Abstract
Cryptococcus neoformans is an encapsulated yeast that causes a life-threatening meningoencephalitis in immunocompromised individuals. The ability to survive and proliferate at the human body temperature is an essential virulence attribute of this pathogen. This trait is controlled in part by the Ca2+-calcineurin pathway, which senses and utilizes cytosolic calcium for signaling. In the present study, the identification of the C. neoformans gene VCX1, which encodes a vacuolar calcium exchanger, is reported. The VCX1 knockout results in hypersensitivity to the calcineurin inhibitor cyclosporine A at 35°C, but not at 30°C. Furthermore, high concentrations of CaCl2 lead to growth inhibition of the vcx1 mutant strain only in the presence of cyclosporine A, indicating that Vcx1 acts in parallel with calcineurin. The loss of VCX1 does not influence cell wall integrity or capsule size but decreases secretion of the major capsular polysaccharide glucuronoxylomannan (GXM) in culture supernatants.Vcx1 also influences C. neoformans phagocytosis by murine macrophages and is required for full virulence in mice. Analysis of cellular distribution by confocal microscopy confirmed the vacuolar localization of Vcx1 in C. neoformans cells.
Calcium (Ca2+) is an intracellular messenger that controls numerous cellular processes. Two essential mediators of calcium signals in eukaryotic cells are the Ca2+ binding protein calmodulin and the Ca2+/calmodulin-activated serine/theronine protein phosphatase calcineurin (4, 21). When intracellular calcium levels are low, calcineurin is inactive. An increase in calcium levels in the external environment leads to elevate cytosolic calcium. This process is sensed by calmodulin, which binds the C-terminal region of calcineurin. Then, activated calcineurin prompts transcription of genes whose products allow the cell to survive under stress conditions and maintain calcium homeostasis (3, 5). In mammalian T cells, calcineurin activates the transcription factor NFAT (nuclear factor of activated T cells). This pathway is the target of the immunosuppressive drugs cyclosporine A (CsA) and FK506, which inhibit T-cell activation (16).
An in silico comparative analysis of fungal Ca2+ signaling components showed that fungi share well-conserved key regulators of Ca2+ signaling. These include Ca2+ channels, pumps, transporters, and exchangers, calmodulin, calmodulin-dependent kinases, and calcineurin. The Ca2+ signaling apparatus of Saccharomyces cerevisiae regulates the cell cycle, mating, sensing of glucose starvation, resistance to salt stress, and cell survival (41). The stress response in S. cerevisiae leads to calcineurin-mediated dephosphorylation and activation of the transcription factor Crz1, which regulates the transcription of more than 160 genes (30, 36). In the filamentous fungi Neurospora crassa and Magnaporthe grisea, there is evidence for the involvement of Ca2+ in many physiological processes, including the cell cycle, sporulation, spore germination, and hyphal growth (41).
The Ca2+-calcineurin signaling pathway in the human pathogenic fungus Cryptococcus neoformans has been characterized (reviewed in reference 19). In this fungus calcineurin is required for mating, morphogenesis, growth at 37°C, and virulence (7, 13, 33). C. neoformans calmodulin is essential for viability and acts in response to high temperatures by two distinct mechanisms. Only one of these pathways, however, involves Ca2+ and calcineurin (22). Furthermore, two important components of the C. neoformans Ca2+ signaling network have been described (10, 24). The first is Cch1, a Ca2+-permeable channel that mediates calcium entry in C. neoformans cells. This plasma membrane calcium channel is required for calcium uptake in low-calcium environments (24). The second is EcaI, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase that participates in stress tolerance (10). Both components, Cch1 and EcaI, are involved in C. neoformans virulence (10, 24), emphasizing the importance of calcium signaling in this pathogen.
Ca2+ exchangers regulate the concentration of cytosolic Ca2+ and its transport to storage organelles. This process is achieved by exchanging positive ions across membranes (41). S. cerevisiae possesses one such previously identified H+/Ca2+ exchanger, named Vcx1 (ScVcx1), which localizes to the vacuolar membrane (9, 32). ScVcx1 is negatively regulated by calcineurin, acting in Ca2+ tolerance and Ca2+ sequestration efficiently when calcineurin is inactivated (9). Additionally, ScVcx1 may also function in Cd2+ transport (35). Here we report the identification of the C. neoformans gene VCX1, which encodes a vacuolar calcium exchanger. The VCX1 knockout results in hypersensitivity to the calcineurin inhibitor cyclosporine A at 35°C, but not at 30°C. Growth analysis of the vcx1 mutant strain with high concentrations of CaCl2 in the presence or absence of cyclosporine A indicated that Vcx1 confers a much larger degree of calcium tolerance when calcineurin has been inhibited. Importantly, Vcx1 influences C. neoformans phagocytosis by murine macrophages and is required for full virulence in mice.
MATERIALS AND METHODS
Fungal strains, plasmids, and media.
C. neoformans serotype A strain H99 was the recipient for construction of the mutant strain. Strains were maintained on YPD medium (1% yeast extract, 2% peptone, 2% dextrose, and 1.5% agar). YPD plates with hygromycin (200 μg/ml) were used to select C. neoformans VCX1 deletion transformants (vcx1 strain). YPD plates with nourseothricin (100 μg/ml) were used to select C. neoformans VCX1 complementation transformants (vcx1::VCX1 strain). Plasmid pJAF15 (14) was the source of a hygromycin resistance cassette, and pAI4 (17) was the source of a nourseothricin resistance cassette. Plasmids were maintained in Escherichia coli grown at 37°C in LB broth or on agar supplemented with 50 μg/ml of kanamycin.
In silico analysis of the C. neoformans VCX1 ortholog.
