Abstract
The purpose of this study was to determine the occurrence and genotypic relatedness of Salmonella enterica isolates recovered from feed and fecal samples in commercial swine production units. Of 275 feed samples, Salmonella was detected in 10 feed samples that originated from 8 of 36 (22.2%) barns, with a prevalence of 3.6% (10/275 samples). In fecal samples, a prevalence of 17.2% was found at the early finishing stage (1,180/6,880 samples), with a significant reduction in prevalence (7.4%) when pigs reached market age (392/5,321 samples). Of the 280 Salmonella isolates systematically selected for further characterization, 50% of the feed isolates and 55.3% of the isolates of fecal origin showed similar phenotypes based on antimicrobial resistance patterns and serogrouping. About 44% of the isolates were multidrug resistant. Pulsed-field gel electrophoresis (PFGE) genotyping grouped the 46 representative isolates into five genotypic clusters, of which four of the clusters consisted of genotypically related isolates recovered from feed and fecal samples. The occurrence of genotypically related and, in some cases, clonal strains, including multidrug-resistant isolates in commercially processed feed and fecal samples, suggests the high significance of commercial feed as a potential vehicle of Salmonella transmission.
Contaminated foods of animal and plant origin have been incriminated as sources of Salmonella enterica infections in humans. Animal feed is an important component in preharvest Salmonella control programs (3, 7, 13, 17). Animal feed has been considered one transmission vehicle for food-borne pathogens such as Salmonella in various food animals, including cattle (6, 14, 16), swine (4, 7, 8, 20, 23), and poultry (2, 15, 21). Food-producing animals, including swine, are one of the primary sources of Salmonella enterica human infections, and contaminated feed is a recognized source of such infection of food animals (5) and thus present a potential public health hazard. Several outbreaks of human illness incidents due to Salmonella infections that were traced back to contaminated animal feed have also been reported, implying the high significance of feed as a vehicle for Salmonella transmission (3). While there is evidence of feed contamination, there has been a paucity of data on the significance of commercially processed feed as a source of Salmonella infection.
While overall, the genotypic approaches are known to have high discriminatory power and reproducibility (10), previous studies of animal feed contamination rarely used genotypic approaches to track contamination from feed to the food animal or food. Phenotypic approaches are commonly implemented. These include serogrouping, serotyping, antimicrobial resistance profiling (antibiotyping), and phage typing (in limited serotypes). Among these, serotyping is the most commonly used typing system. Application of genotypic approaches such as pulsed-field gel electrophoresis (PFGE) has been employed for subtyping of Salmonella isolates of diverse origin and has been very useful to investigate molecular epidemiology of the pathogen. In previous studies, PFGE has been used to investigate the possible risk of cross-contamination of various food-borne pathogens, such as Salmonella and Escherichia coli O157:H7 recovered from various sources, including cattle, broilers, fish meal and other feed types, animals, and humans (2, 6, 19). The objective of the current study was to determine the phenotypic and genotypic relatedness of Salmonella isolates recovered from commercially processed swine feed and compare it with that of isolates of fecal and barn environmental origin recovered from the same swine barns as well as from different ones in commercial swine production systems.
MATERIALS AND METHODS
Study design and sample collection.
The current study was part of a longitudinal group-randomized controlled study designed to investigate the association of various ecologic factors with the occurrence and persistence of multidrug-resistant (MDR) Salmonella. In three vertically integrated commercial swine production systems (systems 1, 2, and 3), three farms per system (n = 9) were selected. At each farm, four barns were selected randomly (n = 36) and assigned to four different treatment groups (BioSentry [0.4%]; Synergize [33%], Virkon S [1%], and control [pressurized water]). From each of the selected barns (n = 36), pooled feed samples (1 per barn), barn floor swabs (10 per barn) before and after disinfection, and fecal samples (48 per barn) from swine at early and late finishing stages of production were collected.
Each production system runs its own feed mill. There was no report of a common source of feed ingredients among the three production systems. The feed was delivered in two forms: fine ground or pelleted type. Detailed information on the ingredients in each feed type prepared for different stages in production systems (nursery and finishing) was not available. A farm survey assessment including basic production information for each farm at nursery and finishing stages was conducted. The information indicated that only ractopamine HCl (Paylean) and heavy metals (copper and zinc) were added to the feed at the finishing stages.
