Abstract
Filamentous fungi synthesize bioactive secondary metabolites with major human health and economic impacts. Little is known about the mechanisms that mediate the export of these metabolites to the cell exterior. Aspergillus parasiticus synthesizes aflatoxin, a secondary metabolite that is one of the most potent naturally occurring carcinogens known. We previously demonstrated that aflatoxin is synthesized and compartmentalized in specialized vesicles called aflatoxisomes and that these subcellular organelles also play a role in the export process. In the current study, we tested the hypothesis that aflatoxisomes fuse with the cytoplasmic membrane to facilitate the release of aflatoxin into the growth environment. Microscopic analysis of A. parasiticus grown under aflatoxin-inducing and non-aflatoxin-inducing conditions generated several lines of experimental evidence that supported the hypothesis. On the basis of the evidence, we propose that export of the mycotoxin aflatoxin in Aspergillus parasiticus occurs by exocytosis, and we present a model to illustrate this export mechanism.
Secondary metabolites are chemically diverse natural products synthesized by plants, fungi, bacteria, algae, and animals. Secondary metabolites have an enormous impact on humans. Antibiotics, for example, are essential elements of the multibillion-dollar pharmaceutical industry, whereas mycotoxins cause hundreds of millions of dollars in damage to agriculture annually (11, 15). These chemicals help the producing organism to survive nutrient limitation (16). They also contribute to cellular defense mechanisms and development (11, 12), reduce cellular oxidative stress (10), and help maintain cellular homeostasis by regulating carbon flow in the cell (17).
Many fungal secondary metabolites are exported outside the cell; examples include antibiotics and mycotoxins (3, 14). We and others conducted extensive studies on the regulation of fungal secondary metabolism at the molecular (11, 15) and cellular (3, 7) levels. However, little is known about the mechanisms that mediate secondary metabolite export or why export occurs.
The filamentous fungus Aspergillus parasiticus produces aflatoxin, a secondary metabolite and the most potent naturally occurring carcinogen known. More than 90% of aflatoxin is exported to the cell exterior (3), making A. parasiticus an excellent model for studying secondary metabolite export. We recently demonstrated that specialized trafficking vesicles called aflatoxisomes play a key role in aflatoxin synthesis and export (3). As synthesis initiates, vesicle-vacuole fusion is downregulated by the global regulator Velvet, resulting in the accumulation of aflatoxisomes which contain at least the last two functional enzymes in the aflatoxin pathway and sequester aflatoxin (3). Treatments that block vesicle-vacuole fusion increase the number of aflatoxisomes, increase the quantity of aflatoxin accumulated in aflatoxisomes, and increase aflatoxin export to the cell exterior (3). On the basis of these previous observations, we hypothesized that aflatoxisomes play a direct role in aflatoxin export.
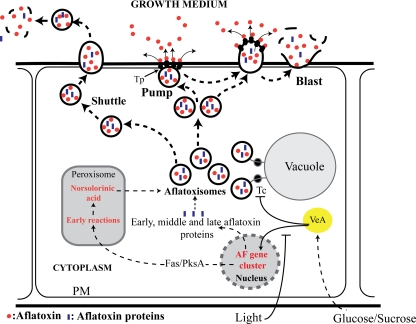
Vesicle-mediated export could theoretically occur by one (or more) of at least three mechanisms (Fig. 1). (i) Vesicles pass across the cytoplasmic membrane intact and “shuttle” their contents into the external environment. This proposed mechanism mediates virulence factor release in Cryptococcus neoformans and Histoplasma capsulatum (1) during pathogenesis. (ii) Vesicles fuse to the cytoplasmic membrane and “pump” vesicle contents to the exterior using transporter proteins similar to those that mediate resistance to antifungal agents (4, 5). (iii) Vesicles fuse with the cytoplasmic membrane, which evaginates, bursts, and “blasts” vesicle contents to the exterior. This process is similar to exocytosis, a proposed secretory mechanism for specific proteins in filamentous fungi (18). We conducted the current study to determine which, if any, of these possible mechanisms most accurately reflects the process of aflatoxin export in A. parasiticus.