The putative C. neoformans VCX1 gene sequence was identified by a BLAST search of the C. neoformans var. grubii strain H99 genomic database at the Broad Institute by using the Vcx1 sequence of S. cerevisiae (GenBank accession number NP_010155.1). The amino acid sequences of Vcx1 orthologs from S. cerevisiae, Candida albicans, Aspergillus fumigatus, Schizosaccharomyces pombe, Coprinus cinereus, Ustilago maydis, Arabidopsis thaliana, Oryza sativa, and C. neoformans were aligned using Clustal X2 (23). Mega4 was utilized for phylogenetic analysis, applying the neighbor-joining method, and the tree architecture was inferred from 1,000 bootstraps (37). A search for conserved domains in the ortholog proteins was performed using the Pfam database (http://pfam.sanger.ac.uk/). Prediction of putative transmembrane segments was conducted by using the TMHMM server (http://www.cbs.dtu.dk/services/TMHMM/).
Disruption and complementation of C. neoformans VCX1.
Disruption of VCX1 was achieved with DNA constructs generated by the Delsgate methodology (15). A Gateway cloning system donor vector (Invitrogen) carrying the hygromycin-selectable marker for C. neoformans transformation was constructed. A 2.2-kb PCR product encompassing the hygromycin marker cassette was amplified from plasmid pJAF15 and cloned into the EcoRV site of pDONR201 (Invitrogen), resulting in a vector named pDONRHYG. The 5′ and 3′ VCX1 flanks (900 bp each) were PCR amplified and gel purified using the Illustra GFX PCR DNA and gel band purification kit (GE Healthcare). Approximately 300 ng of vector pDONRHYG and 30 ng of each PCR product were submitted to a BP clonase reaction, according to the manufacturer's instructions (Invitrogen). This reaction mixture was transformed into E. coli OmniMAX 2-T1 cells. After confirmation of the correct deletion construct, the plasmid was linearized by I-SceI digestion prior to C. neoformans biolistic transformation (38). The transformants were screened by colony PCR, and the deletion was confirmed by Southern blot analysis and semiquantitative reverse transcription-PCR (RT-PCR). For complementation of the mutation, a 4.4-kb genomic PCR fragment carrying the wild-type VCX1 gene was cloned into the SmaI site of vector pAI4. The resulting plasmid was used for transformation of the vcx1 mutant strain, and the transformants were selected in the presence of nourseothricin (200 μg/ml). Random genomic insertion of the complemented gene was confirmed by Southern blot analysis and semiquantitative RT-PCR. The primers used in these constructions are listed in Table S1 of the supplemental material.
Phenotypic characterization assays.
For phenotypic characterization, wild-type (WT), vcx1 mutant, and complemented strains were grown on YPD medium for 16 h, washed, and adjusted to a cell density of 107 cells/ml. The cell suspensions were serially diluted 10-fold, and 3 μl of each dilution was spotted onto YPD agar supplemented with CaCl2 (50, 100, 150, or 200 mM), MnCl2 (2, 4, 6, or 8 mM), CdCl2 (20 or 50 μM), and/or cyclosporine A (100 μg/ml). The plates were incubated for 2 days at 30°C, 35°C, or 37°C and photographed. Fungal cells were also grown at 37°C in 5% CO2, in YPD medium with alkaline, neutral, or acidic pH (8.5, 7, and 4, respectively), or in the presence of Congo Red (0.5%). Melanin production was examined on niger seed agar plates containing 0.1% glucose. Capsule formation was examined by microscopy after incubation for 24 h at 30°C in a minimal medium (12) and staining with India ink. Relative capsule size was defined as the distance between the cell wall and the capsule outer border by cell diameter. ImageJ software was utilized to determine capsule measurements of 100 cells of each strain. The content of extracellular GXM in these culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA) according to a protocol previously described (11). The content of extracellular GXM in culture supernatants in the presence of cyclosporine A (25 to 150 μg/ml) was also determined. Phenotypic analysis of the cell surface architecture was performed after incubation of yeast cells with the wheat germ lectin (WGA), calcofluor white, and the monoclonal antibody (MAb) 18B7 (12). These probes were used to visualize, by fluorescence microscopy, the surface distribution of N-acetylglucosamine (GlcNAc) oligomers (with WGA), cell wall chitin (with calcofluor), and GXM (with 18B7), following a previously described protocol (12).
Virulence assay.
Virulence studies were conducted according to a previously described intranasal inhalation infection model (6) using eight female BALB/c mice (approximately 5 weeks old) for each strain tested. Fungal cells were cultured in 50 ml of YPD medium at 30°C overnight with shaking, washed twice, and resuspended in phosphate-buffered saline (PBS). Mice were infected with 107 yeast cells suspended in 50 μl PBS and monitored daily. Kaplan-Meier analysis of survival was performed using GraphPad Prism software. Animal studies were approved by the Federal University of Rio Grande do Sul Ethics Committee.
Macrophage infection assay.
The susceptibility of fungal cells to the antifungal action of phagocytes was determined by counting CFU after interaction of WT, vcx1 mutant, and vcx1::VCX1-complemented strains with the murine macrophage-like cell line RAW 264.7. Murine cells were maintained at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% heat-inactivated fetal bovine serum (FBS). Fungal cells were opsonized with monoclonal antibody 18B7 (1 μg/ml). Macrophages were seeded at a concentration of 105 cells/well in a 96-well cell culture plate and incubated overnight. Then, 106 fungal cells were inoculated in each well, and after 1 h the wells were washed to remove unattached, extracellular fungal cells. After 20 h of incubation, infected cultures were again washed, and sterile ice-cold distilled H2O was added to each well to promote macrophage lysis. Fungal viability was measured by plating the lysates on YPD for CFU determination after cultivation of the plates for 48 h at 30°C. The assay was performed in triplicate sets for each strain. Student's t test was used to determine the statistical significance of differences in fungal survival. To determine the rate of phagocytosis of WT, vcx1 mutant, and complemented cells during interaction with RAW 264.7 cells, yeast cells were incubated with fluorescein isothiocyanate (FITC) at 0.5 mg/ml in PBS for 10 min at room temperature. Then, the cells were washed with PBS and incubated with macrophage-like cells for 1 h at a 5:1 fungus/host cell ratio, followed by extensive washing with PBS for removal of nonadherent fungi. The fluorescence intensity of the macrophage-like cells was therefore a function of association with FITC-labeled C. neoformans. The infected cells were then detached from tissue culture plates by scraping, fixed with 4% paraformaldehyde, and analyzed in a FACScalibur (BD Biosciences). The data were analyzed using the winMDI 2.9 software.
