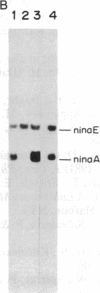
Abstract
Mutations in the ninaA gene of Drosophila severely reduce the amount of rhodopsin specifically in R1-6 photoreceptors. Isolation of the ninaA gene by chromosomal walking revealed that it is expressed only in the eye and encodes a 237-amino acid polypeptide that shows strong sequence similarity to cyclophilin, a putative molecular target for cyclosporine A, a potent immunosuppressant used in human organ transplantations. Unlike most cyclophilins characterized to date, the ninaA-encoded protein has a putative signal sequence and a transmembrane domain. Each of the three ehtyl methanesulfonate-induced ninaA mutant alleles analyzed shows a single nucleotide change in the mRNA coding region leading to either a nonsense or a missense mutation. We find no evidence that the ninaA-encoded protein is directly involved in phototransduction. The only detectable mutant phenotype that correlates with the severity of molecular defects in the three mutants is the amount of depletion of R1-6 rhodopsin. The above results and the recent findings that cyclophilin is a peptidylprolyl cis-trans-isomerase suggest that the ninaA-encoded protein may be required for proper folding and stability of R1-6 rhodopsin.
Full text
PDF
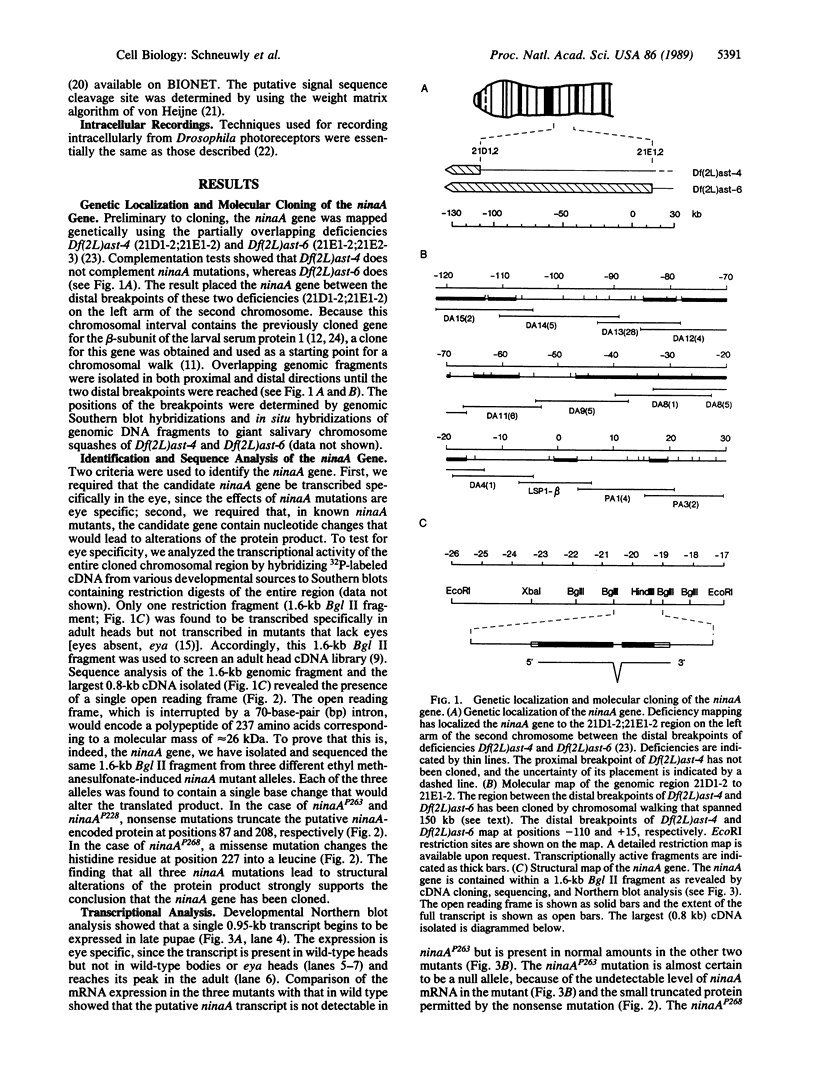

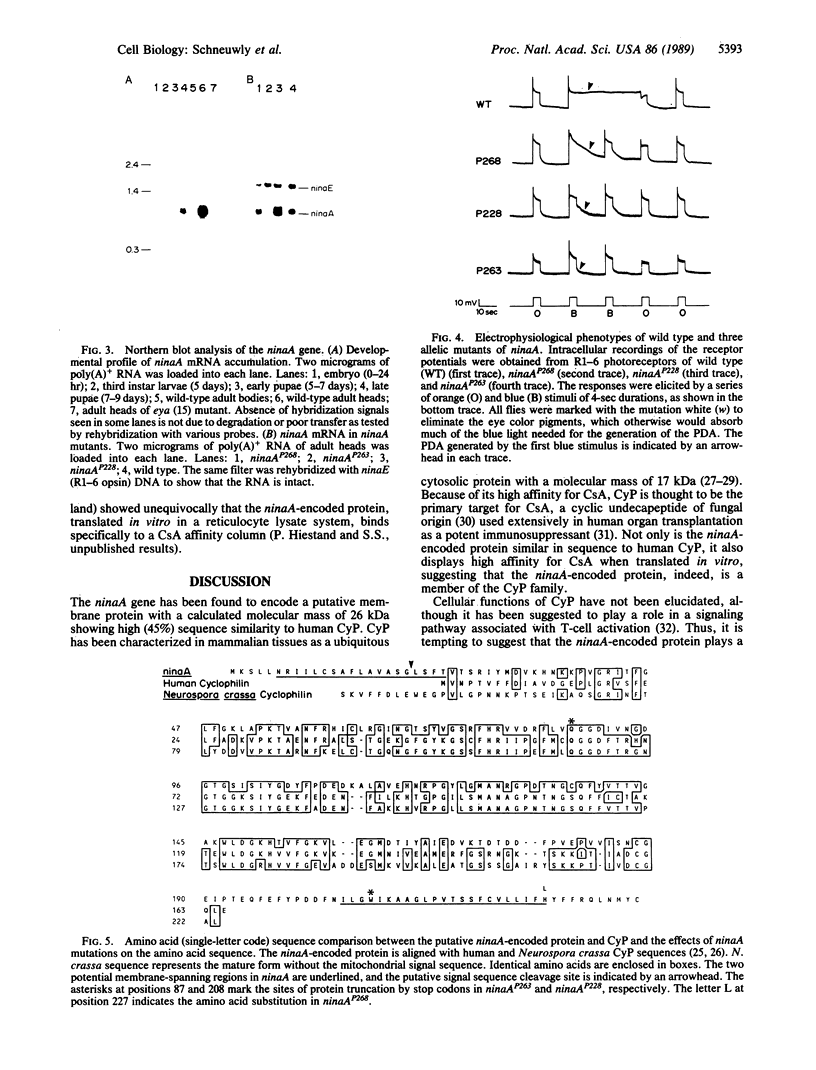

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender W., Spierer P., Hogness D. S. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. J Mol Biol. 1983 Jul 25;168(1):17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- Beveridge T. Clinical transplantation--overview. Prog Allergy. 1986;38:269–292. [PubMed] [Google Scholar]
- Borel J. F. Ciclosporin and its future. Prog Allergy. 1986;38:9–18. [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Haendler B., Hofer-Warbinek R., Hofer E. Complementary DNA for human T-cell cyclophilin. EMBO J. 1987 Apr;6(4):947–950. doi: 10.1002/j.1460-2075.1987.tb04843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E., Speicher D. W. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986 Jun 25;261(18):8547–8555. [PubMed] [Google Scholar]
- Johnson E. C., Pak W. L. Electrophysiological study of Drosophila rhodopsin mutants. J Gen Physiol. 1986 Nov;88(5):651–673. doi: 10.1085/jgp.88.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletsky A. J., Harding M. W., Handschumacher R. E. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. J Immunol. 1986 Aug 1;137(3):1054–1059. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrivee D. C., Conrad S. K., Stephenson R. S., Pak W. L. Mutation that selectively affects rhodopsin concentration in the peripheral photoreceptors of Drosophila melanogaster. J Gen Physiol. 