Fig. 1.
Theoretical models for vesicle-mediated export. Aflatoxigenic vesicles (aflatoxisomes) arise due to downregulation of tethering complex (Tc) activity mediated by VeA (1). Aflatoxin synthesized in aflatoxisomes could theoretically be released to the cell exterior by one or more of three mechanisms: the shuttle (in which aflatoxisomes shuttle cargo across cytoplasmic membrane), pump (in which transmembrane transporter [Tp] proteins mediate the release of secondary metabolites as vesicles adhere to the inner surface of the cytoplasmic membrane), and burst-and-blast (in which vesicles protrude from the cell surface and blast their cargo into the medium) mechanisms. PM, plasma membrane.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Aspergillus parasiticus strain SU-1 (ATCC 56775) was the wild-type aflatoxin producer used in this study. A. parasiticus strain AFS10 (aflR), derived from SU-1, was used as a non-aflatoxin-producing control strain. A. parasiticus strain B62 (nor-1 brn-1; ATCC 24690) was derived from SU-1 and carries a genetic block in the aflatoxin biosynthetic pathway (9). Strain B62 accumulates large quantities of the bright red aflatoxin pathway intermediate norsolorinic acid (NA) as well as significantly reduced quantities of aflatoxin compared to SU-1 (9). YES liquid medium (2% yeast extract and 6% sucrose, pH 5.8) was used as an aflatoxin-inducing growth medium. YEP liquid medium (2% yeast extract and 6% peptone, pH 5.8) was used as a non-aflatoxin-inducing medium. Conidiospores (spores) from frozen stocks of A. parasiticus strains were inoculated into liquid growth medium at 104 spores per ml and incubated for appropriate time periods at 30°C with shaking at 150 rpm in the dark (standard growth conditions). Sortin1 treatment of A. parasiticus strain V-86 (synthesizes the aflatoxin enzyme Ver-1 fused with enhanced green fluorescent protein [EGFP] at the C-terminal end [13]) was conducted with established dosages (3).
Protein analysis.
Protein concentration measurements and Western blot analysis were performed as described previously (3).
Microscopy.
FUN-1 stain (Invitrogen, Carlsbad, CA) was used according to the manufacturer's instructions, as described previously (6). For fluorescence microscopy, microscope slides carrying samples were observed under a Labophot fluorescence microscope (Nikon, Inc., Melville, NY). For bright-field microscopy, we observed hyphal filaments under a Nikon Eclipse E600 microscope. For confocal laser scanning microscopy, MDY-64 (a vacuole and vesicle membrane dye) and Cell Tracker Blue CMAC (a vacuolar lumen stain based on peptidase activity) (Molecular Probes, Invitrogen, Carlsbad, CA) were used to visualize vacuoles and vesicles. These dyes were utilized according to the manufacturer's protocols, with modifications, as described previously (2). Images were acquired using an Olympus FluoView 1000 confocal laser scanning microscope (CLSM) (Olympus, Center Valley, PA) using a 60×/1.42-numerical-aperture oil objective and a 430- to 470-band-pass (BP) emission filter set under excitation with the 405-nm diode laser line for CMAC fluorescence (353-nm excitation/466-nm emission) and a 505- to 525-BP emission filter set under excitation with the 488-nm diode laser line for MDY-64 fluorescence (451-nm excitation/497-nm emission).
Surface immunofluorescence.
Rabbit antibodies against aflatoxin B1 (Sigma, St. Louis, MO) were used as primary antibodies to detect aflatoxin on the hyphal surface. Fluorescein isothiocyanate (FITC)-labeled anti-rabbit IgG (Sigma) was used as a secondary antibody to detect the binding pattern of the primary antibody. Rabbit antibodies against satratoxin (kindly provided by James J. Pestka) were used as a control to demonstrate immunoassay specificity. To conduct the assay, a mycelial pellet harvested from liquid growth medium was untangled with sterile forceps until single hyphae were obtained. Several hyphae were added to 50 μl of fresh YES with anti-AFB1 (α-AFB1) antibodies (diluted 1:10) and incubated for 30 min at 30°C. Labeled mycelia were centrifuged at 13,000 rpm at 4°C, washed three times with fresh YES medium at room temperature (RT), and treated for 15 min with FITC-labeled goat anti-rabbit IgG (secondary antibody; diluted 1:50 in fresh YES) at RT. Finally, mycelia were centrifuged and washed three times with fresh YES medium at RT before being observed under a Labophot fluorescence microscope (Nikon, Inc., Melville, NY).