Construction of C. neoformans Vcx1-mCherry fusion strain.
In order to assess the subcellular localization of Vcx1 in C. neoformans cells, a cassette carrying a Vcx1-mCherry fusion was constructed. Briefly, a 2-kb fragment encompassing the VCX1 promoter and coding sequences without the stop codon was amplified using genomic DNA from strain H99 as template. The pLKB25 plasmid (18) was utilized as a template to amplify a second fragment containing the mCherry-GPD1 terminator and the neomycin resistance gene. These fragments, which overlap by ∼40 bp, were combined and utilized as template for the overlap PCR. The product of the overlap PCR was cloned into the pCRTOPO2.1 vector. This final construct was transformed in the C. neoformans vcx1 mutant strain by biolistic transformation (38). Transformants (Vcx1-mCherry strains) were selected in YPD plates with 200 μg/ml of neomycin. These cells were visualized and photographed using an Olympus FluoView 1000 confocal laser scanning microscope.
Quantitative real time RT-PCR analysis.
For RNA extraction, cultures of WT and vcx1 mutant cells were grown overnight in YPD medium at 37°C with shaking. Three independent sets of RNA samples from independent experiments were prepared using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. After DNase treatment, RNA preparations were purified using RNAeasy minicolumns (Qiagen), and reverse transcription reactions were performed. Real-time PCRs were performed in an Applied Biosystems 7500 real-time PCR system. PCR thermal cycling conditions were an initial step at 95°C for 5 min followed by 40 cycles at 95°C for 15 s, 60°C for 20 s, and 72°C for 20 s. Platinum SYBR green qPCR Supermix (Invitrogen) was used as reaction mix, supplemented with 5 pmol of each primer and 2 μl of the cDNA template in a final volume of 25 μl. All experiments were performed in three independent cultures, and each cDNA sample was analyzed in duplicate with each primer pair. Melting curve analysis was performed at the end of the reaction to confirm a single PCR product. Data were normalized to actin cDNA levels amplified in each set of PCR experiments. Relative expression was determined by the 2−ΔΔCT method (25). The primers utilized in these experiments are listed in Table S1 of the supplemental material.
Determination of relative levels of intracellular calcium concentration.
The relative intracellular free calcium concentration was determined using the acetoxymethylester of Fura-2 (Fura-2-AM; Invitrogen). Briefly, WT and vcx1 mutant cells were cultured in YPD medium overnight with shaking. Then, 107 cells of each strain were incubated for 1 h in fresh YPD amended or not with 100 mM CaCl2. The cells were washed three times with PBS and loaded with 10 μM Fura-2-AM for 30 min at 37°C. After extensive washing, Fura-2 fluorescence was measured by alternating the excitation wavelengths at 340 and 380 nm with an emission wavelength fixed at 505 nm. The relative intracellular calcium concentration is expressed as the ratio between fluorescence intensities with excitation wavelengths at 340 and 380 nm. All data presented are representative of three independent experiments.
RESULTS
Identification of the vacuolar calcium exchanger Vcx1 ortholog in C. neoformans.
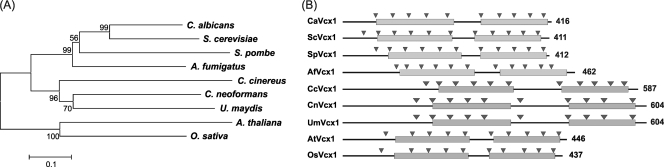
The VCX1 gene was identified in the C. neoformans var. grubii H99 genomic database of the Broad Institute (accession number CNAG_00025.1) (http://www.broadinstitute.org/annotation/genome/cryptococcus_neoformans /MultiHome.html), based on its similarity to the vacuolar calcium exchanger Vcx1 from S. cerevisiae. The C. neoformans VCX1 coding region is 2,399 bp long, contains nine introns, and encodes a putative 604-amino-acid protein. Ca2+ exchanger proteins are characterized by the presence of calcium/hydrogen antiporter (ChaA; COG0387), calcium/proton exchanger (caca2 [TIGR00846], cax [TIGR00378]), and sodium/calcium exchanger superfamily (Na_Ca_ex; pfam01699) domains (41). BLAST search using the Conserved Domain Database at NCBI (http://www.ncbi.nlm.nih.gov/structure/cdd/cdd.shtml) revealed that these domains are also present in the C. neoformans Vcx1 ortholog. Furthermore, a phylogenetic analysis including Vcx1 sequences from distinct eukaryotic organisms was performed (Fig. 1A). C. neoformans Vcx1 has higher similarity to Vcx1 of U. maydis (36% identity and 52% similarity) and lowest similarity to that in A. thaliana (21% identity and 39% similarity) when the amino acids of each protein are compared. The phylogeny tree splits into two clades, corresponding to fungal and plant Vcx1 orthologs. As expected, C. neoformans Vcx1 clusters with other basidiomycete fungal Vcx1 proteins. The domain architecture of the C. neoformans Vcx1 was compared to the orthologs herein analyzed. All of them possess two well-conserved domains related to the sodium/calcium exchanger superfamily (Na_Ca_ex; pfam01699). Moreover, prediction of transmembrane (TM) regions revealed that all the Vcx1 proteins analyzed had 10 or 11 predicted TM domains (Fig. 1B), in agreement with known Ca2+ exchangers (41).
