Abstract
Mating in hemiascomycete yeasts involves the secretion of pheromones that induce sexual differentiation in cells of the opposite mating type. Studies in Saccharomyces cerevisiae have revealed that a subpopulation of cells experiences cell death during exposure to pheromone. In this work, we tested whether the phenomenon of pheromone-induced death (PID) also occurs in the opportunistic pathogen Candida albicans. Mating in C. albicans is uniquely regulated by white-opaque phenotypic switching; both cell types respond to pheromone, but only opaque cells undergo the morphological transition and cell conjugation. We show that approximately 20% of opaque cells, but not white cells, of laboratory strain SC5314 experience pheromone-induced death. Furthermore, analysis of mutant strains revealed that PID was significantly reduced in strains lacking Fig1 or Fus1 transmembrane proteins that are induced during the mating process and, we now show, are necessary for efficient mating in C. albicans. The level of PID was also Ca2+ dependent, as chelation of Ca2+ ions increased cell death to almost 50% of the population. However, in contrast to S. cerevisiae PID, pheromone-induced killing of C. albicans cells was largely independent of signaling via the Ca2+-dependent protein phosphatase calcineurin, even when combined with the loss of Cmk1 and Cmk2 proteins. Finally, we demonstrate that levels of PID vary widely between clinical isolates of C. albicans, with some strains experiencing close to 70% cell death. We discuss these findings in light of the role of prodeath and prosurvival pathways operating in yeast cells undergoing the morphological response to pheromone.
Mating in the model yeast Saccharomyces cerevisiae, as well as in the related hemiascomycete Candida albicans, involves communication between a and α cells via sexual pheromones. The program of sexual differentiation has been particularly well defined in S. cerevisiae, where pheromone signaling activates a conserved mitogen-activated protein kinase (MAPK) cascade culminating in the transcriptional response of almost 500 genes (44). During mating, yeast cells increase the expression of genes required for conjugation, undergo arrest in G1 of the cell cycle, and produce polarized mating projections in preparation for cell fusion (52). Mating also requires partial degradation of the cell wall to allow direct membrane fusion between a and α cells, followed by nuclear karyogamy and formation of a diploid cell. Previous studies have established that a subpopulation of S. cerevisiae a cells responding to high levels of α pheromone undergoes cell death (23, 48, 58). In these studies, pheromone treatment resulted in 25 to 30% cell death. However, prevention of a Ca2+ ion influx resulted in cell death in up to 95% of the population (23, 58). High levels of pheromone-induced death (PID) were also observed in mutants lacking components required for calcium signaling, including the high-affinity Ca2+ influx channel (Cch1/Mid1), calmodulin (Cmd1), and calcineurin (Cnb1). Calcineurin is a highly conserved protein phosphatase whose activity is regulated by Ca2+/calmodulin and is known to play a role in the cellular response of yeast cells to environmental stresses (13). In general, it is thought that a rise in cytosolic Ca2+ levels activates calcineurin, leading to dephosphorylation of key target proteins, including the transcription factor Crz1p (54). The calcineurin signaling pathway also plays a central role in protecting yeast cells from the toxic effects of multiple antifungal drugs (9, 55).
In addition to Ca2+ signaling, several other pathways influence the sensitivity of S. cerevisiae cells to pheromone toxicity, including multiple factors upregulated during the morphological response to pheromone. These include Fus1 and Fig1, two cell membrane proteins required for efficient cell fusion (16, 35, 58). Fus1 localizes to the cell tip of mating projections and coordinates the process of cell fusion, including the secretion of cell wall hydrolases that degrade the cell wall (36, 56). Fig1 forms part of a low-affinity Ca2+ influx system, although its role in PID appears independent of its function in Ca2+ transport (58). Mutants lacking either Fig1 or Fus1 exhibit significantly reduced levels of PID, indicating that although these factors are necessary for efficient mating, their expression can also compromise cell viability.
To combat the potentially lethal effects of pheromone treatment, S. cerevisiae cells activate repair pathways that help maintain cell viability. Cell wall degradation induces the cell wall integrity (CWI) signaling pathway, which involves a protein kinase cascade culminating in the activation of the MAPK, Slt2 (8, 27). Activation of the CWI pathway enhances chitin and glucan synthesis to compensate for cell wall damage induced by the mating program. Loss of CWI signaling therefore leads to greater death in the presence of pheromone, whereas hyperactivation of the CWI pathway limits pheromone-induced death (8, 58).
Candida albicans is a related hemiascomycete yeast that last shared a common ancestor with S. cerevisiae around 700 million years ago (19). C. albicans is a prevalent human fungal pathogen and was originally categorized as an asexual species. Work during the past decade has uncovered an elaborate mating cycle that, while similar to that of S. cerevisiae, also shows fundamental differences. For example, C. albicans mating takes place between a and α cells but requires that cells switch from the regular “white” state to the alternative “opaque” state (33). The regulation of mating by a phenotypic switch is unique to C. albicans (and the sister species Candida dubliniensis) and may have evolved due to the close association of this yeast with its mammalian host (26, 42). Though only opaque cells secrete sex-specific pheromones, both white and opaque cells can respond to pheromone. Opaque cells form polarized mating projections and subsequently undergo cell fusion, while white cells exhibit increased adhesion and biofilm formation (3, 4, 14, 29, 59). It is speculated that the matrix formed by responding white cells helps maintain pheromone gradients and thereby promotes cell fusion between mating opaque cells in vivo (14, 50).
In this work, we examine the response of C. albicans cells to pheromone and demonstrate that cell death occurs in a subpopulation of cells similar to that shown in S. cerevisiae. C. albicans opaque cells, but not white cells, experience cell death, consistent with opaque cells undergoing the morphological response to pheromone leading to the formation of polarized mating projections. We also tested whether the same genetic pathways acting in S. cerevisiae contribute to C. albicans cell death, including the cell integrity pathway, the calcineurin signaling pathway, and the mating factors Fus1 and Fig1. Notable differences were found between the two yeast species in response to pheromone treatment, including a limited role for calcineurin signaling in protecting C. albicans cells compared to that in S. cerevisiae. These results demonstrate that the phenomenon of pheromone-induced death is shared by two diverse hemiascomycete yeasts but that the mechanisms protecting yeast cells from pheromone toxicity differ. We further demonstrate that clinical isolates exhibit a remarkable range in their susceptibility to pheromone killing (from 2% to almost 70%), indicating that some C. albicans strains are highly susceptible to pheromone-induced cell death while others are resistant to pheromone killing.
MATERIALS AND METHODS
Media and reagents.
Standard laboratory media were prepared as described previously (18). Spider medium contained 1.35% agar, 1% nutrient broth, 0.4% potassium phosphate (pH 7.2) supplemented with 2% mannitol (28). SCD medium refers to synthetic complete medium supplemented with 2% glucose. α pheromone MF13 (GFRLTNFGYFEPG) was synthesized by Genemed Synthesis. Propidium iodide (PI; BD Biosciences), BAPTA (Invitrogen), and geldanamycin (InvivoGen) were added at the indicated concentrations.
Strains.
The C. albicans strains used in this study are listed in Table 1. Unless stated otherwise, strains are derived from SC5314, the laboratory strain of C. albicans. Newly constructed strains were generated in SNY152 (37). First, a and α derivatives of SNY152 were generated by growth on sorbose medium, as previously described (25), to create RBY1132 (a/a) and RBY1133 (α/α). Gene knockouts of FUS1, FIG1, CNB1, MKC1, CMK1, and CMK2 were generated using either the fusion PCR (37) or SAT1-flipper (43) method described previously. For each gene targeted using the fusion PCR method, oligonucleotides were used to PCR amplify approximately 500 bp of the 5′ and 3′ flanking sequences of the gene (Tables 1 and 2). In addition, the Candida dubliniensis HIS1, C. dubliniensis ARG4, and Candida maltosa LEU2 markers were PCR amplified from plasmids pSN52, pSN69, and pSN40, respectively, using universal primers 2 and 5 (37). Gene disruption cassettes were then generated by fusion PCR, combining the flanking PCR products with a selectable marker PCR product (37). RBY1132 and RBY1133 were transformed with the gene disruption cassettes, and correct integration was confirmed by PCR using check oligonucleotides (Table 2) that flank the disruption cassette in combination with primers internal to the marker gene, as previously described (37). Subsequent transformation with a second marker fusion construct was similarly used for deletion of the remaining copy of the gene, and loss of the open reading frame (ORF) was confirmed by PCR with primers internal to the ORF (Table 2). To delete CMK1 and CMK2 genes by using the SAT1-flipper method, oligonucleotides were used to amplify approximately 500 bp of the 5′ and 3′ homologous flanks of each gene. The resulting PCR products were digested with ApaI/XhoI and SacI/SacII, respectively, and cloned into the plasmid pSFS2a (43). The resulting plasmids were digested with ApaI/SacI to liberate cassettes containing the 5′ and 3′ gene flanks as well as the SAT1 selectable marker and used for transformation. Correct genomic integration was confirmed by PCR of the 5′ and 3′ disruption junctions. The SAT1 marker was then counterselected via outgrowth in yeast extract-peptone-dextrose (YPD) medium followed by selection on plates containing low concentrations of nourseothricin (43). The transformation process was repeated to delete the remaining copy of the gene, and loss of the ORF was confirmed by PCR. Multiple gene knockouts were generated for each mutant strain to confirm that the observed phenotype was due to the targeted gene deletion.
