, B. S. Fields, R. F. Breiman, and J. M. Barbaree. 1992. Incubation of water samples containing amoebae improves detection of legionellae by the culture method. Appl. Environ. Microbiol. 58:2001-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shadrach, W. S., K. Rydzewski, U. Laube, G. Holland, M. Ozel, A. F. Kiderlen, and A. Flieger. 2005. Balamuthia mandrillaris, free-living ameba and opportunistic agent of encephalitis, is a potential host for Legionella pneumophila bacteria. Appl. Environ. Microbiol. 71:2244-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solomon, J. M., A. Rupper, J. A. Cardelli, and R. R. Isberg. 2000. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect. Immun. 68:2939-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steele, T. W., and A. M. McLennan. 1996. Infection of Tetrahymena pyriformis by Legionella longbeachae and other Legionella species found in potting mixes. Appl. Environ. Microbiol. 62:1081-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinert, M., U. Hentschel, and J. Hacker. 2002. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol. Rev. 26:149-162. [DOI] [PubMed] [Google Scholar]
- 51.Thomas, V., K. Herrera-Rimann, D. S. Blanc, and G. Greub. 2006. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 72:2428-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas, V., J. F. Loret, M. Jousset, and G. Greub. 2008. Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ. Microbiol. 10:2728-2745. [DOI] [PubMed] [Google Scholar]
- 53.Tyndall, R. L., and E. L. Domingue. 1982. Cocultivation of Legionella pneumophila and free-living amoebae. Appl. Environ. Microbiol. 44:954-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valster, R. M., B. A. Wullings, G. Bakker, H. Smidt, and D. van der Kooij. 2009. Free-living protozoa in two unchlorinated drinking water supplies, identified by phylogenic analysis of 18S rRNA gene sequences. Appl. Environ. Microbiol. 75:4736-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van der Kooij, D., H. R. Veenendaal, and W. J. Scheffer. 2005. Biofilm formation and multiplication of Legionella in a model warm water system with pipes of copper, stainless steel and cross-linked polyethylene. Water Res. 39:2789-2798. [DOI] [PubMed] [Google Scholar]
- 56.Van der Kooij, D., H. R. Veenendaal, N. P. G. Slaats, and D. Vonk. 2002. Biofilm formation and multiplication of Legionella on synthetic pipe materials in contact with treated water under static and dynamic conditions, p. 176-180. In R. Marre et al. (ed.), Legionella. ASM Press, Washington, DC.
- 57.Van der Kooij, D., J. H. M. van Lieverloo, J. Schellart, and P. Hiemstra. 1999. Maintaining quality without a disinfectant residual. J. Am. Water Works Assoc. 91:55-64. [Google Scholar]
- 58.Veenendaal, H. R., and A. J. Brouwer-Hanzens. 2007. A method for the concentration of microbes in large volumes of water. Techneau, KWR Watercycle Research Institute, Nieuwegein, Netherlands. http://tki.techneau.org/knowledge-packages/a-method-for-the-concentration-of-microbes-in-large-volumes-of-water.
- 59.Wadowsky, R. M., L. J. Butler, M. K. Cook, S. M. Verma, M. A. Paul, B. S. Fields, G. Keleti, J. L. Sykora, and R. B. Yee. 1988. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl. Environ. Microbiol. 54:2677-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wadowsky, R. M., R. Wolford, A. M. McNamara, and R. B. Yee. 1985. Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl. Environ. Microbiol. 49:1197-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wullings, B. A., and D. van der Kooij. 2006. Occurrence and genetic diversity of uncultured Legionella spp. in drinking water treated at temperatures below 15°C. Appl. Environ. Microbiol. 72:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamoto, N., T. Kubota, M. Tateyama, M. Koide, C. Nakasone, M. Tohyama, T. Shinzato, F. Higa, N. Kusano, K. Kawakami, and A. Saito. 2003. Isolation of Legionella anisa from multiple sites of a hospital water system: the eradication of Legionella contamination. J. Infect. Chemother. 9:122-125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.