RESULTS
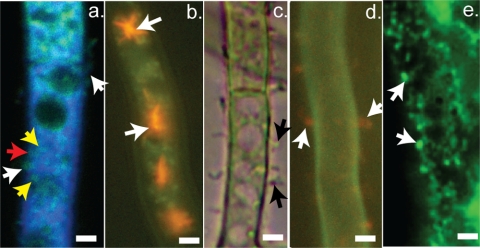
To shed light on possible export mechanisms in A. parasiticus, the fungus was grown for 36 h in an aflatoxin-inducing liquid medium (yeast extract-sucrose [YES] medium) under standard conditions (30°C, with shaking at 150 rpm, in the dark) (3). Samples of mycelia were treated with a vesicle-vacuole membrane stain (MDY64) (Invitrogen, Carlsbad, CA) and a vesicle-vacuole lumen stain (CMAC) (Invitrogen, Carlsbad, CA) (Fig. 2a) and then analyzed by confocal laser scanning microscopy (z series). We observed a shift of vesicles (green membrane with blue lumen) from the cytoplasm to the inner surface of the cytoplasmic membrane (Fig. 2a, yellow arrows) in A. parasiticus at the onset of peak levels of aflatoxin synthesis. At this time (36 h), a large number of vesicles fused with the cytoplasmic membrane (Fig. 2a, red arrows), and some of these appeared to evaginate into the external growth environment (white arrows). Our previous data (3) strongly suggest that these vesicles are aflatoxisomes that carry aflatoxin and functional aflatoxin enzymes. In support of this idea, we previously observed that aflatoxin synthesis increases during a transition from active growth to stationary phase (24 to 36 h) (8). During this transition, the number of vesicles carrying the EGFP-tagged aflatoxin enzyme Ver-1 increased significantly (8) and these adhered to the inner surface of the cytoplasmic membrane. Under these conditions, intact vesicles were not detected in the growth medium either by light microscopy or by fluorescence microscopy.
Fig. 2.
Microscopic analysis of vesicle interaction with the cytoplasmic membrane. A. parasiticus SU-1 (SU-1) was grown in aflatoxin-inducing growth medium (YES) under standard conditions for appropriate periods of time. (a) Strain SU-1 was stained with MDY-64 and CMAC and observed after 36 h of growth. The panel presents a CLSM image derived from a z-series stack of images and demonstrates the presence of blue- and green-stained vesicles at the cell surface (white arrows). (b) Visualization of vacuoles in SU-1 during early stages of aflatoxin biosynthesis using fluorescence microscopy. Strain SU-1 was grown for 24 h in YES and stained with FUN-1; FUN-1 accumulates in red-orange CIVS within vacuoles (white arrows). (c) Bright-field image showing protrusions from the cell surface (black arrows) in strain SU-1 after 72 h of growth in YES. (d) Fluorescence images of SU-1 stained with FUN-1 during aflatoxin synthesis and observed after 72 h of growth in YES (peak levels of aflatoxin synthesis occur between 36 and 48 h under these conditions). Diffuse patches of red/orange fluorescence are observed in protrusions near the hyphal surface (white arrows). (e) Fluorescence image showing aflatoxin on the cell surface. Surface immunofluorescence was conducted on A. parasiticus SU-1, a wild-type aflatoxin-producing strain (see Materials and Methods). White arrows indicate the sites for specific antiaflatoxin binding on the cell surface.
Interestingly, treatment of the fungus with Sortin1 (19), a low-molecular-weight protein sorting inhibitor, induced a transmembrane vesicle shuttle in A. parasiticus (see Fig. S1 in the supplemental material). This observation strongly suggests that transmembrane shuttle machinery exists in A. parasiticus but is likely not involved in aflatoxin export. However, Sortin1 treatment also inhibited aflatoxin production and growth severely (data not shown), suggesting that the vesicles observed in the medium under Sortin1 treatment may contribute to primary metabolism.