Table 1.
Strains used in this study
| Strain | Genotype or description | MTL | Reference or source |
|---|---|---|---|
| RBY1118 | leu2/leu2 his1/his1 | a/a | 47 |
| RBY1132 | leu2/leu2 his1/his1 arg4/arg4 | a/a | 47 |
| RBY1133 | leu2/leu2 his1/his1 arg4/arg4 | α/α | 47 |
| RBY1180 | arg4::hisG/arg4::hisG his1::hisG/HIS1 | α/α | 47 |
| RBY1220 | leu2::hisG/leu2::hisG his1::hisG/his1::hisG arg4::hisG/arg4::hisG bar1::LEU2/bar1::HIS1 | a/a | 47 |
| DSY162 | Sorbose-selected AM2003/0191 clinical isolate | a/a | 1 |
| DSY164 | Sorbose-selected L1086 clinical isolate | a/a | 1 |
| DSY168 | Sorbose-selected IHEM16614 clinical isolate | a/a | 1 |
| DSY194 | Sorbose-selected J981315 clinical isolate | a/a | 1 |
| DSY810/839 | fig1::HIS1/fig1::LEU2 arg4/arg4 | a/a | This study |
| DSY812/814 | mkc1::HIS1/mkc1::LEU2 arg4/arg4 | a/a | This study |
| DSY817/819 | cnb1::HIS1/cnb1::ARG4 leu2/leu2 | α/α | This study |
| DSY834/837 | cnb1::HIS1/cnb1::LEU2 arg4/arg4 | a/a | This study |
| DSY835/838 | fus1::HIS1/fus1::ARG4 leu2/leu2 | α/α | This study |
| DSY840/866 | fus1::HIS1/fus1::LEU2 arg4/arg4 | a/a | This study |
| DSY855/869 | fig1::HIS1/fig1::ARG4 leu2/leu2 | α/α | This study |
| DSY861/871 | mkc1::HIS1/mkc1::ARG4 leu2/leu2 | α/α | This study |
| DSY872/873 | leu2/leu2 his1/his1 pACT1-WOR1:SAT | a/a | This study |
| DSY874/875 | fig1::HIS1/fig1::LEU2 pACT1-WOR1:SAT1 arg4/arg4 | a/a | This study |
| DSY876 | mkc1::HIS1/mkc1::LEU2 pACT1-WOR1:SAT1 arg4/arg4 | a/a | This study |
| DSY877 | cnb1::HIS1/cnb1::LEU2 pACT1-WOR1:SAT1 arg4/arg4 | a/a | This study |
| DSY878/879 | fus1::HIS1/fus1::LEU2 pACT1-WOR1:SAT1 arg4/arg4 | a/a | This study |
| DSY886/887 | leu2::hisG/leu2::hisG his1::hisG/his1::hisG arg4::hisG/arg4::hisG bar1::LEU2/bar1::HIS1 pACT1-WOR1:SAT1 | a/a | This study |
| KAY485/486 | cmk1/cmk1::SAT1 pACT1-WOR1:SAT1 his1/his1 leu2/leu2 | a/a | This study |
| KAY487/488 | cnb1::HIS1/cnb1::LEU2 cmk1/cmk1::SAT1 pACT1-WOR1:SAT1 arg4/arg4 | a/a | This study |
| KAY489/490 | cmk2/cmk2::SAT1 pACT1-WOR1:SAT1 his1/his1 leu2/leu2 | a/a | This study |
| KAY491/492 | cnb1::HIS1/cnb1::LEU2 cmk2/cmk2::SAT1 pACT1-WOR1:SAT1 arg4/arg4 | a/a | This study |
| KAY502/504 | T101 clinical isolate pACT1-WOR1:SAT1 | a/a | This study |
| CAY320 | leu2::hisG/leu2::hisG his1::hisG/his1::hisG arg4::hisG/arg4::hisG bar1::LEU2/bar1::ARG4 | a/a | 1 |
| CAY829/832 | cnb1::HIS1/cnb1::LEU2 cmk1/cmk1::SAT1cmk2/cmk2::SAT1arg4/arg4 | a/a | This study |
| CAY831/833 | cmk1/cmk1::SAT1cmk2/cmk2::SAT1his1/his1 leu2/leu2 | a/a | This study |
| CAY1078 | AM2003/0191 bar1/bar1::SAT1 | a/a | This study |
| AM2005/0377 | Clinical isolate | a/a | 1 |
| L26 | Clinical isolate | a/a | 1 |
| P37005 | Clinical isolate | a/a | 1 |
Table 2.
Oligonucleotides used in this study
| Name | Sequence (5′ to 3′)a |
|---|---|
| Universal primer 2 | CCGCTGCTAGGCGCGCCGTGACCAGTGTGATGGATATCTGC |
| Universal primer 5 | GCAGGGATGCGGCCGCTGACAGCTCGGATCCACTAGTAACG |
| FIG1 5′ flank for | CCATTTTTAGCTACCATTGC |
| FIG1 5′ flank rev | CACGGCGCGCCTAGCAGCGGGAATACAATGGTGAATTTCAATGG |
| FIG1 3′ flank for | GTCAGCGGCCGCATCCCTGCCAACAACCACTGGTGGATAC |
| FIG1 3′ flank rev | AATGTCTCAATTCGCCCTCG |
| FIG1 5′ check | GGTGAAGATGTTGATGATATTG |
| FIG1 3′ check | CTTGGTATAATCACTGCCAC |
| FIG1 5′ ORF | CATAACTGCTGCGTTATTAAG |
| FIG1 3′ ORF | CATTGATCCTAATCCCCAAAG |
| FUS1 5′ flank for | AGAGGGAAATATGCAGTGCC |
| FUS1 5′ flank rev | CACGGCGCGCCTAGCAGCGGGGTAGTATCACCGTCATCTC |
| FUS1 3′ flank for | GTCAGCGGCCGCATCCCTGCATCTCTGGATATCGGCTTGG |
| FUS1 3′ flank rev | TCCATTCGTGGCATATACAG |
| FUS1 5′ check | GGAGGAGCAATAGGTATCAG |
| FUS1 3′ check | GTTAGTAGTTGTGTTCACTGC |
| FUS1 5′ ORF | AAAGCTCTCCAAATGCGAGC |
| FUS1 3′ ORF | TTGCATCACTTTCTCTGGGC |
| CNB1 5′ flank for | CAGGTTGTAATGGGTTGTTG |
| CNB1 5′ flank rev | CACGGCGCGCCTAGCAGCGGGTCAGTAGTTGTTGAAGTCG |
| CNB1 3′ flank for | GTCAGCGGCCGCATCCCTGCACCGATACAATTGCCAACAC |
| CNB1 3′ flank rev | GGTACGTGTATGTGCAGATC |
| CNB1 5′ check | GACTGAAAGCGCAACCTTTG |
| CNB1 3′ check | GATGAGAGTTCTAGAATCTC |
| CNB1 5′ ORF | GAGATTGATAGATTGCGCAAG |
| CNB1 3′ ORF | CTTGCCATCACCGTCCAAAT |
| MKC1 5′ flank for | CAGAAAGCCGTTCAAACGTTC |
| MKC1 5′ flank rev | CACGGCGCGCCTAGCAGCGGTGGGTGCTTCTTGTTGATCC |
| MKC1 3′ flank for | GTCAGCGGCCGCATCCCTGCCGACCACCAGTAGTTGAAAC |
| MKC1 3′ flank rev | AGCTCCACACAACTAGCATG |
| MKC1 5′ check | TGATAACGACCTGGTTGCA |
| MKC1 3′ check | CCCAAGAGAGAGTCGTTCTA |
| MKC1 5′ ORF | GCATCGTTTGTGGCAATCAA |
| MKC1 3′ ORF | CAACCAACAGACCAGATGTC |
| CMK1 5′ for (ApaI) | GGAGCGGGGCCCAATGTCGTTACAGTGTTCGC |
| CMK1 5′ rev (XhoI) | GGCCGCCTCGAGACTTGCCTGTCTTGGTCATC |
| CMK1 3′ for (SacII) | GGAGCGCCGCGGGCATTACCGTTGAATACCTTG |
| CMK1 3′ rev (SacI) | GGCCGCGAGCTCTGCTGAATCGTAGGTTTGTG |
| CMK1 5′ check | CCAGAACTCATGACTGACAC |
| CMK1 3′ check | CCCTAAGTGATGCATTGAGC |
| CMK1 5′ ORF | CACATCTACTGGAAGACACT |
| CMK1 3′ ORF | CTTCTGAAGAAACATCGACCC |
| CMK2 5′ for (ApaI) | GGAGCGGGGCCCCAGCTACATACACTTGTGTG |
| CMK2 5′ rev (XhoI) | GGCCGCCTCGAGGGGAATGATGATCAGTTGAC |
| CMK2 3′ for (SacII) | GGACCGCCGCGGGTGAATTCTGGTGGTTGGAG |
| CMK2 3′ rev (SacI) | GGCCGCGAGCTCCGTTTAGAAGGCAGGCTAGT |
| CMK2 5′ check | GACATGATTCGTGTAATTGTACG |
| CMK2 3′ check | GACGTTGGCCGATCCAACAT |
| CMK2 5′ ORF | GGTAAGACTTTAGGAGCAGG |
| CMK2 3′ ORF | CATGACCAGTACCTAATATGAC |
| LEU2 5′ (SacII) | GGCGCCCCGCGGGTGCTAAATCATCCGATGCAG |
| LEU2 3′ (SacI) | GCCGGCGAGCTCGAGAAATCTGTAGACTTTGGAG |
| ADH1 Term 5′ (XhoI/BamHI) | GCGGCGCTCGAGGCGGGGGATCCGGCAAATAGCTAAATTATATACGAATTAATA |
| ADH1 Term 3′ (BglII) | GGGCGCAGATCTACGCCTTCCAGCAATTGTCTCAAA |
| ACT1 Prom 5′ (KpnI) | GGCGCCGGTACCGGGGTTCCTATACTTTCTAGAGAATAGGAACTTCGGAGATAGAGCTATTAAGATCACCAG |
| ACT1 Prom 3′ (XhoI) | GCCGGCCTCGAGTTTGAARGATTATATTTTTTTAATATTAAT |
| WOR1 5′ ORF (XhoI) | GGCGCCCTCGAGTAAAATGTCTAATTCAAGTATAGTCCCTAC |
| WOR1 3′ ORF (BglII) | GCCGGCAGATCTCGAATTCACACACAGACCCAC |
Underlined sequences indicate the introduced restriction sites.