Each farm was visited at two stages of production (early and late finishing stages) in four replicate visits (repeated visits to the same barns during the study period of October 2007 to November 2009). One replicate visit consists of sampling assigned barn floors before and after disinfection, pigs per treatment group at early and late finishing stages, and pooled feed. About 100 g of pooled feed samples was aseptically collected per barn from the feeder bin. All the feed samples were collected from the feeder bin pipe outlet aseptically before they had a chance for contamination within the barn. Samples were collected in sterile Whirl-Pak bags. Barn floor swab samples were aseptically collected using conventional drag swabs (Tyco Healthcare/Kendall, Mansfield, MA) pre- and postdisinfection from randomly selected pens (n = 10 per barn) in 36 barns. From each barn, a total of 10 drag swab samples and negative-control samples (pre- and postdisinfection) were collected as described previously (24). Fecal samples (approximately 10 g) were aseptically collected with a gloved hand from the rectums of individual pigs per pen (48 samples per barn) at the following two stages: 6 to 9 weeks (F1) and market age (26 to 28 weeks of age [F2]). All the samples were placed on ice and submitted to the laboratory via an overnight shipment.
Salmonella isolation and identification.
Salmonellae were isolated and identified using conventional methods, as described previously (9, 24). Briefly, each fecal and feed sample (10-g portions) was preenriched in 90 ml of buffered peptone water (BPW) and incubated at 37°C overnight. To each Whirl-Pak bag containing individual drag swabs, 90 ml of BPW (Becton Dickinson, Sparks, MD) was added and incubated at 37°C for overnight. A total of 100 μl of the preenriched suspension was then added into 9.9 ml of Rappaport-Vassiliadis (RV) enrichment broth (Becton Dickinson) and incubated at 42°C for 24 h. A loopful of the suspension was plated onto xylose-lactose-Tergitol 4 (XLT-4) agar (Becton Dickinson) plates and incubated at 37°C for 24 to 48 h. Three isolated Salmonella presumptive colonies were selected from each positive sample for biochemical testing. Each selected colony was inoculated onto triple sugar iron (TSI) agar (Becton Dickinson) slants and urea broth (Becton Dickinson) and incubated at 37°C overnight. All biochemically confirmed Salmonella isolates were stored at −80°C until further testing.
Phenotyping.
Biochemically confirmed Salmonella isolates recovered from swine barn floors, feces, and feed were serogrouped using commercially available polyvalent O and group-specific antisera (Mira Vista, Copenhagen, Denmark) by following the recommendations of the manufacturer. Antibiotyping was used as a secondary subtyping approach. The antimicrobial resistance profiles of Salmonella isolates were tested using the Kirby-Bauer disc diffusion method, according to the guidelines established by the NCCLS (18). All isolates were tested for antimicrobial susceptibility to a panel of 12 antimicrobials (BD Diagnostics, Sparks, MD). The following antimicrobials and their respective disc potencies were used: ampicillin (Am; 10 μg), amoxicillin-clavulanic acid (Ax; 30 μg), amikacin (An; 30 μg), ceftriaxone (Ce; 30 μg), cephalothin (Ch; 30 μg), chloramphenicol (Cl; 30 μg), ciprofloxacin (CIP; 5 μg), gentamicin (Gm; 10 μg), kanamycin (Km; 30 μg), streptomycin (St; 10 μg), sulfisoxazole (Su; 250 μg), and tetracycline (Te; 30 μg). We used Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 25923, and Pseudomonas aeruginosa ATCC 27853 as reference strains. Each resistant isolate showing resistance to three or more antimicrobials was classified as multidrug resistant (MDR).
PFGE genotyping.