Fig. 1.
Results of the in silico analysis of the C. neoformans Vcx1 ortholog. (A) Phylogenetic analysis results, applying the neighbor-joining method and including Vcx1 sequences from distinct eukaryotic organisms, as follows: S. cerevisiae (NCBI accession number NP_010155.1), C. albicans (NCBI accession number XP_711893.1), A. fumigatus (NCBI accession number XP_750174.2), S. pombe (NCBI accession number O59768), C. cinereus (Broad Institute accession number CC1G_02539), U. maydis (Broad Institute accession number UM_05132), A. thaliana (NCBI accession number Q945S5), O. sativa (NCBI accession number NP_00105030.1), and C. neoformans (Broad Institute accession number CNAG_00025.1). The bar marker indicates the genetic distance, which is proportional to the number of amino acid substitutions. Bootstrap values obtained with 1,000 resamplings are shown at the nodes. (B) Domain architecture in the Vcx1 orthologs of different eukaryotic organisms (with two-letter species abbreviations for each Vcx1; species are the same as those listed in panel A). The sodium/calcium exchanger superfamily (Na_Ca_ex; pfam01699) domain, a characteristic domain in Ca2+ exchangers, is represented by gray boxes. The transmembrane domains are represented by arrowheads. The lengths (in amino acids) of each of the sequence proteins are indicated on the right.
The vcx1 mutant displayed calcineurin-dependent Ca2+ sensitivity.
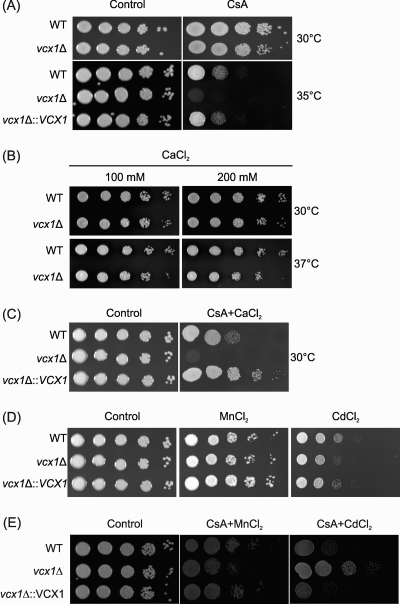
In order to investigate the functional role of VCX1 in C. neoformans, knockout and complemented strains were constructed. Deletion and complementation of VCX1 were confirmed by Southern blot analysis and semiquantitative RT-PCR (see Fig. S1 in the supplemental material). The ability to grow at the human body temperature (36° to 37°C) is one of the most important virulence factors of C. neoformans (28). This trait is controlled in part by the Ca2+-calcineurin pathway, which senses and utilizes cytosolic calcium for signaling (19). We then asked whether the C. neoformans Vcx1 participates in this signaling pathway during growth at high temperatures. Our results demonstrated that Vcx1 is required for C. neoformans growth at 35°C (semipermissive temperature) only in the presence of cyclosporine A, a calcineurin inhibitor (Fig. 2A). Furthermore, the role of VCX1 in Ca2+ tolerance was evaluated by monitoring growth of the vcx1 mutant strain in YPD agar plates supplemented with increasing concentrations of CaCl2 amended or not with cyclosporine A (Fig. 2B and C). The growth of the vcx1 mutant strain was inhibited only in the presence of cyclosporine A, indicating that Vcx1 confers tolerance to a wider range of calcium concentrations when calcineurin is inhibited (Fig. 2C). Together, these results suggest that Vcx1 influences the Ca2+-calcineurin signaling pathway in C. neoformans. Since S. cerevisiae calcineurin regulates the transport of cations by variants of Vcx1 (35), we evaluated the Cd2+ and Mn2+ tolerance of the C. neoformans vcx1 mutant strain. No differences in fungal growth were observed under conditions of a high concentration of CdCl2 or MnCl2 (Fig. 2D). However, when calcineurin was inhibited by cyclosporine A, the vcx1 mutant strain exhibited a slightly increased resistance against high concentrations of CdCl2 compared to the WT and complemented strains (Fig. 2E).
Fig. 2.
The vcx1 mutant displays calcineurin-dependent Ca2+ sensitivity. Ten-fold serial dilutions of WT H99, vcx1 mutant (vcx1Δ), and vcx1::VCX1-complemented (vcx1Δ::VCX1) cells were plated in YPD agar containing 100 μg/ml of CsA (A), 100 or 200 mM CaCl2 (B), 50 mM CaCl2 amended with 100 μg/ml of CsA (C), 4 mM MnCl2 or 50 μM CdCl2 (D), or 4 mM MnCl2 or 50 μM CdCl2 amended with 100 μg/ml of CsA (E). The plates were incubated for 2 days at 30°C, 35°C, or 37°C, as indicated. As control, cells were grown in YPD agar only.
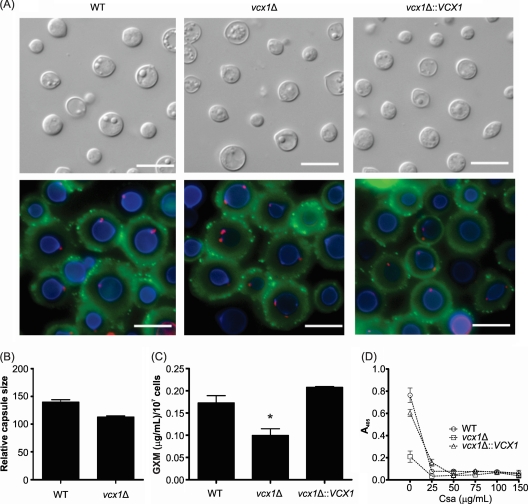
Disruption of VCX1 does not influence capsule size, but it decreases extracellular GXM secretion.