Plasmid pACT1-WOR1, which provides constitutive expression of Wor1p, was constructed as follows. First, a region of the C. albicans LEU2 ORF sequence was PCR amplified using primers LEU2 5′ (SacII) and LEU2 3′ (SacI) (Table 2). The PCR product was then digested with SacII and SacI and ligated into pSFS2a between these restriction enzyme sites, creating plasmid pRS1. Next, the ADH1 terminator sequence was PCR amplified and XhoI/BamHI and BglII sites were introduced at the 5′ and 3′ ends, respectively. The ADH1 terminator sequence was digested with XhoI and BglII and ligated into plasmid pRS1 digested with XhoI and BamHI, creating plasmid pRS2. The ACT1 promoter sequence was PCR amplified and restriction enzyme digested with KpnI and XhoI, and the WOR1 ORF was PCR amplified and digested with XhoI and BglII restriction enzymes. Plasmid pRS2 was digested with KpnI and BamHI, and both ACT1 and WOR1 PCR fragments were inserted into this plasmid, creating pACT1-WOR1. The vector was linearized by cutting within the LEU2 ORF sequence with BsgI, or within the ACT1 promoter sequence with BglII, in preparation for transformation.
Pheromone response and mating assays.
Overnight cultures of C. albicans cells were grown in SCD medium at room temperature (22 to 25°C) following inoculation from single colonies. Cells were washed in water and resuspended in Spider medium at a concentration of 2 × 107 cells per ml. α pheromone was added to a 10 μM concentration, and cultures were incubated for 5 h at room temperature prior to analysis by microscopy. Pictures were taken using a Zeiss Axioplan 2 microscope equipped with a Hamamatsu ORCA camera.
Quantitative mating experiments were performed by crossing strains with different auxotrophic markers as previously described (47). For evaluating the effect of geldanamycin on mating, matings were performed in liquid Spider medium containing the indicated concentrations of geldanamycin and the cells were incubated at room temperature for 24 h. Cells were then plated on selective media and analyzed for the formation of mating products.
Cell death assays.
Overnight cultures of C. albicans cells were grown in SCD medium at room temperature, and cells were washed in water and resuspended in Spider medium at a concentration of 2 × 107 cells/ml. α pheromone was added to a final concentration of 10 μM, unless stated otherwise, and then cultures were incubated at room temperature for 1 to 6 h. At different time points, 0.5 ml of the culture was removed, washed and resuspended in Spider medium, and treated with 1 μg/ml propidium iodide for 15 min. Cells were then washed with water and resuspended in SCD medium for analysis by flow cytometry. At least 10,000 cells were counted in the FL3 channel on a FACSCalibur cell sorter, where PI-positive cells represent dead yeast cells (15).
RESULTS
Pheromone-induced cell death in C. albicans.
In S. cerevisiae, treatment of a cells with α pheromone leads to cell death in approximately 25% of cells in the population (58). To test whether C. albicans cells also experience pheromone-induced death (PID), MTLa cells derived from the laboratory strain SC5314 were treated with synthetic α pheromone (10 μM) and cell death was determined by staining cells with propidium iodide and analyzing them by flow cytometry (see Materials and Methods). A subset of cells in the opaque (mating-competent) form began to lose viability between 2 and 3 h, and by 4 to 6 h approximately 20% of the cells were dead (PI-positive cells) (Fig. 1A and B). Similar levels of inviable cells were detected when cells were stained with an alternative vital dye, eosin Y (see Fig. S1 in the supplemental material). Cell death in opaque cells occurred after they began to exhibit polarized mating projections, which are first observed at 2 h and are more clearly evident by 4 h (6). At time points later than 6 h, levels of cell death began to decline, possibly due to continued growth of nonresponding cells. Cell death was also dependent on Ste2, the receptor for α pheromone (see Fig. S1), suggesting that death required activation of the canonical pheromone signaling MAPK cascade. In addition, no cell death was observed when white-phase a cells were treated with the synthetic α pheromone (see Fig. S1). C. albicans white-phase cells upregulate a limited set of genes in response to pheromone but do not form polarized mating projections or undergo efficient cell-cell conjugation (6, 14, 29).
Fig. 1.
Pheromone-induced cell death in C. albicans. (A) Picture of responding opaque cells and those that are dead (staining with propidium iodide, right panel). (B) Time course of cell death in C. albicans MTLa cells in the presence of α pheromone. Opaque a cells (derived from strain SC5314) were treated with 10 μM α pheromone, aliquots were removed, and cells were tested for viability by staining with propidium iodide. Both native opaque cells and cells locked in the opaque form by constitutive expression of Wor1 (pACT1-WOR1) were quantitated for cell death.
The observation that cell death occurs only in opaque-phase cells led us to design a construct that locks cells into this phenotypic state. The central regulator of the white-opaque switch is the Wor1 protein, a putative transcription factor that is expressed only in opaque cells and not in white cells (20, 53, 60, 61). Constitutive expression of Wor1 protein can maintain cells in the opaque state, and we therefore designed a vector in which WOR1 was expressed under the control of the ACT1 promoter (see Materials and Methods). Insertion of this construct into a cells ensured that >99% of cells were maintained in the opaque state. A time course of pheromone-induced death showed that wild-type opaque strains with and without the constitutive Wor1 expression vector showed very similar levels of cell death (Fig. 1B). These results allowed us to utilize the Wor1 expression vector as a tool to ensure that we were examining >99% pure opaque populations in further analyses.
Factors affecting mating efficiency and cell conjugation in C. albicans.
In S. cerevisiae, multiple genetic pathways have been shown to influence the sensitivity of cells to pheromone-induced death. These include the calcineurin signaling pathway, the cell wall integrity (CWI) pathway, and mating-specific factors upregulated during the morphological response to pheromone (8, 35, 58). We tested mutants in each of these pathways by examining C. albicans strains lacking CNB1 (encoding the regulatory subunit of calcineurin) or MKC1 (encoding the CWI protein kinase), as well as FUS1 and FIG1 (encoding mating-specific cell membrane proteins). These mutants were constructed in MTLa and MTLα strains of SC5314 and were tested first for their ability to undergo polarized growth in response to pheromone and subsequently for their ability to undergo efficient cell-cell conjugation with a mating partner.