Salmonella isolates from feed (18 of 30 isolates) showing phenotypic characteristics (serogrouping and antimicrobial resistance patterns) similar to those of isolates from fecal samples (28 out of 179 isolates) were selected and genotyped using PFGE. PFGE was performed following the PulseNet protocol (22) of the Centers for Disease Control and Prevention (CDC). In brief, Salmonella isolates were grown overnight at 37°C on Trypticase soy agar (TSA) plates (Becton Dickinson, MD). We prepared bacterial cell suspensions in Falcon tubes containing 2 ml of cell suspension buffer (CSB; 100 mM Tris and 100 mM EDTA [pH 8.0]). Each adjusted cell suspension (optical density [OD] at 610 nm of 1.3 to 1.4) was transferred to a microcentrifuge tube containing proteinase K (20 mg/ml) and mixed gently. Agarose-embedded cells were then lysed with cell lysis buffer (CLB; 50 mM Tris and 50 mM EDTA [pH 8.0], 1% sarcosyl [Sigma, St. Louis, MO], 0.1 mg/ml proteinase K), and intact genomic DNA was digested with 50 U of the XbaI restriction enzyme (New England Biolabs, Ipswich, MA) for about 2 h at 37°C. The fragments were then separated using the CHEF-DR III pulsed-field gel electrophoresis system (Bio-Rad Laboratories, Hercules, CA). The PulseNet “universal” standard marker strain, Salmonella enterica serovar Braenderup H9812, was used as a molecular reference marker. PFGE images (stained with ethidium bromide) were analyzed with BioNumerics software version 4.6 (Applied Maths NV, Belgium). Cluster analysis was performed using the unweighted-pair group method using average linkages (UPGMA), with 2.0% band position tolerances and 1.5% optimization values. All isolates with PFGE banding patterns having similarity indexes of >85% were grouped within the same cluster.
RESULTS
A total of 275 pooled swine feed samples from nine farms representing three vertically integrated commercial swine production systems were examined, of which 3.6% were Salmonella positive. Salmonella was detected in feed from 8 of the 36 (22.2%) barns included in the study. In addition to swine feed samples, we also examined drag swab samples collected during pre- and postdisinfection of barn floors and fecal samples collected at early and late finishing stages from the same barns from which the feed samples were collected. Salmonella was detected in 15.3% (206/1,350) and 7.5% (101/1,350) of the drag swab samples pre- and postdisinfection, respectively, and detailed results have been previously reported (24). In fecal samples collected from pigs at two stages of production, a significant reduction of Salmonella (7.4%; 392/5,321) was detected when pigs reached market age compared to those collected from pigs at the early finishing stage (17.2%: 1,180/6,880).
Table 1 shows the sources and phenotypic characteristics of isolates (serogrouping and antimicrobial resistance profiles) recovered from feed, drag swab, and swine fecal samples. Of the total of 30 Salmonella isolates (3 colonies per positive sample) recovered from 10 of the feed samples, 90% (n = 27) were resistant to one or more of the antimicrobials tested, and 44.4% (n = 12) were multidrug resistant (MDR; resistance to ≥3 antimicrobials). The proportions of antimicrobial-resistant Salmonella isolates recovered from swine feed, feces, and barn floor swabs are shown in Table 1. The main MDR type (40%) was resistance type [R-type] Am St Te/Km, while another 40% were resistant to tetracycline (R-type Te only). The proportion of antimicrobial-resistant Salmonella isolates recovered from barn floors and feces was high compared to that recovered from feed (Table 1).
TABLE 1.
