Abstract
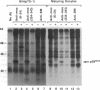
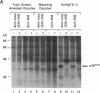
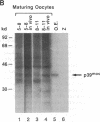
We have identified the mouse Mos-encoded protein product, p39mos, in maturing mouse oocytes and have shown that it is indistinguishable from the product expressed in Mos-transformed NIH 3T3 cells. p39mos is detected in oocytes arrested in the first meiotic prophase, during germinal-vesicle breakdown, metaphase I, anaphase I, and in ovulated eggs. We show that microinjection of three different Mos antisense (but not sense) oligodeoxyribonucleotides into germinal vesicle-stage oocytes prevents first polar-body emission and therefore interrupted the normal progression of meiosis. These results show that in mouse oocytes, as in the amphibian Xenopus [Sagata, N., Oskarsson, M., Copeland, T., Brumbaugh, J. & Vande Woude, G.F. (1988) Nature (London) 335, 519-525], the product of Mos is necessary for normal meiotic maturation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair D. G., McClements W. L., Oskarsson M. K., Fischinger P. J., Vande Woude G. F. Biological activity of cloned Moloney sarcoma virus DNA: Terminally redundant sequences may enhance transformation efficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3504–3508. doi: 10.1073/pnas.77.6.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Oskarsson M., Wood T. G., McClements W. L., Fischinger P. J., Vande Woude G. G. Activation of the transforming potential of a normal cell sequence: a molecular model for oncogenesis. Science. 1981 May 22;212(4497):941–943. doi: 10.1126/science.7233190. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Donahue R. P., Szollosi D. Germinal vesicle breakdown in the mouse oocyte. J Cell Sci. 1972 Mar;10(2):369–385. doi: 10.1242/jcs.10.2.369. [DOI] [PubMed] [Google Scholar]
- Cho W. K., Stern S., Biggers J. D. Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J Exp Zool. 1974 Mar;187(3):383–386. doi: 10.1002/jez.1401870307. [DOI] [PubMed] [Google Scholar]
- Dash P., Lotan I., Knapp M., Kandel E. R., Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekel N., Beers W. H. Rat oocyte maturation in vitro: relief of cyclic AMP inhibition by gonadotropins. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4369–4373. doi: 10.1073/pnas.75.9.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs S. M., Coleman D. L., Ward-Bailey P. F., Eppig J. J. Hypoxanthine is the principal inhibitor of murine oocyte maturation in a low molecular weight fraction of porcine follicular fluid. Proc Natl Acad Sci U S A. 1985 Jan;82(2):454–458. doi: 10.1073/pnas.82.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs S. M., Daniel S. A., Eppig J. J. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool. 1988 Jan;245(1):86–96. doi: 10.1002/jez.1402450113. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Downs S. M. The effect of hypoxanthine on mouse oocyte growth and development in vitro: maintenance of meiotic arrest and gonadotropin-induced oocyte maturation. Dev Biol. 1987 Feb;119(2):313–321. doi: 10.1016/0012-1606(87)90037-6. [DOI] [PubMed] [Google Scholar]
- Froehler B. C., Ng P. G., Matteucci M. D. Synthesis of DNA via deoxynucleoside H-phosphonate intermediates. Nucleic Acids Res. 1986 Jul 11;14(13):5399–5407. doi: 10.1093/nar/14.13.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Goldman D. S., Kiessling A. A., Millette C. F., Cooper G. M. Expression of c-mos RNA in germ cells of male and female mice. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4509–4513. doi: 10.1073/pnas.84.13.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Kishimoto T. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Dev Biol. 1988 Apr;126(2):242–252. doi: 10.1016/0012-1606(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Herzog N. K., Singh B., Elder J., Lipkin I., Trauger R. J., Millette C. F., Goldman D. S., Wolfes H., Cooper G. M., Arlinghaus R. B. Identification of the protein product of the c-mos proto-oncogene in mouse testes. Oncogene. 1988 Aug;3(2):225–229. [PubMed] [Google Scholar]
- Jessus C., Cazenave C., Ozon R., Hélène C. Specific inhibition of endogenous beta-tubulin synthesis in Xenopus oocytes by anti-messenger oligodeoxynucleotides. Nucleic Acids Res. 1988 Mar 25;16(5):2225–2233. doi: 10.1093/nar/16.5.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M., Bosselman R. A., van der Hoorn F. A., Berns A., Fan H., Verma I. M. Identification and molecular cloning of Moloney mouse sarcoma virus-specific sequences from uninfected mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2651–2655. doi: 10.1073/pnas.77.5.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S. Quantitative hybridization-arrest of mRNA in Xenopus oocytes using single-stranded complementary DNA or oligonucleotide probes. Nucleic Acids Res. 1985 Jul 11;13(13):4991–5004. doi: 10.1093/nar/13.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Rosenberg M. P., Mercer J. A., Propst F., Vande Woude G. F., Jenkins N. A., Copeland N. G. Developmental regulation of ovarian-specific Mos expression. Oncogene. 1988 Mar;2(3):235–240. [PubMed] [Google Scholar]
- Kishimoto T., Kuriyama R., Kondo H., Kanatani H. Generality of the action of various maturation-promoting factors. Exp Cell Res. 1982 Jan;137(1):121–126. doi: 10.1016/0014-4827(82)90014-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M., Nurse P. Cell cycle control genes in fission yeast and mammalian cells. Trends Genet. 1988 Oct;4(10):287–290. doi: 10.1016/0168-9525(88)90171-0. [DOI] [PubMed] [Google Scholar]