FUN-1 (Invitrogen, Carlsbad, CA), a fluorescent vital dye, accumulates in vacuoles via endocytosis and appears as bright red cylindrical intravacuolar structures (CIVS) (6). As expected, CIVS were observed in vacuoles during the early stages of aflatoxin synthesis (up to 24 h of growth) (Fig. 2b). However, later in stationary phase, when aflatoxin synthesis and aflatoxisome development passed peak levels (72 h), very few intense CIVS were observed within vacuoles. Instead, diffuse patches of red fluorescence were observed within vesicles near the hyphal surface and approximately 40 to 50% of these vesicles appeared to protrude outward from the mycelial surface (Fig. 2c and d).
Antibodies that specifically recognize aflatoxin (AFB1) were applied to intact mycelia at 48 h (Fig. 2e). Anti-AFB1 bound specifically to a series of discrete patches on the exterior cell surface (Fig. 2e). Binding was not observed in cells grown on non-aflatoxin-inducing medium (yeast extract-peptone [YEP] growth medium) or on the surface of AFS10, a regulatory mutant that does not make aflatoxin (data not shown) or pathway intermediates. These data strongly support the involvement of the blast mechanism in aflatoxin export.
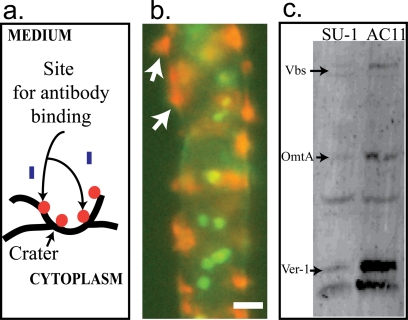
On the basis of these observations, we hypothesize (Fig. 1) that initially, aflatoxisomes carrying aflatoxin and aflatoxin enzymes are transported to the inner surface of the cytoplasmic membrane, where they fuse with this structure. The cytoplasmic membrane at this site then evaginates (protrudes) outward, bursts, and blasts vesicle contents into the growth medium; this series of events is similar to exocytosis (18). As a result of the blast, a “crater” forms on the exterior hyphal surface as illustrated in Fig. 3a. In support of this proposed series of events, we observed that while most aflatoxin is blasted into the growth medium, some aflatoxin remains bound to the crater surface and this bound toxin provides binding sites for anti-AFB1 antibodies (Fig. 2e).
Fig. 3.
Deposition of aflatoxin and norsolorinic acid at the cytoplasmic membrane and evidence for transport of aflatoxin enzymes to the cell exterior. (a) Model explaining the specificity of antibody binding shown in Fig. 2e and the fluorescence pattern shown in panel b. (b) Fluorescence image of A. parasiticus B62 grown for 72 h in YES. Norsolorinic acid accumulation on the cell surface enhances the intensity of red fluorescent patches in vesicles near the hyphal surface (white arrows). Nuclei (green) were stained with SYTOX green. (c) Western blot analysis. A. parasiticus SU-1 (wild type) and an avaA mutant (AC11) derived from SU-1 were grown in YES medium for 72 h. AC11 produces significantly higher numbers of aflatoxisomes an d larger quantities of aflatoxin than SU-1 (1). Aflatoxin enzymes Ver-1, Vbs, and OmtA in the growth medium were detected by Western blot analysis.