Phenotypic characteristics of Salmonella isolated from swine feed, barn floor swabs, and feces from the same barns, farms, and production systemsd
| Farmb | Stagec | Replicatea | Phenotypic characteristics of Salmonella isolates (no. of samples)e |
|||||
|---|---|---|---|---|---|---|---|---|
| Feed (30) |
Swabs (71) |
Feces (179) |
||||||
| Serogroup(s) | R type | Serogroup(s) | R type | Serogroup(s) | R type | |||
| RW7 | F1 | R1 | C | Su Te (3) | E | Te (3) | C | St Su Te (1) |
| C | Su Te (36) | |||||||
| A to I | Am Te (6) | |||||||
| B | Te (2) | |||||||
| RW6 | F2 | R1 | E | Te (3) | A to I | Te (1) | E | Te (15) |
| E | Su Te (3), St Su Te (1) | |||||||
| D | St Su Te (2), Te (1) | |||||||
| C | Te (2), St Su (2), Su (6) | |||||||
| C | Am St Su Te Km (1) | |||||||
| B | Am Cl St Su Te (4), Su (1) | |||||||
| B | Am Su Te Km (3) | |||||||
| RW4 | F1 | R1 | A to E | Am St Te (3) | B | Am Cl St Su Te (4) | E | Te (5) |
| B | Am Cl St Su Te/Ax (5) | |||||||
| C | Su Te (3) | |||||||
| A to I | Te (1), Am Te (2) | |||||||
| A to I | Am Su Te (1) | |||||||
| TT2A | R1 | A to I | Pansusceptible (3) | B | St Su Te (14) | |||
| B | Am Cl St Su Te (3) | |||||||
| C | St Su Te (1) | |||||||
| BH3 | R1 | A to I | Te (3) | B | St Su Te (4) | |||
| B | Am St Te Km (4) | |||||||
| Am St Su Te Km (1) | ||||||||
| RW4 | F1 | R3 | B | Te (3) | E | Te (1) | B | Te (20) |
| B | St Su Te (2) | C | Su Te (1) | |||||
| C | St Su Te (2) | |||||||
| E | Am Te (2) | |||||||
| E | Am Su Te (1) | |||||||
| RW6 | F1 | R3 | B | Te (3) | A to I | St Su Te (4) | B | Te (5) |
| B | St Su Te (6) | |||||||
| A to E | St Su Te (1) | |||||||
| GO2A | F2 | R3 | B | Am Cl St Su Te (3) | C | St Su Te (12) | A to I | Su Te (7) |
| C | Su Te (4) | A to I | Te (1) | |||||
| C | St Su (1) | E | Am St Su Te (1) | |||||
| G | Te (3) | |||||||
| E | Te (6) | |||||||
| FF12 | F2 | R3 | B | Am St Te Km (3) | B | Am St Te Km (23) | ||
| TE6A | F2 | R4 | B | Am St Te Km (3) | E | Te (1) | B (F2) | St Su Te (3) |
| B | Pansusceptible (3) | |||||||
Each replicate consists of sampling of designated barn floors before and after disinfection, sampling of pigs at early and late finishing stages per treatment group, and pooled feed sampling. We made a total of four replicate visits (R1 to R4) to each farm (n = 9) during the study period.
Includes the farm code (RW, TT, BH, FF, GO, or TE) and barn number (7, 6, 6A, 3, 4, 2A, or 12).
F1 = early finishing stage (6 to 9 weeks of age); F2 = late finishing stage (market age).
Salmonella isolates sharing similar serogrouping and antimicrobial resistance patterns are indicated in boldface.
Am, ampicillin; Ax, amoxicillin-clavulanic acid; Cl, chloramphenicol (30 μg); Km, kanamycin; St, streptomycin; Su, sulfisoxazole; Te, tetracycline.
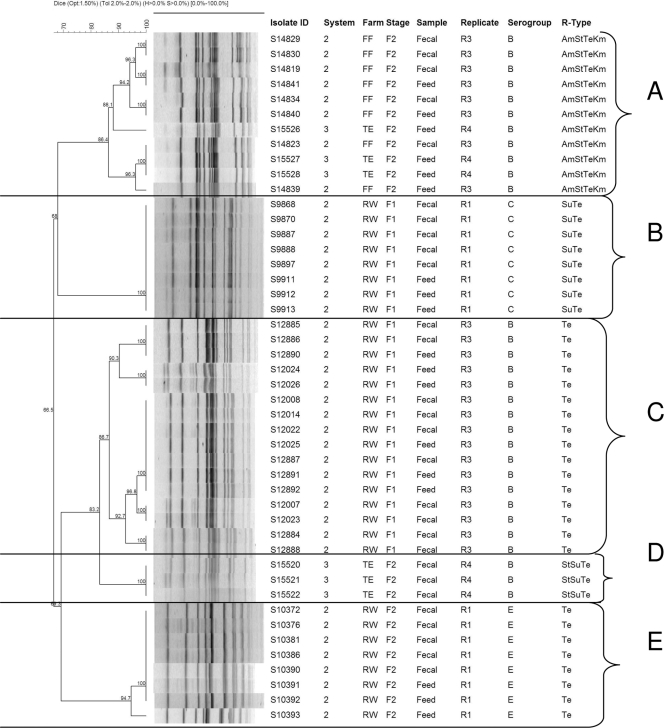
To determine the genotypic relatedness of Salmonella isolates recovered from feed and swine from the same farm, a total of 46 isolates (18 of 30 feed isolates; 28 of 179 fecal isolates) which showed similar phenotypic characteristics were subjected to PFGE DNA fingerprinting analysis. The isolates were selected systematically based on the source (farm, stage of production, and replicate), antimicrobial resistance profile, and serogroup identity. Isolates obtained from barn floor swabs (n = 71) did not share the same antimicrobial resistance patterns and serogrouping as the feed isolates (Table 1) and, hence, were not included in the PFGE analysis. PFGE DNA fingerprinting profiles grouped the 46 isolates into five genotypic clusters (clusters A, B, C, D, and E) at the 85% genetic relatedness threshold (Fig. 1).
