Abstract
Ubiquitous among eukaryotes, lipid droplets are organelles that function to coordinate intracellular lipid homeostasis. Their morphology and abundance is affected by numerous genes, many of which are involved in lipid metabolism. In this report we identify a Trypanosoma brucei protein kinase, LDK, and demonstrate its localization to the periphery of lipid droplets. Association with lipid droplets was abrogated when the hydrophobic domain of LDK was deleted, supporting a model in which the hydrophobic domain is associated with or inserted into the membrane monolayer of the organelle. RNA interference knockdown of LDK modestly affected the growth of mammalian bloodstream-stage parasites but did not affect the growth of insect (procyclic)-stage parasites. However, the abundance of lipid droplets dramatically decreased in both cases. This loss was dominant over treatment with myriocin or growth in delipidated serum, both of which induce lipid body biogenesis. Growth in delipidated serum also increased LDK autophosphorylation activity. Thus, LDK is required for the biogenesis or maintenance of lipid droplets and is one of the few protein kinases specifically and predominantly associated with an intracellular organelle.
Trypanosoma brucei is a single-celled eukaryotic pathogen responsible for human African trypanosomiasis (also known as African sleeping sickness) and nagana in domestic animals. More than 50,000 cases of human disease occur yearly, with over 70 million people at risk. No vaccine exists, and chemotherapy is difficult to administer and prone to pathogen resistance. As T. brucei transits between the mammalian bloodstream and the tsetse fly vector during its life cycle, the organism encounters and adapts to profoundly different environmental conditions. The parasite undergoes dramatic changes in both energy (7, 51) and lipid biosynthesis and metabolism (39, 47, 49) as it shifts between these environments.
Protein kinases function in numerous regulatory aspects of the cell, including control of the cell cycle and morphology, responses to stress, and transmission of signals from the extracellular environment or between compartments of the cell. As is the case in other eukaryotes, protein kinases, particularly those associated with membranes, are expected to play pivotal roles in the cell's ability to sense and appropriately respond to its environment. Trypanosoma brucei possesses over 170 protein kinases (16, 44). Most of these can be assigned to the standard groups of protein kinases based on sequence similarity within the kinase domain. However, sequence similarities with kinases from more well-studied organisms are rarely strong enough to allow one-to-one orthologous relationships to be determined (44), and even those which appear orthologous by sequence have sometimes shown functional divergence (46). Hence, an understanding of the roles of specific protein kinases of trypanosomatids requires an individualized assessment. The initial genome analysis of the trypanosomatids (16) showed a lack of receptor tyrosine kinases, but nine T. brucei predicted serine/threonine kinases were annotated as possessing transmembrane domains. One of these was recently shown to be strategically located at a key interface between the host and parasite: the flagellar pocket (38). This eukaryotic translation initiation factor 2α (eIF2α) family kinase was postulated to play a sensory role in monitoring protein transport.
Only a very small number of protein kinases of various organisms have been observed to localize to the membranes of intracellular organelles, most of them to the endoplasmic reticulum (ER) (14, 27, 50). Lipid droplets (also known as lipid bodies, adiposomes, or oil bodies in plants) are thought to arise from the ER, although the routes of protein localization to them are not well understood. They are increasingly recognized as legitimate organelles due to their dynamic roles in energy metabolism (40), lipid trafficking (41), and protection against toxic effects of nonesterified lipids and sterols (18). Studies also suggest that they function as potential protein storage depots (12) and in antigen presentation (10). Although recent efforts to expand the lipid droplet proteome have resulted in a vastly increased and in many cases surprising catalogue of potentially associated proteins (3, 5, 11, 12, 23, 37), relatively little is known as to how these structures form and are regulated within the cell.
We examine here a novel T. brucei protein kinase with a predicted transmembrane domain. Surprisingly, this protein is localized intracellularly in association with lipid droplets. RNAi-mediated knockdown of this newly identified kinase, dubbed LDK (for lipid droplet kinase), reveals a role in the formation or maintenance of lipid droplets in both mammalian bloodstream-form (BF) and insect procyclic-form (PF) stages of the parasite life cycle.
MATERIALS AND METHODS
Cell culture.