To begin to test the details of this model, we analyzed toxin export in A. parasiticus B62 grown under standard conditions (30°C, with shaking at 150 rpm, in the dark). A. parasiticus B62 carries a nonfunctional Nor-1 enzyme and accumulates norsolorinic acid (NA), a bright red visible pigment and the first stable intermediate in aflatoxin synthesis (9). NA exhibits poor solubility in aqueous solution; hence, NA binds to the mycelium and is not detected in the growth medium. The accumulation of NA is a useful method for visualizing the accumulation of aflatoxin, which is not visible under light in the visible wavelength. After 72 h of growth in YES growth medium, we stained A. parasiticus B62 with FUN-1 and observed the mycelium by use of a fluorescence microscope. At 72 h, A. parasiticus B62 accumulated CIVS in patches on (or within) the exterior surface of the mycelium (Fig. 3b), and these patches appeared similar to patches detected by anti-AFB1 antibodies in A. parasiticus SU-1 (wild type) (Fig. 2e). This observation tends to support the exocytosis (blast) model. Because NA is reported to be synthesized in peroxisomes (13), we hypothesize that NA in A. parasiticus B62 is transported by aflatoxisomes to the cytoplasmic membrane for export. Because of its low-level solubility in aqueous solution, we also propose that NA accumulates on the mycelial surface and blocks the ability of CIVS to exit cells; the localized increases in NA and CIVS result in the more-intense red staining observed at the cell surface in A. parasiticus B62 (compare Fig. 3b with 2d and e).
We previously demonstrated that aflatoxisomes in A. parasiticus SU-1 (wild type) carry at least three aflatoxin enzymes (OmtA, Ver-1, and Vbs) in aflatoxisomes (3). The current study demonstrates that these proteins can be detected in the growth medium at 72 h of growth after the rate of aflatoxin synthesis declines (Fig. 3c). We previously disrupted avaA (which encodes one protein in the tethering complex that drives vesicle vacuole fusion) in A. parasiticus AC-11 (ΔavaA) (3); this strain accumulates higher numbers of aflatoxisomes and exports aflatoxin at significantly higher levels than SU-1. In the current study, we demonstrated a 2-fold increase in aflatoxin enzymes present in the growth medium (Fig. 3c). In contrast to aflatoxin, the aflatoxin enzymes could not be detected on the exterior cell surface in the current study using enzyme-specific antibodies (data not shown). These observations also tend to support the exocytosis (blast) mechanism.
DISCUSSION
In contrast to the significant quantity of data in support of the proposed exocytosis (blast) mechanism presented here, we have yet to obtain any direct evidence supporting or disproving a “pump” export mechanism in A. parasiticus. We recently completed proteomic profile analysis (MudPIT) of a pure vesicle-vacuole (V) fraction containing aflatoxisomes (unpublished data), and the data suggest that AflT, an MFS-like protein, is present in this fraction. However, Chang et al. disrupted aflT in A. parasiticus and reported no effect on aflatoxin secretion (4). Although these observations tend to support our model, we cannot rule out the possibility that transporter proteins might play a role in secondary metabolite export in the initial stages of aflatoxin secretion. It is possible that removal of an MFS transporter by gene disruption would not block export completely (for example, see penicillin and cephalosporin secretion [14]), since the blast mechanism would still theoretically operate and compensate for the impaired pump. This alternative export pathway (pump/blast) is depicted in Fig. 1.
This work may have important practical implications. We demonstrated that we can increase or decrease aflatoxin export by modifying the growth medium composition or by introducing a specific mutation into the transport machinery. These observations suggest that control of the export pathway provides a great opportunity to manipulate fungal secondary metabolite export to increase production of beneficial secondary metabolites or to reduce production of detrimental ones. Future research will now focus on the export mechanism in greater detail to test our hypotheses and the accuracy of our model. In addition, the methods that we developed for detection of surface immunofluorescence in our current work may serve as a useful diagnostic tool to monitor the level of vesicle-mediated secondary metabolite export in filamentous fungi.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant R01 52003-19 and the Michigan Agricultural Experiment Station.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 24 September 2010.