FIG. 1.
Dendrogram of PFGE profiles of Salmonella recovered from swine feed and fecal samples and their association with sources (farm, production stage, and replicate), serogroups, and antimicrobial resistance patterns. The following are terms used in the dendrogram: system, swine production system (three vertically integrated production systems were included in the study, production systems 1, 2, and 3); farm, symbols FF, TE, and RW are used to represent farm names; stage, stage of production (F1, early finishing stage; F2, late finishing stage [market age]).
DISCUSSION
In this study, out of the total number of feed samples we examined, Salmonella was detected in 3.6% of them, and previous investigations also reported swine feed and feed ingredients as sources of Salmonella transmission (8, 11, 20, 21). The contamination of animal feed with important food-borne pathogens such as Salmonella could easily result in infection of food-producing animals and thereby increase the risk of food-borne infections in humans (15, 21). Crump and colleagues (3) reported that the presence of Salmonella in animal feeds indicates a failure to control Salmonella contamination early in the human food chain and contributes to the burden of food-borne illness. Thus, production of safe feed is one of the first steps in ensuring food safety and consumer protection. In those barns in which we recovered Salmonella in feed, Salmonella was also detected in drag swab and fecal samples, except for two instances in which Salmonella was recovered only from feed and drag swabs from two barns (Table 1), indicating the widespread occurrence of Salmonella in feed, swine, and swine production environments. Details on Salmonella distribution on barn floors before and after disinfection and antimicrobial resistance profiles have been reported previously (24). The feed samples collected from farms belonging to production system 1 were less contaminated with Salmonella than those from farms belonging to other production systems (2 and 3). It should be mentioned that not only feed samples but also fecal samples obtained from pigs at early and late finishing stages and barn floor swabs collected from farms belonging to production system 1 were relatively less contaminated with Salmonella than samples collected from farms under production systems 2 and 3. This could most probably be partly associated with better biosecurity systems at those farms. In another two barns (TT2A and BH3), Salmonella was isolated from swab and feed samples but not from fecal samples. The isolates from feed and swabs were also phenotypically different from each other, suggesting the possibility of different sources of Salmonella contamination.
The main MDR type exhibited among Salmonella isolates recovered from feed was R-type Am St Te/Km, while another 40% were resistant to tetracycline (R-type Te only). On the other hand, those isolates of fecal origin were R-type Am St Te Km (21.8%), followed by R-type St Su Te (9%). In contrast, those isolates recovered from barn floor swabs showed R-type St Su Te (52.1%). Serogrouping results showed that 50%, 45.1%, and 42% of the feed, drag swab, and fecal isolates, respectively, belonged to Salmonella serogroup B. Of the feed, barn floor swab, and fecal isolates from the same farm, 50% and 55.3% of those recovered from feed and feces, respectively, shared similar phenotypic characteristics (serogrouping and antimicrobial resistance profiles). In spite of the widespread occurrence of Salmonella in the barn floor swabs in this study, genotypic relatedness could not be demonstrated between feed and barn floor Salmonella isolates based on phenotypic traits (serogrouping and antimicrobial resistance patterns), and this could partly be associated with the limited number of Salmonella-positive feed samples from the farms included in the study or potentially due to the ecologic niche predilection of various strains, an area which has not yet been fully elucidated. Huehn and colleagues (12) reported that the inclusion and reassortment of specific genes (e.g., prophage-associated virulence genes) could enable some Salmonella serovars to adapt to different environmental conditions and conquer ecological niches, which could be reflected in serovar-specific ecology. It is often understood that a given Salmonella serovar could be represented in multiple ecological compartments in a given animal production site (1).