The single marker BF line of T. brucei, which is derived from the 427 strain (52), was grown in HMI-9 supplemented with 10% fetal calf serum and 2.5 μg of G418/ml. PF T. brucei line 29-13, also a derivative of 427 (52), was grown in SDM-79 (JRH Biosciences) media and 15% fetal calf serum. G418, hygromycin, and phleomycin were added to final concentrations of 15, 50, and 2.5 μg/ml, respectively. PF were induced for lipid droplet formation by growth in 1.5 μM myriocin (Sigma) for 24 h as previously described (20) or by substituting 10% delipidized bovine calf serum (Equitech) in the indicated experiments. Tetracycline (Tet) was used at 1 to 2 μg/ml for inductions.
DNA constructs.
The Tb11.01.0670 (LDK) ORF was obtained by PCR from T. brucei strain 29-13 genomic DNA by using the primers LDK 5′ AvrII (5′-CGGCCTAGGATGTCTACGGGAAAGATAATTGGTG-3′) and LDK 3′ HindIII (5′-CCCAAGCTTGTTCTTCTCCAGCCAACGGAGCAC-3′). The LDK open reading frame (ORF) was cloned into pGEM-T Easy and sequenced. The ORF was then subcloned into the AvrII and HindIII sites in the inducible expression plasmids pLew79-3V5(PAC), which contains the puromycin resistance marker in lieu of the phleomycin marker in pLEW79, as well as sequences encoding three V5 epitopes. The hydrophobic domain at residues 432 to 455 was deleted by site-directed mutagenesis in the T. brucei pLew79-LDK-3V5(PAC) expression plasmid (primers 5′-CTCCAGATACACCTAAAGGTGATCGGTTTTTGTTTGACGTCCCTG-3′ and 5′-CAGGGACGTCAAACAAAAACCGATCACCTTTAGGTGTATCTGGAG-3′). Lysine 41 was mutated to alanine by using the sense primer (5′-GTGGACTTGACAACAGACAAGGTATACGCGGTAATGGTTATAAGTAAATCGGTAATTG-3′) and antisense primer (5′-CAATTACCGATTTACTTATAACCATTACCGCGTATACCTTGTCTGTTGTCAAGTCCAC-3′).
RNA interference (RNAi) constructs were based on the plasmid p2T7TAblue (PAC), in which the hygromycin gene of the original plasmid (1) was replaced by the puromycin resistance gene. The plasmid contains cloning sites between opposing T7 promoters under the control of the Tet operator, as well as sequences mediating integration into the ribosomal DNA intergenic spacer region. A region of the LDK ORF (nucleotides 1 to 528), which was predicted to have little homology to other T. brucei mRNAs, was amplified by PCR and using the primers LDK 5′ AvrII and LDK 3′ XhoI RNAi (5′-GCGCTCGAGCGTGCCGCACGAATGGTGAAG) and cloned directly into Eam11051 linearized p2T7TAblue(PAC).
RNAi.
PF 29-13 and BF single-marker lines each contain integrated copies of genes encoding the T7 RNA polymerase and the tetracycline (Tet) repressor, allowing Tet-regulated expression of introduced sequences (52). p2T7TAblue-LDK was linearized with NotI and transfected into BF and PF parasites as described previously (52). Subsequent multiwell plating and selection with puromycin produced stably transfected clonal T. brucei BF. Multiple independently derived clonal cell lines were assayed in BF RNAi experiments. Procyclic transfectants were selected with puromycin for 3 to 4 weeks prior to RNAi studies. Four independent transfectant populations were assayed in RNAi experiments. Cell densities were determined by using a Z1 Coulter Counter, and growth curves were plotted using duplicate or triplicate cultures. Cultures in log phase were diluted to 5 × 105 cells/ml (PF) or 105 cells/ml (BF) to begin the experiment. RNAi was induced by the addition of 2 μg of Tet/ml. Tet was subsequently added every 24 h (BF) or 48 h (PF), and cultures were maintained below 2 × 106 (BF) or 1.5 × 107 (PF). To assess knockdown of mRNA, cells uninduced or induced for RNAi, were collected on day 0 and day 5 (PF). Washed parasites were resuspended in TRIzol, and RNA preparation and Northern analyses were carried out as described previously (30).
Immunofluorescence analysis.