The vcx1 mutant strain was tested for its ability to grow at 37°C, for capsule size, and for melanin production, since these traits are thought to be the major virulence factors in C. neoformans (31). The VCX1 knockout apparently did not interfere with any of these features, compared to WT cells (Fig. 3 and data not shown). Moreover, the deletion of VCX1 also did not cause defects in cell wall integrity, as assessed by staining of chitin and GlcNAc oligomers (Fig. 3A) and by a spot growth assay on plates containing Congo red (see Fig. S2 in the supplemental material). However, mutant cells showed an impaired ability to produce extracellular GXM, as concluded from the lower polysaccharide contents in culture supernatants of the vcx1 mutant strain than in WT or complemented cells (P < 0.05) (Fig. 3C). We believe that the antibody-based lower GXM detection in supernatants of vcx1 mutant cells reflects a decreased production of this extracellular polysaccharide rather than the production of a structurally modified GXM, since the surface polysaccharide was regularly recognized by MAb 18B7. This observation, which echoed previous findings with a secretion mutant of C. neoformans (34), suggests a role for Vcx1 in the release of capsular polysaccharides to the extracellular environment. The levels of extracellular GXM in culture supernatants of WT, vcx1 mutant, and complemented cells treated with cyclosporine A were also quantified. Different concentrations of cyclosporine A (ranging from 25 to 150 μg/ml) completely inhibited GXM secretion by all strains tested (Fig. 3D).
Fig. 3.
Disruption of VCX1 does not influence capsule size but decreases extracellular GXM secretion. (A) Fluorescence microscopy of WT, vcx1 mutant, and vcx1::VCX1-complemented mutant cells stained with the lectin WGA (red spots), calcofluor white (blue staining), and the monoclonal antibody 18B7 (green), to visualize GlcNAc oligomers, cell wall, and GXM, respectively. Bars, 10 μm. (B) Relative capsule sizes of WT and vcx1 mutant cells. (C) Content of extracellular GXM in culture supernatants of WT, vcx1 mutant, and vcx1::VCX1-complemented mutant cells, as determined by ELISA. *, P < 0.05. (D) Content of extracellular GXM in culture supernatants of WT, vcx1 mutant, and vcx1::VCX1-complemented mutant cells incubated with different concentrations of CsA, as determined by ELISA.
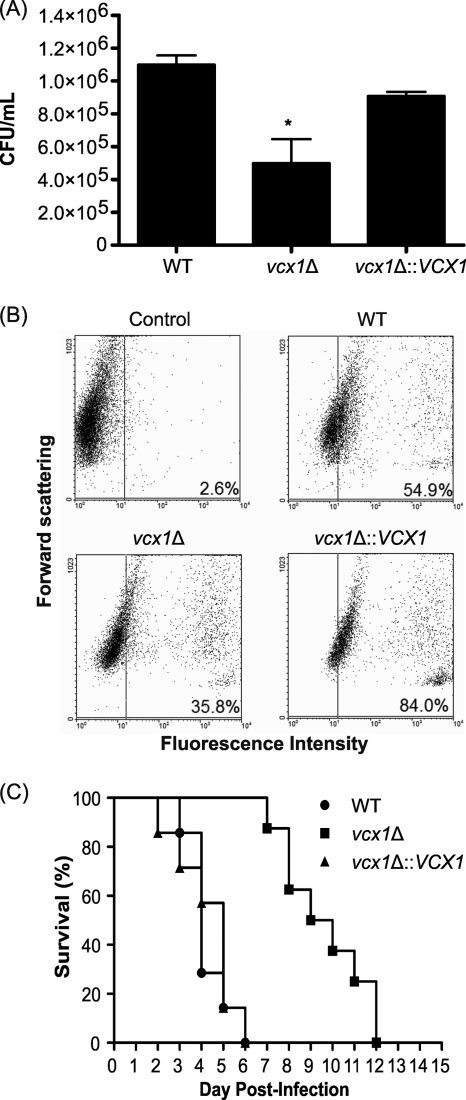
VCX1 influences C. neoformans phagocytosis by macrophages and is required for full virulence in animal cryptococcosis.
The susceptibility of WT, vcx1 mutant, and vcx1::VCX1-complemented strains to the antimicrobial action of macrophage-like cells was evaluated in vitro. In comparison to WT and complemented cells, the vcx1 deletion mutant showed decreased rates of survival after interaction with the phagocytes (Fig. 4A) (P < 0.05). To exclude the possibility that the lower rate of vcx1 mutant survival inside macrophages was due to its hypersensitivity to extreme pH or to 5% CO2, fungal cells were grown in YPD medium with alkaline, neutral, or acidic pH (8.5, 7, and 4, respectively) or in 5% CO2. However, no growth differences were observed under these conditions (see Fig. S2 in the supplemental material). The lower CFU counts for the vcx1 mutant could be due to a decreased survival within the macrophages (lower viability or slower proliferation within the macrophages) or, alternatively, they may be due to a lower rate of phagocytosis. To test this hypothesis, the rates of phagocytosis of WT, vcx1 mutant, and complemented cells were measured by flow cytometry. In fact, the rate of phagocytosis of the vcx1 mutant cells was significantly lower than in WT or complemented cells (Fig. 4B), indicating that VCX1 is required for efficient phagocytosis and perhaps has some minor role in survival within the macrophages.
Fig. 4.