PGFE genotyping results were consistent with the phenotypic findings (serogrouping, antimicrobial resistance patterns, and sources of isolates). Results from the PFGE fingerprinting profiling indicated that the 46 isolates belonged to five genotypic clusters (clusters A, B, C, D, and E) at the 85% genetic relatedness threshold (Fig. 1). In four of five genotypic clusters (A, B, C, and E), we found genotypically related isolates of feed and fecal origin, indicating the potential of Salmonella dissemination via processed feed. Cluster A consisted of feed (n = 6) and fecal (n = 4) isolates which belonged to the same serogroup (serogroup B) and had the same antimicrobial resistance pattern (Am St Te Km). These isolates originated from two production systems, showing the genotypic relatedness of isolates from different production systems and sources (feed and feces). In addition, the feed and fecal isolates from both farms (farms FF and TE) were recovered from pigs in the late finishing stage (F2; market age) and from feed presented to pigs in the F2 stage in replicates 3 (farm FF) and 4 (farm TE). However, fecal isolates from the same farm (TE) were grouped in a different cluster (cluster D) and had different antimicrobial resistance patterns (R-type St Su Te) than feed isolates from the same farm showing a different antimicrobial resistance type (R-type Am St Te Km), demonstrating some diversity of origin of the Salmonella isolates recovered from the same barn and potentially the emergence and dissemination of antimicrobial-resistant strains within the swine production system. A similar finding was observed in four of the five clusters, as depicted in Fig. 1.
The PFGE patterns of feed isolates were indistinguishable from those of fecal isolates collected on the same day from the same farm. PFGE has already been used as a tool to track sources of Salmonella contamination in livestock feed, as well as for large-scale nationwide monitoring purposes (PulseNet). For instance, Davis et al. (6) utilized PFGE with a sufficient level of discrimination to demonstrate whether the contamination of cattle feeds with Salmonella and Escherichia coli O157:H7 has a role as a vehicle in the transmission of these pathogens to cattle on farms. The findings of the study by Davis et al. (6) provided support for the hypothesis in our current study that feedstuff serve as a vehicle to introduce Salmonella and other food-borne pathogens to food animals, which could be a significant public health hazard. In poultry (2), PFGE was employed to demonstrate if the same Salmonella enterica strains present in broiler feed could be detected in raw, frozen chicken nuggets and strips available for human consumption. PFGE has been used as one of the reliable and discriminatory genotyping methods in subtyping food-borne pathogens such as Salmonella. It is considered the “gold standard” method specifically for food-borne pathogens, has been standardized, and is used routinely by laboratories in PulseNet (22). It should also be noted that PFGE is an indirect way of detecting genomic variations based on macrorestriction patterns. Therefore, its utility for genomic purposes is very limited, though it is well accepted as a genotyping approach. For large-scale epidemiologic investigations, other subtyping methods such as amplified fragment length polymorphism (AFLP) could be a better choice for genotyping purposes since they are high throughput (10).