Immunofluorescence was performed as previously described (15, 32). BF parasites were prefixed in 4% paraformaldehyde for 5 min on ice prior to placing on poly-l-lysine coverslips. The V5 epitope tag was detected by using mouse monoclonal anti-V5 (Invitrogen) at 1 μg/ml, followed by goat anti-mouse IgG conjugated with fluorescein isothiocyanate (FITC) (Southern Biotechnology). Anti-BiP (4), a gift from Jay Bangs, was used at 1:400, followed by goat anti-rabbit immunoglobulin conjugated to Texas Red (Southern Biotechnology). To detect lipid droplets, slides bearing paraformaldehyde-fixed cells were incubated in phosphate-buffered saline (PBS) or SDM-79 containing 1.5 μg of Nile Red (Sigma)/ml for 30 min at 25°C as described previously (24). Slides were washed twice in PBS prior to mounting using Prolong Antifade (Molecular Probes) for fluorescence microscopy. DAPI (4′,6′-diamidino-2-phenylindole) staining was used to identify the nucleus and kinetoplast. Fluorescence of Nile Red in the red channel (617 nm) is brighter but less specific to lipid bodies (36); therefore, data were collected in the FITC channel (528 nm) except in colocalization studies. Slides were viewed on a Deltavision RT deconvolution microscope with an Olympus UPlan/Apo 100× 1.35 NA objective. Deltavision images were deconvolved using standard parameters and the conservative ratio algorithm. Single deconvolved planes are shown.
Extractions, immunoblots, and protein kinase assays.
Samples were analyzed by SDS-PAGE (8 to 16% acrylamide gradient). Immunoblots were probed with mouse anti-V5 at 2 μg/ml, rabbit anti-T. brucei phosphoglycerate kinase (1:4,000) (43), rabbit anti-vacuolar H+ pyrophosphatase (VP1, 1:5,000, a gift from Roberto Docampo) (35), or a mixture of two monoclonal antibodies against EP procyclin (antibodies 16 and 418 at 1:10,000) (45). Bound antibodies were revealed with goat anti-mouse immunoglobulin (IRDye800) and goat anti-rabbit immunoglobulin (IRDye700). Blots were scanned with a LiCor Odyssey.
For phase partitioning of LDK in Triton X-114 (9), 5 × 108 PF were pelleted, washed in PBS plus glucose, and resuspended in 200 μl of 1% Triton X-114, 150 mM NaCl, and 10 mM Tris (pH 7.4). After 10 min on ice, debris was removed by centrifugation at 16,000 × g for 10 min. The supernatant was incubated at 30° for 3 min to condense the Triton-X114. The lysate was then centrifuged for 3 min at 12,000 × g at room temperature. The upper aqueous phase was removed. The detergent phase (∼20 μl) was reextracted with 200 μl of the above buffer (without Triton X-114). All samples were brought to 200 μl and mixed with sample buffer for SDS-PAGE.
For treatment with carbonate (22), 5 × 108 PF were washed with PBS plus glucose as described above. The cells were resuspended in 2 ml of 10 μM Tris (pH 7.4) with protease inhibitors on ice for 2 min and Dounce homogenized to achieve hypotonic lysis. Cell debris was pelleted at 1,000 × g, and the supernatant was transferred to a fresh tube. An equal volume of 0.2 M sodium carbonate (pH 11) was added to the supernatant and incubated on ice for 1 h. The membranes were then pelleted at 100,000 × g for 1 h at 4°C. The soluble protein fraction was removed, and the membrane fraction was resuspended in an equal volume of PBS.
Immunoprecipitation of LDK-V5 from PF cell lysates (108 cells) of Tet-induced (24 h) and uninduced cultures was performed as previously described (15). Kinase assays were performed as described elsewhere (42) using [32γ-P]ATP and in some cases casein (5 μg/reaction) as substrate. Reactions were separated by SDS-PAGE, transferred to nitrocellulose, and labeled proteins detected by phosphorimaging. Signals were quantified by using ImageQuant (Molecular Dynamics). The same blots were probed with anti-V5 as described above to allow normalization of activity.