VCX1 influences C. neoformans phagocytosis by macrophages and is required for full virulence in mice. (A) CFU counts after macrophage infection with WT, vcx1 mutant, or vcx1::VCX1-complemented strains. *, P < 0.05. (B) Rates of phagocytosis of WT, vcx1 mutant, and complemented cells as measured by flow cytometry. (C) Virulence assay of WT, vcx1 mutant, and vcx1::VCX1-complemented strains in an intranasal inhalation infection model with BALB/c mice.
These observations encouraged us to test the role of Vcx1 in the pathogenesis of C. neoformans in a mouse inhalation model of cryptococcosis. Mice inoculated with WT or complemented strains had mean survival times of 4 and 5 days, respectively. Statistical analysis revealed no significant difference between mortality rates caused by these two strains (P = 0.79). In contrast, mice inoculated with vcx1 mutant cells survived longer, with a mean survival time of 9.5 days (P = 0.002) (Fig. 4C). This result demonstrates that Vcx1 is required for the virulence of C. neoformans in animal cryptococcosis.
Vcx1 localizes to the vacuole of C. neoformans cells.
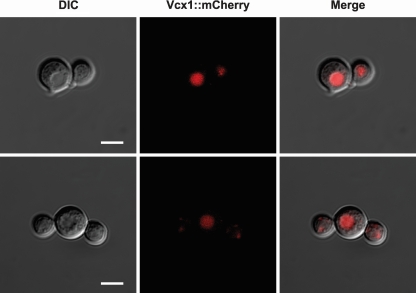
In order to confirm the predicted vacuolar localization of Vcx1 in C. neoformans cells, an mCherry-tagged Vcx1 strain was constructed. Figure 5 illustrates the colocalization of mCherry-tagged Vcx1 with vacuole organelles, which appear as a depression in the differential interference contrast (DIC) image. This result confirmed that Vcx1 localizes to the vacuole of C. neoformans cells.
Fig. 5.
Vcx1 localizes to the vacuoles of C. neoformans cells, as shown via confocal microscopy of Vcx1-mCherry fusion strain cells. The mCherry-tagged Vcx1 (red) colocalizes with vacuole organelles, which appear as a depression in the DIC image. Bars, 5 μm.
Disruption of VCX1 influences the expression of other calcium transporters in C. neoformans.
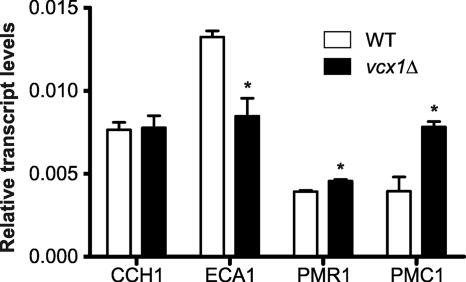
Because C. neoformans Cch1 and EcaI are also calcineurin pathway calcium transporters, the transcript levels of these genes were evaluated in WT and vcx1 mutant strains. Disruption of C. neoformans VCX1 led to a decrease in expression of ECA1, but the CCH1 transcription level was not influenced. Furthermore, we also analyzed the expression of two putative orthologs of well-characterized calcium transporters of S. cerevisiae, PMC1, a vacuolar calcium ATPase, and PMR1, a Golgi apparatus calcium ATPase (8, 29). Interestingly, the transcript levels of the C. neoformans PMC1 ortholog significantly increased in vcx1 mutant cells (P < 0.05). A slight increase in PMR1 ortholog expression was also observed in vcx1 mutant cells (Fig. 6).
Fig. 6.
Disruption of VCX1 influences expression of other calcium transporters in C. neoformans. The relative expression levels of different C. neoformans calcium transporters (CCH1, ECA1, PMR1, and PMC1) in WT and vcx1 mutant cells were quantified by RT-PCR. The measured quantity of the mRNA in each of the samples was normalized using the threshold cycle (CT) values obtained for the actin gene. The accession numbers for the C. neoformans orthologs of PMR1 and PMC1 from the Broad Institute database are CNAG_05135.2 and CNAG_01232.2, respectively. Data shown are means ± standard deviations. *, P < 0.05.
Loss of VCX1 influences the relative level of intracellular calcium concentration in C. neoformans cells.
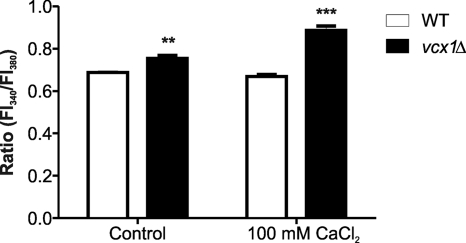
In order to determine the relative concentration of free calcium in WT and vcx1 mutant strains, the calcium-sensitive dye Fura-2-AM was utilized. This fluorescent calcium indicator can passively diffuse across cell membranes. Once inside the cell, the esters are cleaved by intracellular esterases to yield cell-impermeant fluorescent indicators. Upon binding Ca2+, Fura-2 exhibits an absorption shift from 380 to 340 nm of excitation. Therefore, the relative Ca2+ concentration was obtained based on the fluorescence ratio after dual-wavelength excitation. The vcx1 mutant strain had an increased relative level of intracellular calcium concentration compared to the WT strain (Fig. 7). This increase was more pronounced when an excessive amount of CaCl2 was added to the medium, indicating that the loss of VCX1 influences the relative level of intracellular calcium concentration. This phenotype is probably due to a calcium transport defect of vacuoles in the vcx1 mutant strain, because Ca2+ exchangers regulate the concentration of cytosolic Ca2+ and its transport to storage organelles (41).
Fig. 7.