- Magnusson C., Hillensjö T. Inhibition of maturation and metabolism in rat oocytes by cyclic amp. J Exp Zool. 1977 Jul;201(1):139–147. doi: 10.1002/jez.1402010117. [DOI] [PubMed] [Google Scholar]
- Marcus-Sekura C. J., Woerner A. M., Shinozuka K., Zon G., Quinnan G. V., Jr Comparative inhibition of chloramphenicol acetyltransferase gene expression by antisense oligonucleotide analogues having alkyl phosphotriester, methylphosphonate and phosphorothioate linkages. Nucleic Acids Res. 1987 Jul 24;15(14):5749–5763. doi: 10.1093/nar/15.14.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Maxwell S. A., Arlinghaus R. B. A cAMP-independent serine/threonine kinase activity is associated with the mos sequences of ts110 Moloney murine sarcoma virus-encoded P85gag-mos. J Gen Virol. 1985 Oct;66(Pt 10):2135–2146. doi: 10.1099/0022-1317-66-10-2135. [DOI] [PubMed] [Google Scholar]
- Maxwell S. A., Arlinghaus R. B. Serine kinase activity associated with Maloney murine sarcoma virus-124-encoded p37mos. Virology. 1985 May;143(1):321–333. doi: 10.1016/0042-6822(85)90119-9. [DOI] [PubMed] [Google Scholar]
- Mutter G. L., Wolgemuth D. J. Distinct developmental patterns of c-mos protooncogene expression in female and male mouse germ cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5301–5305. doi: 10.1073/pnas.84.15.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Lai M. H., Hunter T., Verma I. M. Analysis of transforming gene products from Moloney murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):109–119. doi: 10.1016/0092-8674(81)90365-2. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Nigg E. A., Hunter T. The transforming protein of Moloney murine sarcoma virus is a soluble cytoplasmic protein. Cell. 1983 May;33(1):161–172. doi: 10.1016/0092-8674(83)90345-8. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Verma I. M., Hunter T. Detection of a transforming gene product in cells transformed by Moloney murine sarcoma virus. Cell. 1982 Jun;29(2):417–426. doi: 10.1016/0092-8674(82)90158-1. [DOI] [PubMed] [Google Scholar]
- Propst F., Rosenberg M. P., Iyer A., Kaul K., Vande Woude G. F. c-mos proto-oncogene RNA transcripts in mouse tissues: structural features, developmental regulation, and localization in specific cell types. Mol Cell Biol. 1987 May;7(5):1629–1637. doi: 10.1128/mcb.7.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst F., Vande Woude G. F. Expression of c-mos proto-oncogene transcripts in mouse tissues. Nature. 1985 Jun 6;315(6019):516–518. doi: 10.1038/315516a0. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987 May 22;49(4):559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Oskarsson M. K., Dunn J. K., Blair D. G., Hughes S., Propst F., Vande Woude G. F. Chicken homolog of the mos proto-oncogene. Mol Cell Biol. 1988 Feb;8(2):923–929. doi: 10.1128/mcb.8.2.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A. C., Eppig J. J. The developmental capacity of mouse oocytes that matured spontaneously in vitro is normal. Dev Biol. 1984 Apr;102(2):493–497. doi: 10.1016/0012-1606(84)90215-x. [DOI] [PubMed] [Google Scholar]
- Seth A., Priel E., Vande Woude G. F. Nucleoside triphosphate-dependent DNA-binding properties of mos protein. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3560–3564. doi: 10.1073/pnas.84.11.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Vande Woude G. F. Nucleotide sequence and biochemical activities of the Moloney murine sarcoma virus strain HT-1 mos gene. J Virol. 1985 Oct;56(1):144–152. doi: 10.1128/jvi.56.1.144-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth J., Colman A. Antisense oligonucleotide-directed cleavage of mRNA in Xenopus oocytes and eggs. EMBO J. 1988 Feb;7(2):427–434. doi: 10.1002/j.1460-2075.1988.tb02830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Wittenberg C., Reed S. I., Arlinghaus R. B. Moloney murine sarcoma virus encoded p37mos expressed in yeast has protein kinase activity. Virology. 1986 Jul 30;152(2):502–506. doi: 10.1016/0042-6822(86)90156-x. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Ecker R. E. The interaction of steroids with Rana pipiens Oocytes in the induction of maturation. Dev Biol. 1971 Jun;25(2):232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Strickland S., Huarte J., Belin D., Vassalli A., Rickles R. J., Vassalli J. D. Antisense RNA directed against the 3' noncoding region prevents dormant mRNA activation in mouse oocytes. Science. 1988 Aug 5;241(4866):680–684. doi: 10.1126/science.2456615. [DOI] [PubMed] [Google Scholar]
- Sunkara P. S., Wright D. A., Rao P. N. Mitotic factors from mammalian cells induce germinal vesicle breakdown and chromosome condensation in amphibian oocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2799–2802. doi: 10.1073/pnas.76.6.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Josefowicz W. J., Letourneau G. E. Meiotic maturation of mouse oocytes in vitro: inhibition of maturation at specific stages of nuclear progression. J Cell Sci. 1976 Dec;22(3):531–545. doi: 10.1242/jcs.22.3.531. [DOI] [PubMed] [Google Scholar]
- Watson R., Oskarsson M., Vande Woude G. F. Human DNA sequence homologous to the transforming gene (mos) of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4078–4082. doi: 10.1073/pnas.79.13.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. G., McGeady M. L., Baroudy B. M., Blair D. G., Vande Woude G. F. Mouse c-mos oncogene activation is prevented by upstream sequences. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7817–7821. doi: 10.1073/pnas.81.24.7817. [DOI] [PMC free article] [PubMed] [Google Scholar]