RESULTS
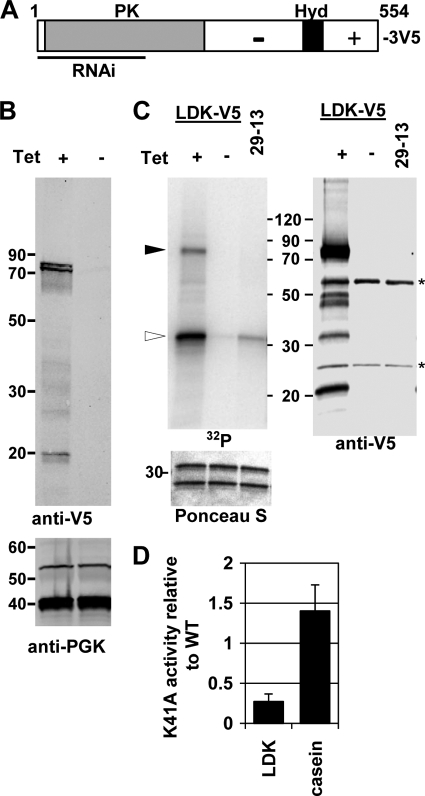
Tb11.01.0670 was identified by searching GeneDB (www.genedb.org) for T. brucei genes encoding proteins possessing a protein kinase catalytic domain, as well as a predicted transmembrane domain(s). The Tb11.01.0670 protein is 554 amino acids (aa) long; the protein kinase domain begins 12 aa from the N terminus (see schematic in Fig. 1A). It contains all of the essential motifs and residues required for protein kinase activity (see Fig. S1 in the supplemental material) (26). The kinase domain is followed by a 267-aa extension, which contains a single predicted transmembrane domain of 24 aa, suggesting potential membrane localization. This extension shows little homology with any known predicted proteins except with the orthologous kinases of trypanosomatids (see Fig. S1 in the supplemental material for alignment and systematic names). The orthologues in the related genus Leishmania are highly similar within the kinase domain, and also have a similarly placed predicted transmembrane domain within the C-terminal extension, but the extension is longer and shows reduced sequence similarity (see Fig. S1 in the supplemental material). These trypanosomatid kinases belong to the calmodulin-dependent kinase (CAMK) group of protein kinases and appear to be most closely related to the CAMKL (for CAMK-like) family (44). However, there is no obvious orthologous relationship to any specific eukaryotic protein kinase outside of the trypanosomatids. Our previous genome-wide microarray analysis showed that the corresponding mRNA was expressed to low levels in both BF and PF T. brucei (31). Based on the presence of a protein kinase domain and the data below, we named the protein lipid droplet kinase (LDK).
Fig. 1.
Lipid droplet kinase structure and kinase activity. (A) Schematic of LDK, showing location of protein kinase (PK domain) and hydrophobic region (Hyd), which lies in the C-terminal extension. Between the kinase domain and the hydrophobic region, the protein is enriched for acidic aa (−), whereas distal to the hydrophobic region it is enriched for basic aa (+). The region included in the RNAi construct is marked. The annotated sequence is shown in Fig. S1 in the supplemental material. (B) Immunoblot. Lysates were prepared from PF induced (+Tet) or uninduced (−Tet) for LDK-V5 expression. After SDS-PAGE, samples were transferred to nitrocellulose. The blot was incubated with anti-V5 and goat anti-mouse immunoglobulin IRDye800 and scanned. The migration of molecular mass markers (in kilodaltons) is indicated in this and other figures. (C) Kinase assay. Anti-V5 immunoprecipitates from cell expressing (Tet+) or not expressing (Tet−) LDK-V5 were collected and assayed for protein kinase activity in the presence of casein as a candidate exogenous substrate. The gel containing the samples was blotted and subjected to overnight phosphorimaging, as well as Western analysis with anti-V5. Left section (32P), kinase assay. Filled arrowhead, LDK-V5; open arrowhead, upper band of casein. Below is the relevant portion of the same blot stained with Ponceau S to reveal casein. Right section (anti-V5), Western analysis. Asterisks mark the heavy and light chain of the antibodies used for immunoprecipitation, which were detected by the second step antibodies. (D) Phosphotransfer activity of LDK K41A. After immunoprecipitation and kinase assay of wild-type and K41A LDK-V5, 32P associated with the LDK-V5 band and the casein bands was quantitated by phosphorimaging. LDK-V5 was quantitated by immunoblotting of the same gel lanes, allowing normalization of activity. The graph depicts the relative level of phosphorylation by LDK K41A immunoprecipitates compared to the wild type.
To assess whether LDK has protein kinase activity, we expressed LDK tagged with three V5 epitopes at its C terminus in PF. Induction of LDK-V5 expression with Tet resulted in expression of an immunoreactive species migrating at ∼75 kDa, slightly larger than the predicted mass of 68 kDa (Fig. 1B). In this experiment and some others, LDK-V5 appeared as a doublet, but this was not consistently observed. We immunoprecipitated LDK-V5 from Tet-induced PF lysates and performed in vitro kinase reactions. As shown in Fig. 1C, immunoprecipitates from cells induced for LDK-V5 expression showed a significant amount of phosphorylation of a species comigrating with LDK-V5, which likely represents autophosphorylation. The LDK-V5 immunoprecipitates also phosphorylated the upper casein band, although some casein phosphorylation from control immunoprecipitates was also observed. The latter may result from casein kinases such as CK1.2, CK2α, and CK2α′, each of which is ranked among the eight most abundant kinases at the RNA level (31). Both LDK and casein phosphorylation were seen with purified TAP-tagged LDK compared to a mock purification from untransfected cells (not shown). We observed modest protein kinase activity directed toward a small synthetic peptide that is a favored substrate for other kinases in the CAMKL kinase family and a lower level of phosphorylation on myelin basic protein, another exogenous substrate (data not shown).