1981 Nov;78(5):521–545. doi: 10.1085/jgp.78.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesant J. A., Levine M., Garen A., Lepesant-Kejzlarvoa J., Rat L., Somme-Martin G. Developmentally regulated gene expression in Drosophila larval fat bodies. J Mol Appl Genet. 1982;1(5):371–383. [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Conrad B. Improved programs for DNA and protein sequence analysis on the IBM personal computer and other standard computer systems. Nucleic Acids Res. 1986 Jan 10;14(1):443–454. doi: 10.1093/nar/14.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tousa J. E., Baehr W., Martin R. L., Hirsh J., Pak W. L., Applebury M. L. The Drosophila ninaE gene encodes an opsin. Cell. 1985 Apr;40(4):839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. B., Brock H. W., Rudden N. C., Evans-Roberts S. A Genetic and Cytogenetic Analysis of the Region Surrounding the Lsp-1 beta-Gene in DROSOPHILA MELANOGASTER. Genetics. 1985 Jan;109(1):145–156. doi: 10.1093/genetics/109.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Kuroiwa A., Gehring W. J. Molecular analysis of the dominant homeotic Antennapedia phenotype. EMBO J. 1987 Jan;6(1):201–206. doi: 10.1002/j.1460-2075.1987.tb04739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh B. H., Stamnes M. A., Seavello S., Harris G. L., Zuker C. S. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature. 1989 Mar 2;338(6210):67–70. doi: 10.1038/338067a0. [DOI] [PubMed] [Google Scholar]
- Smith D. F., McClelland A., White B. N., Addison C. F., Glover D. M. The molecular cloning of a dispersed set of developmentally regulated genes which encode the major larval serum protein of D. melanogaster. Cell. 1981 Feb;23(2):441–449. doi: 10.1016/0092-8674(81)90139-2. [DOI] [PubMed] [Google Scholar]
- Stephenson R. S., O'Tousa J., Scavarda N. J., Randall L. L., Pak W. L. Drosophila mutants with reduced rhodopsin content. Symp Soc Exp Biol. 1983;36:477–501. [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Nicholson D. W., Hartl F. U., Köhler H., Pfanner N., Wachter E., Neupert W. Cyclosporin A-binding protein (cyclophilin) of Neurospora crassa. One gene codes for both the cytosolic and mitochondrial forms. J Biol Chem. 1988 Oct 5;263(28):14433–14440. [PubMed] [Google Scholar]
- Zuker C. S., Cowman A. F., Rubin G. M. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985 Apr;40(4):851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Zuker C. S., Mismer D., Hardy R., Rubin G. M. Ectopic expression of a minor Drosophila opsin in the major photoreceptor cell class: distinguishing the role of primary receptor and cellular context. Cell. 1988 May 6;53(3):475–482. doi: 10.1016/0092-8674(88)90167-5. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]