Each of the mutants was able to efficiently respond to pheromone and form polarized mating projections (approximately 70% of cells formed characteristic projections similar to wild-type cells [Fig. 2A]). Analysis of the shape of the mating projections (average width and length) revealed only subtle differences between the mutants, although Δfus1 strains consistently exhibited projections that were longer (9.3 μm) than those in other strains (average, 6 to 7 μm [Fig. 2B]). Quantitative mating experiments were also performed with each of the mutant strains. These were performed using bilateral crosses, as described in Materials and Methods. C. albicans wild-type strains mated at 77% efficiency when coincubated on solid Spider medium for 2 days and at 58% efficiency when grown in liquid medium (Fig. 2C). Mutant Δcnb1 strains lacking calcineurin mated with a mating efficiency similar to that of wild-type strains. In contrast, mutants lacking FIG1, FUS1, or MKC1 all showed a significant decrease in mating efficiency. The Δfig1 mutant mated with the lowest efficiency, undergoing only 16% or 5% mating on solid or liquid medium, respectively. The Δfus1 and Δmkc1 mutants also showed a reduced mating efficiency, with Δfus1 strains undergoing 14% or 31% mating and Δmkc1 strains undergoing 30% or 22% mating on solid or liquid medium, respectively (Fig. 2C). These results indicate that FIG1, FUS1, and MKC1 genes play important, but nonessential, roles in promoting mating and tetraploid formation in C. albicans. In S. cerevisiae, defects in the homologous genes similarly reduced mating efficiency; loss of ScFIG1 reduced mating 2.5-fold (16), while loss of ScFUS1 or ScMPK1 resulted in moderate decreases in mating efficiency (17, 56).
Fig. 2.
Formation of mating projections and mating efficiency in mutant strains of C. albicans. (A) C. albicans a strains lacking the FIG1, FUS1, CNB1, or MKC1 gene were tested for their ability to form mating projections in response to 10 μM α pheromone. Strains were treated with pheromone in Spider medium, and the percentage of responding cells was determined after 5 h of incubation at room temperature. (B) The length and width of mating projections formed by the mutant strains in response to 10 μM α pheromone were analyzed. (C) Quantitative mating efficiency of mutant strains. Bilateral mating experiments were performed (mutant × mutant) by coincubation of a and α mutant strains on Spider medium plates (Solid) or in liquid Spider medium (Liquid). Opaque cells were coincubated for 2 days, and the frequency of formation of mating products was determined as previously described (47). Asterisks indicate a significant difference between mutant and wild-type strains (P < 0.05) using a two-tailed t test (two-sample test assuming equal variances). WT, wild type.
Genetic pathways influencing pheromone killing in C. albicans.
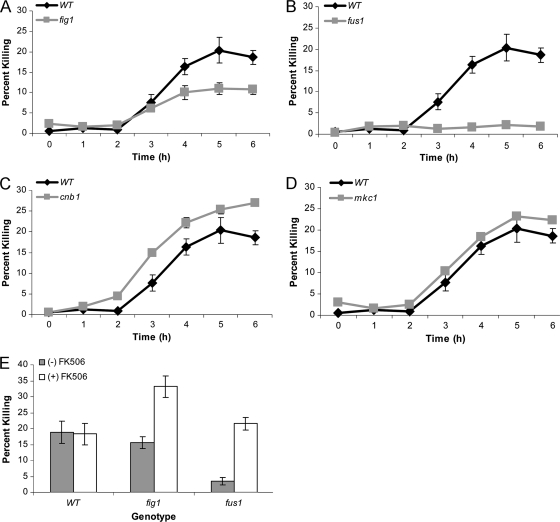
Each of the four mutant strains, the Δfig1, Δfus1, Δcnb1, and Δmkc1 mutants, was tested for its sensitivity to killing during exposure to pheromone. To ensure that pure populations of opaque cells were used in these experiments, each strain was transformed with the Wor1 constitutive expression vector to lock cells into the opaque state. However, we note that the mutant strains showed no obvious defects in white-opaque switching. A time course of pheromone treatment was performed, and cell death was determined by staining cells with propidium iodide and by analysis by flow cytometry, as described above.
The temporal profiles of cell death in response to pheromone were similar in each of the strains, with cell inviability first observed between 2 h and 3 h and maximal levels of death observed at 5 to 6 h. Very little cell death, however, was observed in the Δfus1 mutant, which showed only 2% cell death at the 5-h point (Fig. 3A). The Δfig1 mutant also showed decreased cell death compared to that of wild-type strains, with only 11% of cells becoming inviable (Fig. 3B). In contrast, the Δcnb1 strain showed a very modest, but statistically significant, increase in the frequency of cell death with 25 to 27% of cells inviable at 5 to 6 h (Fig. 3C). The Δmkc1 strain was the only mutant strain that did not exhibit a significant difference in pheromone-induced death from that of the wild-type strain (Fig. 3D).
Fig. 3.
Time course of pheromone-induced cell death in different C. albicans mutants. (A to D) Opaque MTLa strains lacking the FIG1, FUS1, CNB1, or MKC1 gene were treated with 10 μM α pheromone, and the percentage of dead cells was determined by staining with propidium iodide and analysis by flow cytometry. (E) Evaluation of PID in the presence or absence of 2.5 μM FK506. Wild-type, Δfus1, and Δfig1 cells were treated with 10 μΜ pheromone in the presence or absence of FK506 for 5 h and then stained with propidium iodide and analyzed by flow cytometry.
These results show both parallels and notable differences with the program of pheromone-induced cell death in S. cerevisiae. In both C. albicans and S. cerevisiae, loss of Fus1 or Fig1 activity results in a considerable decrease in the level of pheromone killing. In contrast, deletion of CNB1 led to almost complete killing (80 to 95%) of S. cerevisiae cells (22, 58) but only a subtle increase in cell death in C. albicans. Furthermore, compromising the CWI pathway in S. cerevisiae by deletion of MPK1/SLT2 resulted in decreased viability in the presence of pheromone (17, 58), while deletion of the homolog MKC1 in C. albicans had no discernible effect on cell viability. The lack of a significant role for calcineurin signaling in C. albicans cell survival was also confirmed by addition of FK506, a chemical inhibitor of calcineurin in fungal species (9, 55). The presence of FK506 did not significantly affect the level of pheromone-induced cell death in wild-type cells of C. albicans (Fig. 3E), while this compound led to high levels of pheromone killing in S. cerevisiae (58). Interestingly, the addition of FK506 did lead to a significant increase in the frequency of cell death in the Δfus1 (3.5% to 21.5%) and Δfig1 (15.6% to 33.1%) mutants (Fig. 3E). No other strain showed a significant increase in cell death in the presence of FK506. These results indicate that calcineurin signaling can play a role in protecting against pheromone-induced cell death in C. albicans, and this is particularly evident in Δfig1 and Δfus1 backgrounds.
The role of Ca2+ signaling in pheromone-induced cell death in C. albicans.
Several reports have demonstrated that Ca2+ signaling is critical for maintaining the viability of S. cerevisiae cells in the presence of pheromone, including a central role for the calcineurin pathway (22, 23, 35, 58). These studies showed that depletion of extracellular Ca2+ causes S. cerevisiae cells to die during prolonged exposure to pheromone. To test if Ca2+ ions are similarly required for C. albicans cells to survive pheromone exposure, a cell-impermeant Ca2+ chelator (BAPTA) was added to the culture medium. We found that relatively high concentrations of BAPTA (10 mM) were necessary to see any significant effect on the survival of C. albicans cells (cell death increased from 16% to 31% [Fig. 4 ]). BAPTA had little or no effect on cell viability when present in the absence of pheromone regardless of the strain genotype (10 mM BAPTA increased basal levels of cell death, on average, from 0.5% to 1.52%). BAPTA also showed a strong effect on mating efficiency, reducing mating of wild-type strains from 15.9% after 24 h to 0.2%. These results indicate that the loss of Ca2+ ions from the medium causes a major defect in mating in C. albicans but only partially compromises cell survival during the response to pheromone.
Fig. 4.
Increased pheromone-induced cell death following chelation of extracellular Ca2+ ions with BAPTA. (A) C. albicans strains were incubated with 10 μM α pheromone in the presence of increasing concentrations of the Ca2+ chelator BAPTA. Cells were treated with pheromone for 5 h and analyzed by staining with propidium iodide and flow cytometry to determine the percentage of dead cells. (B) Additional C. albicans strains were incubated with 10 μM α pheromone in the presence or absence of 10 mM BAPTA.