With rare exceptions, a lysine in subdomain II is required for activity. Mutation of this residue (K41A, see Fig. S1 in the supplemental material) strongly reduced phosphorylation of LDK, but the phosphorylation of casein was not decreased (Fig. 1D). This finding suggests that LDK possesses kinase activity which autophosphorylates in cis. The activity of the kinase-dead LDK-V5 immunoprecipitates toward casein may result from association with the native kinase or another kinase; hence, casein phosphorylation was not considered further. Autophosphorylation was similar in 50 to 100 mM NaCl and was dependent on Mg2+ (Mn2+ could not substitute). Ca2+ (with or without calmodulin) did not increase activity.
LDK protein localizes to the periphery of lipid droplets.
We then assessed the potential localization of LDK to specific membrane locations in both PF and BF parasites. The expression plasmid uses a promoter that is highly active in PF as opposed to BF, as well as a 3′ untranslated region that is compromised for expression in BF (52). However, LDK-V5 was detected in both developmental stages upon immunofluorescence analysis of stably transfected parasites. In induced cells, a distinctive ringlike pattern, apparently marking the periphery of multiple intracellular structures (Fig. 2 A to D) was observed, and this ring was absent in uninduced cells. In colocalization studies with antibodies specific for glycosomes (43) and acidocalcisomes (17, 35), we eliminated these well-characterized organelles as the location of LDK-V5 (data not shown). Nile Red, a lipophilic fluorescent dye, was then used to specifically stain intracellular lipid droplets (24). LDK-V5 was found to primarily reside at the periphery of Nile Red-stained organelles in both developmental stages, and we conclude that LDK-V5 resides in or is associated with the monolayer membrane of lipid droplets. A similar ringlike staining pattern has been seen for proteins localized to the lipid droplet surface in other eukaryotes (see references 6 and 8 for examples). In the culture conditions used, images of cultured PF showed an average of four lipid droplets, whereas cultured BF typically showed two smaller lipid droplets. Staining of the ER, in addition to lipid droplets, was seen in some cells, most noticeably in those with a higher level of expression (Fig. 2E). This is consistent with the concept that lipid droplets derive from the ER.
Fig. 2.
Localization of LDK-V5 to lipid droplets. Images of control parasites (uninduced, Tet−) and or those induced for expression of LDK-V5 (Tet+) were collected using the same parameters and identically processed. Transfectants expressing LDK-V5 were stained with Nile Red to identify lipid droplets and costained with anti-V5 antibodies (green). Nile Red was collected on the Texas Red filter, while LDK-V5 was visualized in the green channel. Cells were costained with DAPI (blue). All bars, 5 μm. (A) Localization of LDK-V5 to lipid droplets in PF. Left, induced for LDK-V5 expression (+Tet); right, uninduced (−Tet). (B) Localization of LDK-V5 to lipid droplets in BF. (C) Deletion of the hydrophobic region of LDK leads to cytosolic localization in PF. Due to the lower maximum signal intensity, the scaling for brightness is twice that of the images showing wild-type protein. (D) Threefold enlargement of lipid droplet staining of panel cell immediately above. (E) Tet-induced PF cell showing LDK-V5 localization to the ER and lipid droplets (green). Anti-BiP was used as a marker of the ER (red).