Disruption of VCX1 influences the relative levels of intracellular calcium concentration in C. neoformans. The relative levels of intracellular calcium in WT and vcx1 mutant cells were determined using the calcium-sensitive dye Fura-2-AM. The relative Ca2+ concentration was determined based on the fluorescence ratio after dual-wavelength excitation (fluorescence intensity at 340 nm [FI340]/FI380). The control was fresh YPD medium. Data shown are means ± standard deviations. **, P < 0.05; ***, P < 0.01.
DISCUSSION
The calcium-calcineurin signaling pathway in C. neoformans is fundamental to sense and to adapt to the human host milieu. In addition to its importance for high-temperature growth, calcineurin is also essential for cell wall integrity, mating, and monokaryotic fruiting (7, 13, 19, 20, 28, 33). In the present study Vcx1, a newly recognized component of the C. neoformans Ca2+ signaling network, was identified. This vacuolar calcium exchanger is part of a conserved family of Ca2+ exchangers that regulate cytosolic calcium concentration and transport into Ca2+ storage organelles (i.e., vacuoles) in distinct eukaryotes (41).
First, we investigated if C. neoformans Vcx1 acts in the Ca2+-calcineurin pathway under host temperature growth conditions. In fact, Vcx1 is required for C. neoformans growth at 35°C only when calcineurin has been inhibited. Moreover, Vcx1 participates in Ca2+ tolerance in a calcineurin-dependent manner. These findings suggest that Vcx1 influences the Ca2+-calcineurin signaling pathway in C. neoformans, in agreement with findings reported for the S. cerevisiae Vcx1 ortholog (9, 35). Calcineurin significantly affects Vcx1 Ca2+/H+ exchange activity in S. cerevisiae. The negative regulation of ScVcx1 by calcineurin is thought to be posttranslational and independent of the transcription factor Crz1, which regulates genes encoding ion pumps and cell wall biosynthesis enzymes (9, 30, 36). No ortholog of the CRZ1 gene has been identified in C. neoformans. It is possible that C. neoformans contains a different transcription factor or more than one transcription factor responsive to calcineurin (19).
The loss of C. neoformans Vcx1 by knockout did not cause defects in cell wall integrity, melanin production, or capsule size, but the vcx1 mutant showed a reduced ability to produce extracellular GXM. Although the concentration of extracellular GXM is usually related to capsule enlargement, Panepinto and colleagues recently demonstrated that a sec6 mutant of C. neoformans produced a normal capsule even under conditions in which GXM secretion was decreased (34). Our result suggests that, although yeast cells lacking Vcx1 expression are able to assemble a normal capsule, the protein is somehow required for polysaccharide release.
A key feature of cryptococcal pathogenesis is its ability to survive and replicate inside macrophages, in a process that requires GXM release for further accumulation in cytoplasmic vesicles (39). GXM is toxic for the macrophages, which implies that polysaccharide secretion reduces the antimicrobial activity of phagocytes. Furthermore, C. neoformans possesses mechanisms that allow cell-to-cell spread and extrusion from infected macrophages (1, 2, 26, 27, 39, 40). These processes supposedly require production of GXM. Therefore, capsular polysaccharide interferes with macrophage function at multiple levels (reviewed in reference 40). In this context, the defective ability to produce extracellular GXM that was observed in the vcx1 mutant could be related to its reduced rate of phagocytosis and survival after macrophage infection.
In our study, Vcx1 was required for full virulence during infection. This same phenotype was observed for C. neoformans mutants lacking expression of Cch1 and EcaI, a plasma membrane calcium channel and a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, respectively (10, 24). These results emphasize the key role of calcium signaling for cryptococcal virulence. Calcineurin is essential for C. neoformans survival in the host environment (19, 33). Our results showed that in the presence of the calcineurin inhibitor cyclosporine A, the vcx1 mutant failed to grow at the high temperature, suggesting that Vcx1 acts in parallel with calcineurin in response to the host's temperature. C. neoformans Vcx1 also influences the expression of other calcium transporters. The transcript level of the PMC1 ortholog, a putative vacuolar calcium ATPase, was significantly increased in the vcx1 mutant compared to the WT strain, probably due to a compensatory effect, since PMC1 also transports calcium into vacuoles (8).
In conclusion, we have shown that Vcx1, a vacuolar calcium transporter, influences C. neoformans phagocytosis by macrophages and is required for full virulence in animal infection. Additionally, Vcx1 is involved in calcineurin-dependent Ca2+ tolerance, acts in the Ca2+-calcineurin signaling pathway in C. neoformans, and influences the relative intracellular calcium concentration. Further studies are necessary to address the role of Vcx1 in the release of GXM to the extracellular environment and to completely understand how this component of the Ca2+-calcineurin pathway contributes to the pathogenesis of cryptococcosis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo a Pesquisa no Estado do Rio de Janeiro (FAPERJ), and Financiadora de Estudos e Projetos (FINEP).
We thank Joseph Heitman and Alex Idnurm for providing pJAF15, pAI4, and pLKB25 plasmids and Arturo Casadevall for providing the monoclonal antibody anti-GXM (18B7). We also thank the Electron Microscopy Center of the Federal University of Rio Grande do Sul (CME, UFRGS) for the confocal microscopy analysis and Henrique Biehl for technical assistance. Automated DNA sequencing was performed at the facilities of the Brazilian Genome Network at the Center of Biotechnology, CBiot-UFRGS-RS.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 1 October 2010.