BAPTA similarly increased the level of pheromone killing in the Δcnb1 and Δfig1 mutant strains (Fig. 4A). The Δcnb1 mutant result is notable, as it suggests that Ca2+ signaling provides protection against pheromone-induced death but does so via a largely calcineurin-independent mechanism. The most striking result, however, was observed with the Δfus1 strain, as this mutant usually exhibits very small amounts of cell death (3.6% in this experiment) but was hypersensitive to pheromone killing in the presence of BAPTA (21% death at 0.1 mM BAPTA and 49% death at 10 mM BAPTA [Fig. 4A]). In fact, Δfus1 cell death in the absence of Ca2+ ions was equivalent to the highest level of cell death observed in any mutant SC5314 strain and represents an increase of 14-fold over medium containing Ca2+.
To further pursue the role of Ca2+ signaling in cell death, we constructed mutants lacking the CMK1 and CMK2 genes that encode Ca2+/calmodulin-dependent protein kinases (41). Loss of CMK1 and CMK2 in S. cerevisiae can lead to increased sensitivity to pheromone killing and can also further potentiate cell death in strains lacking the CNB1 calcineurin gene (34). Due to the limited levels of PID observed in Δcnb1 mutants of C. albicans, we speculated that CMK1 or CMK2 may play a more important role in the pheromone response in C. albicans than in S. cerevisiae. However, strains lacking both CMK1 and CMK2 genes showed no significant increase in the rates of pheromone-induced death. Additionally, a triple mutant lacking CMK1, CMK2, and CNB1 genes showed levels of cell death that were not significantly increased (Fig. 4B). Each of these mutants (Δcmk1, Δcmk2, and the related double and triple mutants) also responded similarly to 10 mM BAPTA treatment (Fig. 4B), supporting the hypothesis that Ca2+ provides protection against cell death in a calcineurin-independent manner. These results demonstrate that although PID in C. albicans is sensitive to changes in extracellular Ca2+ levels, the signaling pathways regulated by CMK1, CMK2, and CNB1 play relatively minor roles in this process or that additional (yet-to-be-identified) Ca2+ signaling pathways can compensate for the loss of these factors.
Role of Hsp90 in mediating the response to pheromone and cell death.
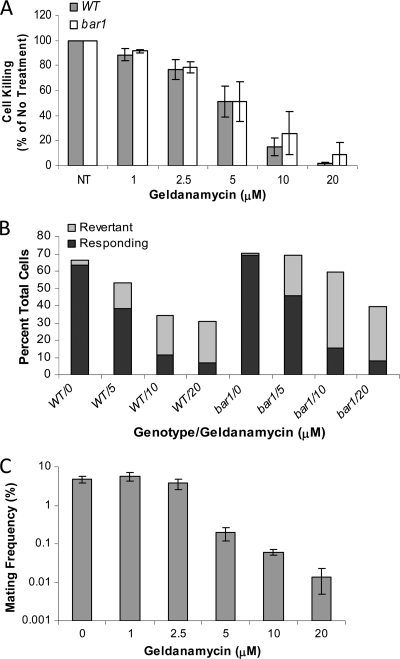
There is now a substantial body of evidence indicating a connection between calcineurin signaling and the heat shock protein Hsp90 in multiple fungal species. In particular, it appears that the Hsp90-calcineurin interaction is necessary for calcineurin activation and subsequent signaling to downstream effectors (24). Thus, inhibition of either Hsp90 activity (e.g., by using the chemical inhibitor geldanamycin) or calcineurin activity (e.g., by using FK506) can abrogate a subset of the same signaling pathways (9). The Hsp90-calcineurin pathway is particularly relevant for cellular responses to stress and has been shown to promote the accumulation of drug-resistant isolates (9–12, 49, 55).
To test the role of C. albicans Hsp90 during pheromone signaling, wild-type or Δbar1 cells were treated with increasing concentrations of the Hsp90 inhibitor geldanamycin. Bar1 protease is a secreted protein that acts to degrade α pheromone in both S. cerevisiae and C. albicans (32, 47, 51). Loss of Bar1 sensitizes S. cerevisiae and C. albicans cells to respond to lower concentrations of α pheromone (discussed below). As shown in Fig. 5, as the concentration of the inhibitor increased, the frequency of pheromone-induced cell death decreased in both wild-type and Δbar1 cells. Thus, at 5 μM geldanamycin, cell death was approximately 50% of that in assays lacking the drug, while at 20 μM geldanamycin cell death in both wild-type and Δbar1 cells was virtually abolished (Fig. 5A). Geldanamycin alone had very little or no effect on C. albicans cell viability when used at these concentrations (data not shown).
Fig. 5.
Inhibition of pheromone-induced death and the morphological response to pheromone by the Hsp90 inhibitor geldanamycin. (A) Geldanamycin inhibits cell killing by pheromone in MTLa cells of wild-type and Δbar1 C. albicans strains. Opaque cells were incubated with 10 μM pheromone for 5 h in the presence of the indicated concentrations of geldanamycin, and cell death was determined by staining with propidium iodide and flow cytometry. NT, no treatment. (B) Fractions of cells responding to α pheromone in the presence of geldanamycin. Opaque cells were treated with pheromone for 5 h and then analyzed microscopically to determine the percentage of cells with mating projections (responding cells) or those cells that had initiated mating projections but then had reverted to budding (revertant cells). (C) Geldanamycin inhibits mating of wild-type C. albicans a and α cells. MTLa and MTLα opaque cells were coincubated for 2 days in the presence of various concentrations of geldanamycin, and the mating frequency was determined by the fraction of prototrophic mating products formed, as previously described (47).
To examine the effect of geldanamycin on the morphological response to pheromone, microscopic analysis of cells was performed. These experiments revealed that geldanamycin also inhibited the morphological response of cells to pheromone. Thus, whereas close to 70% of cells formed polarized mating projections in the absence of geldanamycin, as the concentration of the drug increased, fewer cells exhibited polarized growth (Fig. 5B). In addition, a significant number of cells initiated a mating response but then reverted to dividing as budding yeast in the presence of geldanamycin (Fig. 5B). Consistent with a role for geldanamycin in blocking pheromone signaling, we also observed a decrease in mating frequencies in the presence of high concentrations of the drug. Mating frequencies were unaltered in assays with 1 to 2.5 μM geldanamycin, while higher concentrations of the drug significantly inhibited the formation of conjugation products by nearly 100-fold (Fig. 5C).
These results establish that loss of Hsp90 activity results in inhibition of the morphological response to pheromone. We observed a correlation between inhibition of the pheromone response and decreased cell death, indicating that loss of viability is dependent on processes accompanying the morphological transition. In S. cerevisiae, Hsp90 inhibitors have also been shown to block polarized growth in response to pheromones, possibly due to defective signaling via Ste11, a component of the pheromone MAPK cascade (31). Our findings now indicate that Hsp90 plays an important role in mediating pheromone signaling in a distantly related hemiascomycete. In addition, we demonstrate that Hsp90 and calcineurin play distinct roles in the mating program. Inhibition of Hsp90 activity prevents activation of the pheromone signaling response, while calcineurin is not required for the pheromone response but does play a role (albeit a minor one in wild-type strains) in protecting cells from pheromone toxicity.
Pheromone-induced cell death in clinical isolates of C. albicans.
The experiments described above utilized derivatives of the standard laboratory strain of C. albicans, SC5314. Mating has also been observed in multiple clinical isolates of C. albicans, including those from alternative clades (5, 21, 40). In total, 17 distinct clades of C. albicans strains have been described, although 68% of strains belong to the four most populous clades, numbered 1 through 4 (38). To compare the frequencies of cell death between natural C. albicans strains, we initially treated six clinical opaque a strains with α pheromone and determined the efficiency of the mating response as well as the susceptibility to cell death. These six strains were chosen at random as representatives of the four major clades of C. albicans.
Each of the clinical strains showed a robust morphological response to pheromone, with the majority of cells forming polarized mating projections (Fig. 6B). Despite the similar frequencies of responding cells, there was a marked difference in the susceptibilities of clinical strains to pheromone-induced death. As shown in Fig. 6A, only 2% of J981315 cells became inviable during pheromone treatment, while 40 to 50% of L26 and P37005 cells were killed and ∼70% of AM2003/0191 cells died. These experiments reveal that cell death varies by more than an order of magnitude between strains with different genetic backgrounds. The strain that showed the highest survival rate, J981315, belongs to clade 3, while the strain with the lowest survival rate, AM2003/0191, belongs to clade 2 (38). To test if other isolates from these clades showed similar susceptibilities to cell death, we examined the frequency of killing in several additional strains belonging to clades 1 to 4. The additional strains revealed that high (∼70%) levels of cell death are observed in isolates from each of the four clades. However, low (<7%) levels of cell death, in the presence of an otherwise robust mating response, occurred only in a subset of clade 3 strains (K. Alby and R. J. Bennett, unpublished observations). These data suggest that clade-specific differences may exist in the susceptibilities of strains to pheromone-induced cell death.