Of the total of 30 Salmonella isolates recovered from feed, 18 were randomly selected for PFGE based on phenotypic characteristics (serogrouping and antimicrobial resistance patterns), and only 28 isolates of a total of 179 fecal samples were randomly selected. Using simple probability, the chance that similar phenotypes originated from the same feed sample further genotyped is likely, whereas there is low probability that the isolates of fecal origin originated from the same fecal sample, since only 28 of 179 isolates were genotyped. Regardless, we believe that this has a low impact on the findings, since the focus of the study is comparison between the different types of samples rather than clonality or heterogeneity within the same types of samples. Even though the number of feed samples we analyzed and the number of farms included in the study were limited, the feed samples represented three major commercial swine production systems and nine farms in the study area and demonstrated the presence of Salmonella, including multidrug-resistant (MDR) strains in commercially processed swine feeds. Based on the phenotypic characteristics (antimicrobial resistance profiles and serogrouping) and PFGE fingerprinting, the findings indicated that more than 50% of the Salmonella isolates recovered from feed shared genotypic clonality with those detected in swine fecal samples, suggesting the dissemination of Salmonella via feed. The presence of highly clonal Salmonella isolates with identical phenotypic traits strongly suggests an epidemiological link between Salmonella in swine feed and fecal samples. The circulation of genotypically related strains of Salmonella, including multidrug-resistant isolates in commercial swine feed and swine, indicates the need for sustainable intervention strategies targeting multiple compartments to control feed as a vehicle of MDR Salmonella strains in swine production units and to safeguard human and animal health.
Acknowledgments
This work was funded by the USDA-NRI, Epidemiological Approaches for Food Safety (grant 2007-01778).
We thank members of the Infectious Diseases Molecular Epidemiology Laboratory (IDMEL) for technical assistance in sample processing.
Footnotes
Published ahead of print on 17 September 2010.
REFERENCES
- 1.Barber, D. A., P. B. Bahnson, R. Isaacson, C. J. Jones, and R. M. Weigel. 2002. Distribution of Salmonella in swine production ecosystems. J. Food Prot. 65:1861-1868. [DOI] [PubMed] [Google Scholar]
- 2.Bucher, O., R. A. Holley, R. Ahmed, H. Tabor, C. Nadon, L. K. Ng, and J. Y. D'Aoust. 2007. Occurrence and characterization of Salmonella from chicken nuggets, strips, and pelleted broiler feed. J. Food Prot. 70:2251-2258. [DOI] [PubMed] [Google Scholar]
- 3.Crump, J. A., P. M. Griffin, and F. J. Angulo. 2002. Bacterial contamination of animal feed and its relationship to human foodborne illness. Clin. Infect. Dis. 35:859-865. [DOI] [PubMed] [Google Scholar]
- 4.Davies, R. P., W. E. Morrow, J. F. T. Jones, J. Deen, P. J. Fedorka-Cray, and I. T. Harris. 1997. Prevalence of Salmonella in finishing swine raised in different production systems in North Carolina, U. S. A. Epidemiol. Infect. 119:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies, R. P., H. Scotthurd, J. A. Funk, P. J. Fedorka-Cray, and F. T. Jones. 2004. The role of contaminated feed in the epidemiology and control of Salmonella enterica in pork production. Foodborne Pathog. Dis. 1:202-215. [DOI] [PubMed] [Google Scholar]
- 6.Davis, M. A., D. D. Hancock, D. H. Rice, D. R. Call, R. Digiacomo, M. Samadpou, and T. E. Bessser. 2003. Feedstuffs as a vehicle of cattle exposure to Escherichia coli O157:H7 and Salmonella enterica. Vet. Microbiol. 95:199-210. [DOI] [PubMed] [Google Scholar]
- 7.Farzan, A., R. M. Friendship, C. E. Dewey, K. Warriner, C. Poppe, and K. Klotins. 2006. Prevalence of Salmonella spp. on Canadian pig farms using liquid or dry-feeding. Prev. Vet. Med. 73:241-254. [DOI] [PubMed] [Google Scholar]