Unlike other subcellular organelles, lipid droplets are bounded by a membrane monolayer in which the charged moieties face outwards. The presence of a single hydrophobic, predicted transmembrane domain suggested that this region might be critical for localization of LDK to lipid droplets. We therefore expressed a mutant version of the kinase lacking the 24 aa comprising the hydrophobic region (LDKΔ432-455) in PF. The mutant kinase was found throughout the cell, with no apparent association with lipid droplets (Fig. 2C). We examined the association of LDK-V5 with the lipid droplet using Triton X-114 and carbonate extractions (Fig. 3). In Triton X-114 extractions, LDK-V5 partitioned to the aqueous phase, although a C-terminal degradation fragment predicted to contain the hydrophobic region partitioned equally between the aqueous and detergent phases. EP-procyclin, a heterogeneously glycosylated glycosylphosphatidylinositol-anchored protein, was predominantly in the detergent phase, whereas the cytosolic and glycosomal phosphoglycerate kinase were present in the aqueous phase. When hypotonically lysed cells were extracted with carbonate, pH 11, control proteins fractionated as expected: phosphoglycerate kinases were in the supernatant, whereas the acidocalcisomal H+ pyrophosphatase (VP1), an integral membrane protein, was present only in the pellet. Most LDK-V5 remained in the integral membrane protein fraction (pellet), but some was released into the supernatant. The partial resistance of LDK to carbonate extraction indicates that the protein is strongly associated with the lipid droplet membrane.
Fig. 3.

Extraction of LDK. (A) Triton X-114 extraction. After incubation with Triton X-114 the samples were separated into aqueous (Aq1) and detergent phase by centrifugation extraction, and the detergent phase was re-extracted (Aq2 and Det). Cell equivalents were analyzed by Western blotting with anti-V5, anti-procyclin, and anti-phosphoglycerate kinase (which detects both the 45-kDa cytosolic and the 56-kDa glycosomal matrix isoforms). (B) Carbonate extraction. Cell equivalents of the carbonate supernatant and pellet in SDS-PAGE sample buffer were loaded onto SDS-PAGE gels and blotted. The sample used for detection of the integral membrane protein VP1 was not boiled but heated to 45°C. Blots were probed with anti-V5, anti-phosphoglycerate kinase (anti-PGK), and anti-VP1. TCL, total cell lysate; P, carbonate pellet; S, carbonate supernatant.
LDK RNAi parasites show minimal growth defects but are depleted of lipid droplets.
To further examine the role of LDK and ascertain its importance for survival of T. brucei, we performed RNAi knockdown analysis. A segment of the LDK ORF was cloned into the p2T7TAblue vector between Tet-inducible T7 promoters. Addition of Tet initiates RNAi knockdown. We isolated several clonal lines of BF transfectants, as well as nonclonal lines of PF transfectants. RNAi knockdown of LDK mRNA in PF was verified by Northern analysis and phosphorimaging, which demonstrated ca. 8% of the uninduced control level when normalized to levels of β-tubulin mRNA (Fig. 4A). The average decrease of LDK mRNA in BF was 70% by quantitative PCR. However, the reduction in LDK mRNA did not alter the growth rate significantly under the conditions examined (Fig. 4B). Several independently derived clonal BF lines showed a small change in growth rate upon induction of RNAi; the results obtained with one of these lines are shown in Fig. 4C.
Fig. 4.

RNAi targeting LDK in PF and BF does not abrogate parasite proliferation. (A) Northern analysis of PF transfectants bearing an RNAi cassette targeting LDK. The abundance of full-length 3.32-kb mRNA seen in the uninduced condition (Tet−) is dramatically reduced upon induction of RNAi (Tet+). The small species in the Tet+ lane corresponds to the double-stranded RNA produced upon induction. At right is the control hybridization with α-tubulin. Migration of molecular mass markers is shown (in kb). (B) Growth curve for PF, induced (Tet+) or uninduced (Tet−) for RNAi. Standard deviations of cell counts are obscured by markers. (C) Growth curve for BF (Tet+) or uninduced (Tet−) for RNAi. Standard deviations of cell counts are obscured by markers.
We next analyzed BF and procyclic RNAi transfectants for numbers of lipid droplets, utilizing Nile Red staining and microscopic analysis. Parasites were induced with Tet for RNAi and then examined on day 5 (PF) and day 2 (BF) for the presence of Nile Red staining bodies. The numbers of lipid droplets were enumerated by examining at least 50 randomly selected cells for each condition. In both developmental stages, LDK RNAi knockdown resulted in a 90% reduction in the number of lipid droplets (Fig. 5). In addition, the remaining lipid droplets appeared to be somewhat smaller and stained more faintly than those seen in the control samples. In control experiments with the parental 29-13 PF line and an irrelevant BF RNAi line (CK2α′), Tet treatment gave rise to a small increase in lipid droplet numbers (7 and 16%, respectively), indicating that neither Tet nor the process of RNAi itself are responsible for the reduction in lipid droplets seen upon LDK RNAi. These data show that LDK is required for maintaining normal numbers of lipid droplets in vitro.
Fig. 5.