Fig. 6.
Pheromone-induced death in clinical isolates of C. albicans. Six clinical strains were analyzed to determine their susceptibilities to killing by pheromone. (A) MTLa/a isolates were switched to the opaque phase and treated with 10 μM α pheromone for 5 h, prior to staining with propidium iodide and analysis by flow cytometry. Clade designations for each strain are in parentheses. (B) Formation of mating projections by the clinical isolates. Opaque cells were treated with α pheromone for 5 h and analyzed by microscopy. All isolates were able to efficiently undergo the morphological response to pheromone, despite significant differences in their susceptibilities to killing by pheromone. (C) Evaluation of the presence or absence of FK506 on PID in clinical isolates. Cells were treated with 10 μΜ pheromone in the presence or absence of 2.5 μM FK506 for 5 h and then stained with propidium iodide and analyzed by flow cytometry.
Due to the differential responses of SC5314 mutants to FK506 treatment (Fig. 3B), we tested if clinical isolates of C. albicans showed increased susceptibility to PID in the presence of FK506. None of the isolates showed a significant difference in PID when treated with FK506, although AM2005/0377 was slightly more sensitive to killing in the presence of the drug (Fig. 6C). This indicates that FK506-mediated inhibition of calcineurin does not significantly affect the level of PID in C. albicans, regardless of the genetic background of the strain.
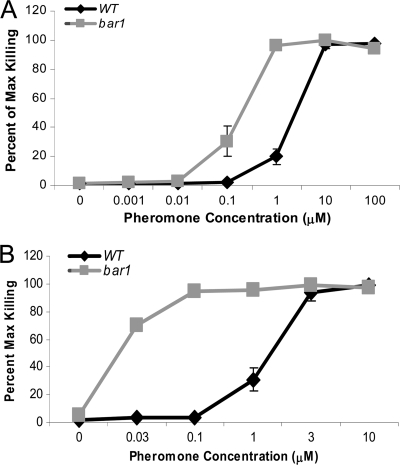
We also compared the roles of the Bar1 protease during PID in both the SC5314 background and the hypersensitive clinical strain AM2003/0191. In S. cerevisiae, loss of Bar1 activity results in strains exhibiting PID at lower concentrations of α pheromone due to reduced degradation of the peptide (58). In C. albicans, we similarly found that Δbar1 mutants experienced cell death at lower concentrations of pheromone than did wild-type cells. Thus, whereas 10 μM pheromone was required to cause maximal levels of cell killing in SC5314 cells (∼23% [Fig. 1B]), 10-fold-less pheromone (1 μM) was sufficient to cause the same frequency of killing in Δbar1 cells (Fig. 7A). Even with 100-fold-less pheromone (0.1 μM), there was still appreciable death in Δbar1 cells but not in wild-type cells. Similarly, maximal killing in AM2003/0191 (∼70% cells inviable [Fig. 6A]) required 3 μM α pheromone in the wild-type strain but only 0.03 μM pheromone in the Δbar1 mutant (Fig. 7B). It is notable that the maximal levels of cell death in SC5314 and AM2003/0191 strains were the same in both wild-type and Δbar1 cells. These results indicate that while Bar1 plays an important role in determining the amount of pheromone necessary to induce cell death, Bar1 activity does not influence the maximal level of death in a given strain. Thus, at least in the case of AM2003/0191, the high susceptibility of this strain to PID is not due to defective production of Bar1 protein.
Fig. 7.
Effect of BAR1 on pheromone-induced death. (A) Titration of α pheromone with wild-type and Δbar1 SC5314 a strains. Strains were analyzed by PI staining at 5 h after the addition of pheromone. (B) Titration of α pheromone with wild-type and Δbar1 AM2003/0191 a strains. Strains were analyzed by PI staining at 5 h after the addition of pheromone.
Finally, we examined the subset of clinical strains to determine if susceptibility to killing by pheromone correlated with sensitivity to other fungal stresses. Strains were incubated on plates containing the cell wall stressors Congo red, sodium dodecyl sulfate (SDS), or calcofluor white. Strains were also tested for susceptibility to killing with the antifungal drug fluconazole, which targets the cell membrane. The subset of clinical strains exhibited a wide range of susceptibilities to these agents (see Fig. S2 in the supplemental material). However, strain AM2003/0191, which was the most sensitive to killing by pheromone, was as resistant to each of these types of cell stress as were isolates from the other clades. This demonstrates that the susceptibility to pheromone killing by this strain is not due to its general sensitivity to stress.
Taken together, these results demonstrate an unexpected variability in the response of natural isolates of C. albicans to pheromone. Each of the clinical strains underwent the morphological response to pheromone, but levels of cell death varied from 2% to 70%, far more than that observed even between mutant derivatives of SC5314. Cell killing by pheromone did not correlate with sensitivity to other cell stresses, indicating that pheromone toxicity is not related to that of other antifungal agents. We note that we have not, however, observed any obvious synergy between killing by pheromone treatment and cell death induced by azole or echinocandin classes of drugs (Alby and Bennett, unpublished).
DISCUSSION
This work is the first to report the phenomenon of pheromone-induced cell death in C. albicans, a hemiascomycete yeast that diverged from S. cerevisiae approximately 700 million years ago (19). Using homozygous a/a derivatives of SC5314, the standard laboratory strain of C. albicans, cell death was first observed 2 to 3 h after pheromone treatment, coincident with the initial formation of polarized mating projections. Cell death gradually increased and was maximal at 5 to 6 h, where it was observed in approximately 20% of cells in a population. Cell death was also dependent on Ste2, the receptor for α pheromone (6), confirming that it required the canonical pheromone signaling pathway.
Unlike S. cerevisiae, a and α cells of C. albicans can exist in two alternative physical states, white and opaque, and cells undergo heritable and reversible switching between these states (30, 33, 50). Both white and opaque forms can respond to pheromone via the MAPK cascade, but signaling results in the upregulation of phase-specific genes. White cells treated with pheromone exhibit increased adhesion and biofilm formation, whereas opaque cells undergo the more conventional program of mating, including polarized growth, cell-cell conjugation, and zygote formation (14, 46, 57). Consistent with cell death being dependent on the morphological response to pheromone, only opaque cells showed a loss of viability in these studies, while white cells were resistant to pheromone-induced death. As predicted by experiments in S. cerevisiae, mutants lacking the BAR1 gene were hypersensitive to killing by low concentrations of pheromone. BAR1 encodes a conserved aspartyl protease that degrades α pheromone in both S. cerevisiae and C. albicans (1, 47).
In S. cerevisiae, multiple genes have been implicated both in mediating the toxic effects of pheromone and in protecting cells against killing. It was recently proposed that three signaling pathways, with distinct kinetics and genetic requirements, modulate the response to pheromone. Loss of calcineurin signaling promotes “slow cell death” and requires mitochondrial respiration, and loss of the cell wall integrity (CWI) pathway leads to “intermediate cell death” due to increased cell wall degradation, while a wave of “fast cell death” is dependent on the Fig1 protein (58). In all three waves of death, reactive oxygen species (ROS) were at least transiently observed, although only slow cell death required mitochondrial function (58).
In comparison, our studies in C. albicans demonstrate that genes functioning in calcineurin signaling (CNB1) and the cell wall integrity pathway (MKC1) play only minor roles in providing overall protection against pheromone-induced death, at least in wild-type cells. Thus, Δmkc1 and Δcnb1 mutants exhibited levels of cell death similar to those of wild-type strains. In S. cerevisiae, the calcineurin signaling pathway is particularly important in counteracting pheromone toxicity, but the level of protection can vary depending on the strain background. For example, in some strains deletion of the Ca2+/calmodulin-dependent kinase Cmk2 is required in addition to Cnb1 in order to see high levels of pheromone-induced killing (K. Cunningham, personal communication). In C. albicans loss of CNB1 did not result in high levels of cell death even when combined with deletion of the CMK2 gene. Furthermore, a triple mutant lacking CNB1, CMK1, and CMK2 genes was also tested and did not exhibit increased sensitivity to pheromone killing. Thus, in contrast to S. cerevisiae, the calcineurin signaling pathway does not appear to play a central role in protecting C. albicans cells from pheromone-induced death, even when combined with loss of Ca2+/calmodulin-dependent kinases. It remains to be seen, however, if other pathways can compensate for the loss of these signaling pathways. The latter is a strong possibility given that chelation of Ca2+ ions by BAPTA significantly increased cell death (even in Δcnb1 strains), suggesting that Ca2+ signaling can activate protective pathways that are independent of calcineurin and Cmk1/2 function.