- 8.Fedorka-Cray, P. J., A. Hogg, J. T. Gray, K. Lorenzen, J. Velasquez, and P. von Behren. 1997. Feed and feed trucks as sources of Salmonella contamination in swine. Swine Health Prod. 5:189-193. [Google Scholar]
- 9.Gebreyes, W. A., R. P. Davies, P.-K. Turkson, W. E. M. Morrow, J. A. Funk, and C. Altier. 2004. Salmonella enterica serovars from pigs on farms and after slaughter and validity of using bacteriologic data to define herd Salmonella status. J. Food Prot. 67:691-697. [DOI] [PubMed] [Google Scholar]
- 10.Gebreyes, W. A., C. Altier, and S. Thakur. 2006. Molecular epidemiology and diversity of Salmonella serovar Typhimurium in pigs using phenotypic and genotypic approaches. Epidemiol. Infect. 134:187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris, I. T., P. J. Fedorka-Cray, J. T. Gray, L. A. Thomas, and K. Ferris. 1997. Prevalence of Salmonella organisms in swine feed. J. Am. Vet. Med. Assoc. 210:382-385. [PubMed] [Google Scholar]
- 12.Huehn, S., R. M. La Ragione, M. Anjum, M. Saunders, M. J. Woodward, C. Bunge, R. Helmuth, E. Hauser, B. Guerra, J. Beutlich, A. Brisabois, T. Peters, L. Svensson, G. Madajczak, E. Litrup, A. Imre, S. Herrera-Leon, D. Mevius, D. G. Newell, and B. Malorny. 2010. Virulotyping and antimicrobial resistance typing of Salmonella enterica serovars relevant to human health in Europe. Foodborne Pathog. Dis. 7:523-535. [DOI] [PubMed] [Google Scholar]
- 13.Isaacson, R. E., L. D. Firkins, R. M. Weigel, F. A. Zuckermann, and J. A. DiPietro. 1999. Effect of transportation and feed withdrawal on shedding of Salmonella Typhimurium among experimentally infected pigs. Am. J. Vet. Res. 60:1155-1158. [PubMed] [Google Scholar]
- 14.Jones, P. W., P. Collins, G. T. Brown, and M. Aitken. 1982. Transmission of Salmonella Mbandaka to cattle from contaminated feed. J. Hyg. (Lond.) 88:255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, F. T., and K. E. Richardson. 2004. Salmonella in commercially manufactured feeds. Poult. Sci. 83:384-391. [DOI] [PubMed] [Google Scholar]
- 16.Kidd, R. S., A. M. Rossignol, and M. J. Gamroth. 2002. Salmonella and other Enterobacteriaceae in dairy-cow feed ingredients: antimicrobial resistance in western Oregon. J. Environ. Health 64:9-16. [PubMed] [Google Scholar]
- 17.Lindqvist, N., S. Heinkainen, A.-M. Toivonen, and S. Pelkonen. 1999. Discrimination between endemic and feedborne Salmonella Infantis infection in cattle by molecular typing. Epidemiol. Infect. 122:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard, 2nd ed. M31-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 19.Nesse, L. L., T. Refusum, E. Heir, K. Nordby, T. Vardund, and G. Holstad. 2005. Molecular epidemiology of Salmonella spp. isolates from gulls, fish-meal factories, feed factories, animals and humans in Norway based on pulsed-field gel electrophoresis. Epidemiol. Infect. 133:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osterberg, J., I. Vågsholm, S. Boqvist, and S. S. Lewerin. 2006. Feed-borne outbreak of Salmonella Cubana in Swedish pig farms: risk factors and factors affecting the restriction period in infected farms. Acta Vet. Scand. 47:13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadopoulou, C., J. J. Carrique-Mas, R. H. Davies, and A. R. Sayers. 2009. Retrospective analysis of Salmonella isolates recovered from animal feed in Great Britain. Vet. Rec. 165:681-688. [PubMed] [Google Scholar]
- 22.Ribot, E. M., M. A. Fair, R. Gautom, D. N. Cameron, S. B. Hunter, B. Swaminathan, and T. J. Barrett. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3:59-67. [DOI] [PubMed] [Google Scholar]
- 23.Wierup, M., and P. Haggblom. 2010. An assessment of soybeans and other vegetable proteins as source of Salmonella contamination in pig production. Acta Vet. Scand. 52:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zewde, B. M., R. Robbins, M. J. Abley, B. House, W. E. M. Morrow, and W. A. Gebreyes. 2009. Comparison of Swiffer wipes and conventional drag swab methods in the recovery of Salmonella from swine production environment. J. Food Prot. 72:142-146. [DOI] [PubMed] [Google Scholar]