REFERENCES
- 1.Alvarez M., Casadevall A. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16:2161–2165 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez M., Casadevall A. 2007. Cell-to-cell spread and massive vacuole formation after Cryptococcus neoformans infection of murine macrophages. BMC Immunol. 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aramburu J., Rao A., Klee C. B. 2000. Calcineurin: from structure to function. Curr. Top. Cell Regul. 36:237–295 [DOI] [PubMed] [Google Scholar]
- 4.Berridge M. J., Lipp P., Bootman M. D. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21 [DOI] [PubMed] [Google Scholar]
- 5.Chin D., Means A. R. 2000. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 10:322–328 [DOI] [PubMed] [Google Scholar]
- 6.Cox G. M., Mukherjee J., Cole G. T., Casadevall A., Perfect J. R. 2000. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 68:443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz M. C., Fox D. S., Heitman J. 2001. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 20:1020–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham K. W., Fink G. R. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124:351–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham K. W., Fink G. R. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2226–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan W., Idnurm A., Breger J., Mylonakis E., Heitman J. 2007. EcaI, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, is involved in stress tolerance and virulence in Cryptococcus neoformans. Infect. Immun. 75:3394–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonseca F. L., Frases S., Casadevall A., Fischman-Gompertz O., Nimrichter L., Rodrigues M. L. 2009. Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet. Biol. 46:496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca F. L., Nimrichter L., Cordero R. J., Frases S., Rodrigues J., Goldman D. L., Andruszkiewicz R., Milewski S., Travassos L. R., Casadevall A., Rodrigues M. L. 2009. Role for chitin and chitooligomers in the capsular architecture of Cryptococcus neoformans. Eukaryot. Cell 8:1543–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox D. S., Cruz M. C., Sia R. A., Ke H., Cox G. M., Cardenas M. E., Heitman J. 2001. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 39:835–849 [DOI] [PubMed] [Google Scholar]
- 14.Fraser J. A., Subaran R. L., Nichols C. B., Heitman J. 2003. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: implications for an outbreak on Vancouver Island, Canada. Eukaryot. Cell 2:1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Pedrajas M. D., Nadal M., Kapa L. B., Perlin M. H., Andrews D. L., Gold S. E. 2008. DelsGate, a robust and rapid gene deletion construction method. Fungal Genet. Biol. 45:379–388 [DOI] [PubMed] [Google Scholar]
- 16.Hogan P. G., Chen L., Nardone J., Rao A. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17:2205–2232 [DOI] [PubMed] [Google Scholar]
- 17.Idnurm A., Reedy J. L., Nussbaum J. C., Heitman J. 2004. Cryptococcus neoformans virulence gene discovery through insertional mutagenesis. Eukaryot. Cell 3:420–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozubowski L., Heitman J. 2010. Septins enforce morphogenetic events during sexual reproduction and contribute to virulence of Cryptococcus neoformans. Mol. Microbiol. 75:658–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozubowski L., Lee S. C., Heitman J. 2009. Signalling pathways in the pathogenesis of Cryptococcus. Cell. Microbiol. 11:370–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraus P. R., Fox D. S., Cox G. M., Heitman J. 2003. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48:1377–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraus P. R., Heitman J. 2003. Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem. Biophys. Res. Commun. 311:1151–1157 [DOI] [PubMed] [Google Scholar]
- 22.Kraus P. R., Nichols C. B., Heitman J. 2005. Calcium- and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryot. Cell 4:1079–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 24.Liu M., Du P., Heinrich G., Cox G. M., Gelli A. 2006. Cch1 mediates calcium entry in Cryptococcus neoformans and is essential in low-calcium environments. Eukaryot. Cell 5:1788–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 26.Ma H., Croudace J. E., Lammas D. A., May R. C. 2006. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 16:2156–2160 [DOI] [PubMed] [Google Scholar]
- 27.Ma H., Croudace J. E., Lammas D. A., May R. C. 2007. Direct cell-to-cell spread of a pathogenic yeast. BMC Immunol. 8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma H., May R. C. 2009. Virulence in Cryptococcus species. Adv. Appl. Microbiol. 67:131–190 [DOI] [PubMed] [Google Scholar]
- 29.Marchi V., Sorin A., Wei Y., Rao R. 1999. Induction of vacuolar Ca2+-ATPase and H+/Ca2+ exchange activity in yeast mutants lacking Pmr1, the Golgi Ca2+-ATPase. FEBS Lett. 454:181–186 [DOI] [PubMed] [Google Scholar]
- 30.Matheos D. P., Kingsbury T. J., Ahsan U. S., Cunningham K. W. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11:3445–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McClelland E. E., Bernhardt P., Casadevall A. 2005. Coping with multiple virulence factors: which is most important? PLoS Pathog. 1:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miseta A., Kellermayer R., Aiello D. P., Fu L., Bedwell D. M. 1999. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 451:132–136 [DOI] [PubMed] [Google Scholar]
- 33.Odom A., Muir S., Lim E., Toffaletti D. L., Perfect J., Heitman J. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panepinto J., Komperda K., Frases S., Park Y. D., Djordjevic J. T., Casadevall A., Williamson P. R. 2009. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol. Microbiol. 71:1165–1176 [DOI] [PubMed] [Google Scholar]
- 35.Pittman J. K., Cheng N. H., Shigaki T., Kunta M., Hirschi K. D. 2004. Functional dependence on calcineurin by variants of the Saccharomyces cerevisiae vacuolar Ca2+/H+ exchanger Vcx1p. Mol. Microbiol. 54:1104–1116 [DOI] [PubMed] [Google Scholar]
- 36.Stathopoulos A. M., Cyert M. S. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 38.Toffaletti D. L., Rude T. H., Johnston S. A., Durack D. T., Perfect J. R. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tucker S. C., Casadevall A. 2002. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. U. S. A. 99:3165–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaragoza O., Rodrigues M. L., De Jesus M., Frases S., Dadachova E., Casadevall A. 2009. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 68:133–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zelter A., Bencina M., Bowman B. J., Yarden O., Read N. D. 2004. A comparative genomic analysis of the calcium signaling machinery in Neurospora crassa, Magnaporthe grisea, and Saccharomyces cerevisiae. Fungal Genet. Biol. 41:827–841 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.