LDK RNAi depletes lipid droplets. Parasites induced for LDK RNAi were stained for lipids using Nile Red after 5 days (PF) or 48 h (BF) and compared to parallel untreated cultures. (A) Representative images of cells stained with Nile Red. Bar, 5 μm. (B) The number of lipid droplets per cell under each condition was enumerated. The means and standard deviations are shown.
Cells respond to an increase in fatty acids or to various stresses in lipid homeostasis by increasing the number of lipid droplets (48). We therefore investigated whether stimuli that normally induce lipid droplet biogenesis were still able to do so when LDK was depleted. Two different conditions were tested, both of which were tolerated by PF, although they were much more toxic to BF. Each condition deprives cells of at least one type of lipid, presumably yielding a compensatory increase in lipid synthesis or uptake and subsequent storage in lipid droplets. Treatment of PF with 1.5 μM myriocin, a potent inhibitor of the first step in sphingolipid biosynthesis, has been reported to increase the number of lipid droplets (20). Reduction of lipids in the medium has been previously shown to induce fatty acid synthesis in T. brucei (34), and we observed that replacing fetal calf serum with delipidized calf serum for 24 h almost doubled the number of lipid droplets in PF (see Table S1 in the supplemental material). Both of these treatments were applied to PF that had been induced for LDK RNAi for 5 days. Although the treated PF remained viable for the treatment period, both treatments increased the proportion of parasites with two, three, or four nuclei as revealed by DAPI staining. We therefore normalized the number of lipid droplets to the number of nuclei in these experiments to more accurately reflect any potential induction of lipid droplets (Fig. 6B; Table S1 in the supplemental material provides detail by number of nuclei per cell). Myriocin increased the number of lipid droplets per nucleus from ∼4 to >10, whereas delipidated serum increased the number to ∼7. Under both inducing conditions, cells depleted of LDK averaged less than one droplet per nucleus (Fig. 6). The reduction was apparent when only “normal” cells (one or two nuclei) were considered (Table S1 in the supplemental material). These findings indicate that LDK plays a critical role in the biogenesis and/or maintenance of lipid droplets.
Fig. 6.

LDK RNAi prevents lipid body induction. Parallel cultures of PF were treated with or without Tet to induce RNAi. After 4 days, the cultures were split into three conditions—control, myriocin (1.5 μM), or delipidated serum—and cultured an additional 24 h. They were compared to parallel untreated cultures. (A) Representative images of cells stained with Nile Red. Bar, 5 μm. Figure S2 in the supplemental material provides additional images. (B) The numbers of lipid droplets per nucleus following the various treatments were determined. The means and standard deviations are shown. Occasional cells with >4 nuclei were not included in the analysis. Detail is provided in Table S1 in the supplemental material.
Induction of lipid droplets increases LDK autophosphorylation.
Given that LDK appears to be important for the induction or maintenance of lipid droplets, we hypothesized that the kinase might show increased activity after cells were exposed to lipid droplet inducing conditions. Because the studies with the K41A mutant suggested that autophosphorylation was a better reflection of LDK activity, we focused on autophosphorylation. Cell lysates were prepared after growing the cells in delipidated serum for 4 h. Immunoprecipitates were prepared and assayed for kinase activity, and the same blots were incubated with anti-V5 to quantitate LDK-V5. As shown in Fig. 7, growth in the absence of lipids resulted in significantly increased phosphorylation of LDK in the assay. Because phosphorylation of LDK was shown to require its catalytic lysine (Fig. 1D), the enhanced phosphorylation is likely attributable to increased activation of LDK.
Fig. 7.

Growth in delipidated serum increases LDK autophosphorylation activity. (A) PF induced for expression of LDK-V5 were grown for 4 h in medium containing delipidated or normal serum. The tagged LDK was immunoprecipitated and subjected to a kinase assay as in Fig. 1, measuring autophosphorylation (32P) and the amount of LDK-V5 by Western blotting (anti-V5) on the same lanes. U, untreated; D, grown in delipidated serum. (B) Graph of the relative activity of LDK from cells grown in delipidated serum versus cells from a parallel culture grown in normal serum. Shown are the data from two independent isolates (Iso 1 and Iso 2), and two independent experiments for isolate 2 (Exp1 and Exp2).