Two genes that did show similar effects on PID in both S. cerevisiae and C. albicans were FIG1 and FUS1. These genes are highly induced as part of the transcriptional response to pheromone in both yeasts (6, 16, 56). In addition, in both species loss of these cell membrane proteins results in a reduction in pheromone-induced death, as well as a reduction in mating efficiency. S. cerevisiae Fig1 has been shown to be part of a low-affinity Ca2+ influx system (35), although recent studies indicate that its role in cell death is independent of Ca2+ uptake (58). Fus1 promotes cell death by mediating the local secretion of hydrolases necessary for cell wall degradation (7, 58). The roles of Fig1 and Fus1 are closely related, as Fus1 activity is required for Fig1-dependent cell death in S. cerevisiae (58). The current work shows that Fus1 and Fig1 also act as prodeath factors during the pheromone response in C. albicans. The result with the C. albicans Δfus1 strain was particularly striking, as cell death occurred in only 2 to 4% of cells. Despite the low frequency of killing, chelation of Ca2+ ions in the medium increased Δfus1 cell death to ∼50% of the population. The high frequency of cell death (even higher than that of wild-type SC5314 cells) indicates that although death is rare in the absence of Fus1, these cells are highly susceptible to loss of the calcium signaling pathway(s). In support of this model, treatment of Δfus1 strains with the calcineurin inhibitor FK506 also resulted in a significant increase in cell death (from 3.5% to 21.5%). Thus, although wild-type strains are not markedly affected by the loss of calcineurin, Δfus1 strains are exquisitely sensitive to the loss of this activity. Furthermore, the very high levels of cell death observed in the absence of both Fus1 and Ca2+ ions indicate that this protein can perform a prosurvival role, in addition to its prodeath function, during the response to pheromone.
We report that the strain background of C. albicans can also have a dramatic effect on the level of PID. In total, we have analyzed cell death in over 20 clinical strains and found that levels varied from ∼2% in J981315 to ∼70% in AM2003/0191. This difference was not due to differences in the abilities of these strains to respond to pheromone, as each of these clinical strains efficiently formed mating projections. In addition, while AM2003/0191 was the strain most susceptible to pheromone-induced death, it was not hypersensitive to other types of cell stress, including the presence of antifungal drugs (azoles) and cell wall stressors (SDS, Congo red, or calcofluor white). Thus, the high levels of cell death seen in the presence of pheromone are not due to a general susceptibility to different forms of cell stress.
Natural isolates of C. albicans strains have been classified into 17 distinct clades, with almost 70% of the isolates belonging to the four largest clades (clades 1 to 4) (38). Clade-specific associations are relatively rare, although one example is the number of tandem repeats in alleles of ALS and HYR family members, genes involved in adhesion to host surfaces (38, 39). Our results suggest that pheromone-induced death may also exhibit clade-specific differences. Multiple strains from clade 3 experience very low levels of PID (2 to 6%) while still exhibiting a robust mating response. Given the limited number of natural MTLa/a strains from clade 3, confirmation of a clade-specific phenotype will require construction of additional MTL homozygous strains from a/α parental strains and testing for their survival in the presence of pheromone.
The role of pheromone-induced death in yeast biology continues to be more of an enigma than are the signaling pathways influencing it. Based on studies in S. cerevisiae, it is thought that PID may act to selectively discard older or weaker cells, allowing only the healthiest cells to survive and propagate (48). In this model, such programmed cell death can potentially benefit the species by increasing overall fitness in the population. An alternative hypothesis suggests that PID is a direct consequence of cells failing to undergo cell-cell conjugation, with decreased viability due to pheromone-induced degradation of cell wall material and subsequent loss of cell integrity (58). A third possibility is that PID is a result of phenotypic variation within a population and that only a subpopulation of cells are actually “programmed” to mate. The remaining cells in the population experience only the stressful consequences of pheromone exposure that can result in cell death. This phenomenon is commonly seen in bacteria in response to external stresses such as antibiotic treatment (2, 45). While these three models are not mutually exclusive, our studies in C. albicans indicate that a simple relationship does not exist between a cell's susceptibility to undergoing pheromone-induced death and its ability to undergo mating. This is most apparent in comparing natural isolates of C. albicans: the J981315 isolate is resistant to PID (∼2% death) whereas the AM2003/0191 isolate exhibits the highest level of PID (∼70% death), yet both strains undergo efficient conjugation in quantitative mating assays (1). Clearly, it will be revealing to determine which pathways (either prodeath or prosurvival) contribute to the marked differences in cell death between C. albicans isolates and to determine if these differences affect the fitness of mating products produced by the parasexual mating cycle.
In summary, our results demonstrate that the phenomenon of pheromone-induced death has been conserved between two diverse species of hemiascomycete yeasts. However, the signaling pathways that influence the susceptibility to killing by pheromone have diverged, and natural isolates can exhibit dramatically different levels of cell death in response to pheromone. It is therefore evident that the study of mechanisms of pheromone-induced killing in C. albicans will help further elucidate the genetic factors that contribute to this phenomenon in hemiascomycetes, as well as the role of PID in yeast biology.
Supplementary Material
ACKNOWLEDGMENTS
We specially thank Kyle Cunningham (John Hopkins University) for discussions and the communication of unpublished data and Donna MacCullum (University of Aberdeen) for providing clinical isolates of C. albicans.
Work in our laboratory was supported by the NIH (R21AI081560AI081560 and AI081704 to R.J.B., F31DE019752 to K.A., and F31AI075607 to R.K.S.). R.J.B. also holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 24 September 2010.
REFERENCES
- 1.Alby K., Schaefer D., Bennett R. J. 2009. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460:890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaumont H. J., Gallie J., Kost C., Ferguson G. C., Rainey P. B. 2009. Experimental evolution of bet hedging. Nature 462:90–93 [DOI] [PubMed] [Google Scholar]
- 3.Bennett R. J., Johnson A. D. 2003. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 22:2505–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett R. J., Johnson A. D. 2006. The role of nutrient regulation and the Gpa2 protein in the mating pheromone response of C. albicans. Mol. Microbiol. 62:100–119 [DOI] [PubMed] [Google Scholar]
- 5.Bennett R. J., Miller M. G., Chua P. R., Maxon M. E., Johnson A. D. 2005. Nuclear fusion occurs during mating in Candida albicans and is dependent on the KAR3 gene. Mol. Microbiol. 55:1046–1059 [DOI] [PubMed] [Google Scholar]
- 6.Bennett R. J., Uhl M. A., Miller M. G., Johnson A. D. 2003. Identification and characterization of a Candida albicans mating pheromone. Mol. Cell. Biol. 23:8189–8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brizzio V., Gammie A. E., Rose M. D. 1998. Rvs161p interacts with Fus2p to promote cell fusion in Saccharomyces cerevisiae. J. Cell Biol. 141:567–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buehrer B. M., Errede B. 1997. Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:6517–6525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowen L. E. 2008. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 6:187–198 [DOI] [PubMed] [Google Scholar]
- 10.Cowen L. E. 2009. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog. 5:e1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowen L. E., Lindquist S. 2005. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309:2185–2189 [DOI] [PubMed] [Google Scholar]
- 12.Cowen L. E., Singh S. D., Kohler J. R., Collins C., Zaas A. K., Schell W. A., Aziz H., Mylonakis E., Perfect J. R., Whitesell L., Lindquist S. 2009. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. U. S. A. 106:2818–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cyert M. S. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 311:1143–1150 [DOI] [PubMed] [Google Scholar]
- 14.Daniels K. J., Srikantha T., Lockhart S. R., Pujol C., Soll D. R. 2006. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 25:2240–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudgeon D. D., Zhang N., Ositelu O. O., Kim H., Cunningham K. W. 2008. Nonapoptotic death of Saccharomyces cerevisiae cells that is stimulated by Hsp90 and inhibited by calcineurin and Cmk2 in response to endoplasmic reticulum stresses. Eukaryot. Cell 7:2037–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdman S., Lin L., Malczynski M., Snyder M. 1998. Pheromone-regulated genes required for yeast mating differentiation. J. Cell Biol. 140:461–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Errede B., Cade R. M., Yashar B. M., Kamada Y., Levin D. E., Irie K., Matsumoto K. 1995. Dynamics and organization of MAP kinase signal pathways. Mol. Reprod. Dev. 42:477–485 [DOI] [PubMed] [Google Scholar]