REFERENCES
- 1.Casadevall A., Nosanchuk J. D., Williamson P., Rodrigues M. L. 2009. Vesicular transport across the fungal cell wall. Trends Microbiol. 17:158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chanda A., Roze L. V., Pastor A., Frame M. K., Linz J. E. 2009. Purification of a vesicle-vacuole fraction functionally linked to aflatoxin synthesis in Aspergillus parasiticus. J. Microbiol. Methods 78:28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanda A., Roze L. V., Kang S., Artymovich K. A., Hicks G. R., Raikhel N. V., Calvo A. M., Linz J. E. 2009. A key role for vesicles in fungal secondary metabolism. Proc. Natl. Acad. Sci. U. S. A. 106:19533–19538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang P. K., Yu J., Yu J. H. 2004. aflT, a MFS transporter-encoding gene located in the aflatoxin gene cluster, does not have a significant role in aflatoxin secretion. Fungal Genet. Biol. 41:911–920 [DOI] [PubMed] [Google Scholar]
- 5.Coleman J. J., Mylonakis E. 2009. Efflux in fungi: la piece de resistance. PLoS Pathog. 5:e1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Essary B. D., Marshall P. A. 2009. Assessment of FUN-1 vital dye staining: yeast with a block in the vacuolar sorting pathway have impaired ability to form CIVS when stained with FUN-1 fluorescent dye. J. Microbiol. Methods 78:208–212 [DOI] [PubMed] [Google Scholar]
- 7.Evers M. E., Trip H., van den Berg M. A., Bovenberg R. A., Driessen A. J. 2004. Compartmentalization and transport in beta-lactam antibiotics biosynthesis. Adv. Biochem. Eng. Biotechnol. 88:111–135 [DOI] [PubMed] [Google Scholar]
- 8.Hong S. Y., Linz J. E. 2008. Functional expression and sub-cellular localization of the aflatoxin pathway enzyme Ver-1 fused to enhanced green fluorescent protein. Appl. Environ. Microbiol. 74:6385–6396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong S.-Y., Linz J. E. 2009. Functional expression and sub-cellular localization of the early aflatoxin pathway enzyme Nor-1 in Aspergillus parasiticus. Mycol. Res. 113:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J. Q., Jiang H. F., Zhou Y. Q., Lei Y., Wang S. Y., Liao B. S. 2009. Ethylene inhibited aflatoxin biosynthesis is due to oxidative stress alleviation and related to glutathione redox state changes in Aspergillus flavus. Int. J. Food Microbiol. 130:17. [DOI] [PubMed] [Google Scholar]
- 11.Keller N. P., Turner G., Bennett J. W. 2005. Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 3:937–947 [DOI] [PubMed] [Google Scholar]
- 12.Kutchan T. M. 2001. Ecological arsenal and developmental dispatcher. The paradigm of secondary metabolism. Plant Physiol. 125:58–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggio-Hall L. A., Wilson R. A., Keller N. P. 2005. Fundamental contribution of beta-oxidation to polyketide mycotoxin production in planta. Mol. Plant Microbe Interact. 18:783–793 [DOI] [PubMed] [Google Scholar]
- 14.Martin J. F., Casqueiro J., Liras P. 2005. Secretion systems for secondary metabolites: how producer cells send out messages of intercellular communication. Curr. Opin. Microbiol. 8:282–293 [DOI] [PubMed] [Google Scholar]
- 15.Miller M. J., Linz J. E. 2006. Genetic mechanisms involved in regulation of mycotoxin biosynthesis, p. 1505–1542InShetty K., Paliyath G., Pometto A., Levin R. E.(ed.), Food biotechnology, 2nd ed.Taylor & Francis, New York, NY [Google Scholar]
- 16.Price-Whelan A., Dietrich L. E., Newman D. K. 2006. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat. Chem. Biol. 2:71–78.17 [DOI] [PubMed] [Google Scholar]
- 17.Roze L. V., Chanda A., Laivenieks M., Beaudry R. M., Artymovich K. A., Koptina A., Valeeva D., Awad D., Jones A. D., Linz J. E. 2010. Volatile profiling reveals intracellular metabolic changes in Aspergillus parasiticus: veA regulates branched chain amino acid and ethanol metabolism. BMC Biochem. 11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoji J. Y., Arioka M., Kitamoto K. 2008. Dissecting cellular components of the secretory pathway in filamentous fungi: insights into their application for protein production. Biotechnol. Lett. 30:7–14 [DOI] [PubMed] [Google Scholar]
- 19.Zouhar J., Hicks G. R., Raikhel N. V. 2004. Sorting inhibitors (Sortins): chemical compounds to study vacuolar sorting in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 101:9497–9501 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.