- 18.Guthrie C., Fink G. R. 1991. Guide to yeast genetics and molecular biology. Academic Press, San Diego, CA [Google Scholar]
- 19.Hedges S. B., Blair J. E., Venturi M. L., Shoe J. L. 2004. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol. Biol. 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang G., Wang H., Chou S., Nie X., Chen J., Liu H. 2006. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 103:12813–12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim A. S., Magee B. B., Sheppard D. C., Yang M., Kauffman S., Becker J., Edwards J. E., Jr., Magee P. T. 2005. Effects of ploidy and mating type on virulence of Candida albicans. Infect. Immun. 73:7366–7374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iida H., Nakamura H., Ono T., Okumura M. S., Anraku Y. 1994. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol. 14:8259–8271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iida H., Yagawa Y., Anraku Y. 1990. Essential role for induced Ca2+ influx followed by [Ca2+]i rise in maintaining viability of yeast cells late in the mating pheromone response pathway. A study of [Ca2+]i in single Saccharomyces cerevisiae cells with imaging of fura-2. J. Biol. Chem. 265:13391–13399 [PubMed] [Google Scholar]
- 24.Imai J., Yahara I. 2000. Role of HSP90 in salt stress tolerance via stabilization and regulation of calcineurin. Mol. Cell. Biol. 20:9262–9270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janbon G., Sherman F., Rustchenko E. 1998. Monosomy of a specific chromosome determines L-sorbose utilization: a novel regulatory mechanism in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 95:5150–5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson A. 2003. The biology of mating in Candida albicans. Nat. Rev. Microbiol. 1:106–116 [DOI] [PubMed] [Google Scholar]
- 27.Levin D. E. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69:262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H., Kohler J., Fink G. R. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723–1726 [DOI] [PubMed] [Google Scholar]
- 29.Lockhart S. R., Zhao R., Daniels K. J., Soll D. R. 2003. Alpha-pheromone-induced “shmooing” and gene regulation require white-opaque switching during Candida albicans mating. Eukaryot. Cell 2:847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohse M. B., Johnson A. D. 2009. White-opaque switching in Candida albicans. Curr. Opin. Microbiol. 12:650–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louvion J. F., Abbas-Terki T., Picard D. 1998. Hsp90 is required for pheromone signaling in yeast. Mol. Biol. Cell 9:3071–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacKay V. L., Welch S. K., Insley M. Y., Manney T. R., Holly J., Saari G. C., Parker M. L. 1988. The Saccharomyces cerevisiae BAR1 gene encodes an exported protein with homology to pepsin. Proc. Natl. Acad. Sci. U. S. A. 85:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller M. G., Johnson A. D. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293–302 [DOI] [PubMed] [Google Scholar]
- 34.Moser M. J., Geiser J. R., Davis T. N. 1996. Ca2+-calmodulin promotes survival of pheromone-induced growth arrest by activation of calcineurin and Ca2+-calmodulin-dependent protein kinase. Mol. Cell. Biol. 16:4824–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller E. M., Mackin N. A., Erdman S. E., Cunningham K. W. 2003. Fig1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J. Biol. Chem. 278:38461–38469 [DOI] [PubMed] [Google Scholar]
- 36.Nelson B., Parsons A. B., Evangelista M., Schaefer K., Kennedy K., Ritchie S., Petryshen T. L., Boone C. 2004. Fus1p interacts with components of the Hog1p mitogen-activated protein kinase and Cdc42p morphogenesis signaling pathways to control cell fusion during yeast mating. Genetics 166:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble S. M., Johnson A. D. 2005. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell 4:298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odds F. C., Bougnoux M. E., Shaw D. J., Bain J. M., Davidson A. D., Diogo D., Jacobsen M. D., Lecomte M., Li S. Y., Tavanti A., Maiden M. C., Gow N. A., d'Enfert C. 2007. Molecular phylogenetics of Candida albicans. Eukaryot. Cell 6:1041–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh S. H., Cheng G., Nuessen J. A., Jajko R., Yeater K. M., Zhao X., Pujol C., Soll D. R., Hoyer L. L. 2005. Functional specificity of Candida albicans Als3p proteins and clade specificity of ALS3 alleles discriminated by the number of copies of the tandem repeat sequence in the central domain. Microbiology 151:673–681 [DOI] [PubMed] [Google Scholar]
- 40.Panwar S. L., Legrand M., Dignard D., Whiteway M., Magee P. T. 2003. MFalpha1, the gene encoding the alpha mating pheromone of Candida albicans. Eukaryot. Cell 2:1350–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pausch M. H., Kaim D., Kunisawa R., Admon A., Thorner J. 1991. Multiple Ca2+/calmodulin-dependent protein kinase genes in a unicellular eukaryote. EMBO J. 10:1511–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pujol C., Daniels K. J., Lockhart S. R., Srikantha T., Radke J. B., Geiger J., Soll D. R. 2004. The closely related species Candida albicans and Candida dubliniensis can mate. Eukaryot. Cell 3:1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reuss O., Vik A., Kolter R., Morschhauser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119–127 [DOI] [PubMed] [Google Scholar]
- 44.Roberts C. J., Nelson B., Marton M. J., Stoughton R., Meyer M. R., Bennett H. A., He Y. D., Dai H., Walker W. L., Hughes T. R., Tyers M., Boone C., Friend S. H. 2000. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 287:873–880 [DOI] [PubMed] [Google Scholar]
- 45.Rotem E., Loinger A., Ronin I., Levin-Reisman I., Gabay C., Shoresh N., Biham O., Balaban N. Q. 2010. Regulation of phenotypic variability by a threshold-based mechanism underlies bacterial persistence. Proc. Natl. Acad. Sci. U. S. A. 107:12541–12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahni N., Yi S., Daniels K. J., Srikantha T., Pujol C., Soll D. R. 2009. Genes selectively up-regulated by pheromone in white cells are involved in biofilm formation in Candida albicans. PLoS Pathog. 5:e1000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaefer D., Cote P., Whiteway M., Bennett R. J. 2007. Barrier activity in Candida albicans mediates pheromone degradation and promotes mating. Eukaryot. Cell 6:907–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Severin F. F., Hyman A. A. 2002. Pheromone induces programmed cell death in S. cerevisiae. Curr. Biol. 12:R233–R235 [DOI] [PubMed] [Google Scholar]
- 49.Slutsky B., Buffo J., Soll D. R. 1985. High-frequency switching of colony morphology in Candida albicans. Science 230:666–669 [DOI] [PubMed] [Google Scholar]
- 50.Soll D. R. 2009. Why does Candida albicans switch? FEMS Yeast Res. 9:973–989 [DOI] [PubMed] [Google Scholar]
- 51.Sprague G. F., Jr., Herskowitz I. 1981. Control of yeast cell type by the mating type locus. I. Identification and control of expression of the a-specific gene BAR1. J. Mol. Biol. 153:305–321 [DOI] [PubMed] [Google Scholar]
- 52.Sprague G. F. J., Thorner J. T. 1992. Pheromone response and signal transduction during the mating process of S. cerevisiae, p. 657–744InPringle J. R., Broach J. R., Jones E. W. (ed.), Molecular and cellular biology of the yeast Saccharomyces. CSH Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 53.Srikantha T., Borneman A. R., Daniels K. J., Pujol C., Wu W., Seringhaus M. R., Gerstein M., Yi S., Snyder M., Soll D. R. 2006. TOS9 regulates white-opaque switching in Candida albicans. Eukaryot. Cell 5:1674–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stathopoulos A. M., Cyert M. S. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinbach W. J., Reedy J. L., Cramer R. A., Jr., Perfect J. R., Heitman J. 2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5:418–430 [DOI] [PubMed] [Google Scholar]
- 56.Trueheart J., Boeke J. D., Fink G. R. 1987. Two genes required for cell fusion during yeast conjugation: evidence for a pheromone-induced surface protein. Mol. Cell. Biol. 7:2316–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi S., Sahni N., Pujol C., Daniels K. J., Srikantha T., Ma N., Soll D. R. 2009. A Candida albicans-specific region of the alpha-pheromone receptor plays a selective role in the white cell pheromone response. Mol. Microbiol. 71:925–947 [DOI] [PubMed] [Google Scholar]
- 58.Zhang N. N., Dudgeon D. D., Paliwal S., Levchenko A., Grote E., Cunningham K. W. 2006. Multiple signaling pathways regulate yeast cell death during the response to mating pheromones. Mol. Biol. Cell 17:3409–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao R., Daniels K. J., Lockhart S. R., Yeater K. M., Hoyer L. L., Soll D. R. 2005. Unique aspects of gene expression during Candida albicans mating and possible G(1) dependency. Eukaryot. Cell 4:1175–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zordan R. E., Galgoczy D. J., Johnson A. D. 2006. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc. Natl. Acad. Sci. U. S. A. 103:12807–12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zordan R. E., Miller M. G., Galgoczy D. J., Tuch B. B., Johnson A. D. 2007. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 5:e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.