DISCUSSION
We describe here a novel protein kinase, LDK, which localizes to the periphery of lipid droplets in both BF and PF T. brucei. LDK was identified by searching T. brucei predicted proteins for those possessing both a protein kinase catalytic domain and at least one putative transmembrane domain. Although we anticipated that our screen might yield genes encoding proteins localized to the plasma membrane, this kinase was instead localized to an intracellular organelle. Previous work has shown some partial localization of mitogen-activated protein kinases to lipid droplets of leukocytes (53), as well as anonymous kinase activity associated with lipid droplets (33). However, these physical associations have not been tied to droplet function. Recently, a genome-wide RNAi screen of Drosophila cells identified over 200 genes that modulate lipid droplet morphology, affecting their size, subcellular distribution, or number (25). Of those that resulted in a ′fewer lipid droplets' phenotype, two were protein kinases: cdc2 and tlk. These protein kinases are involved in cell cycle regulation and are not localized to lipid droplets. Hence, their modulation of lipid droplet number likely results from pleiotropic effects. Neither of these kinases is closely related to LDK at the sequence level. Thus, LDK is unique in that it is both positioned at the lipid droplet surface and is involved in lipid droplet maintenance.
To our knowledge, LDK is the first protein kinase identified as localized to intracellular organelles of trypanosomatids and the first protein shown to primarily reside on lipid droplets of these organisms under normal conditions. Lipid droplet membranes are unusual since they consist of a monolayer of phospholipids. The internal core of the lipid droplet is composed primarily of neutral lipids, an environment unfavorable to almost all proteins. The topology of proteins associated with the lipid droplet membrane is therefore likely to be distinct from transmembrane proteins in lipid bilayers that make up other membranes of the cell. Because deletion of the hydrophobic domain led to diffuse cytosolic staining, we propose that LDK associates with the monolayer membrane via its single hydrophobic domain, leaving both the N-terminal kinase domain and the C-terminal portion extending toward the cytosol. Because LDK-V5 is partially extractable with carbonate and partitions to the aqueous phase upon Triton X-114 extraction (not shown), we suggest it is a tightly associated peripheral membrane protein. However, given the lack of studies on the behavior of proteins in the lipid body monolayer, this topic bears further study. Indeed, a previous publication showed membrane-dipping proteins may be more readily extracted by carbonate than transmembrane proteins (28). Unlike some membrane-dipping domains (13), the LDK hydrophobic domain is not predicted to form an amphipathic alpha helix. However, it is flanked by charged regions, especially basic amino acids. Basic amino acids have been shown to work in concert with the hydrophobic domain of caveolin to mediate its interaction with the lipid body membrane when cells are treated with fatty acids (29).
The location of LDK at the lipid droplet surface suggests it could function as a mediator of the lipidomic status in the cell, reminiscent of the sensing of AMP/ATP ratio by protein kinase AMPK. Indeed, LDK is most similar to members of the CAMKL family, which includes AMPK. We propose that localization to the lipid droplet surface may also be important for modulating activity, similar to what is seen for a key enzyme in phospholipid synthesis, CTP:phosphocholine cytidylyltransferase. This enzyme shows a dramatic increase in kcat and decrease in Km when associated with membranes and specifically associates with lipid droplets as they enlarge (21). Changes in membrane curvature are thought to affect the conformation and hence the activity of the molecule (13). The location of LDK at the lipid droplet membrane suggests it, too, could be modulated by the composition or curvature of the membrane, allowing it to transmit signals regarding the lipid status of the cell. We observed an increase in autophosphorylation activity of LDK when cells were incubated in a lipid droplet inducing condition, indicating activation of the kinase. Phosphorylation of natural substrates by the kinase anchored at the droplet surface might provide a more accurate measure of activation. Identification of the substrates would be a step toward understanding the regulation of lipid droplet homeostasis in the parasite.
The observation that LDK RNAi knockdown causes little growth phenotype despite a dramatic reduction in the number of detectable lipid droplets indicates that droplet loss does not inevitably lead to parasite death. It is possible that small lipid droplets, undetectable by the techniques we used, remained in the cells or that survival is facilitated by other lipid storage and mobilizing mechanisms. The higher sensitivity of BF to knockdown of LDK may result from their high requirement for myristic acid (used to anchor the variant surface glycoprotein that covers the parasite surface) or their upregulated exocytic and endocytic transport systems (2, 19). In either case, it appears that LDK is not essential in vitro, but confirmation will require gene knockout approaches. Using conditional knockouts in studies that manipulate the lipids provided to and synthesized by the cells may elucidate conditions in which the protein provides important regulatory information. In addition, such knockouts will provide a means to dissect the structural features of LDK that are essential for lipid droplet biogenesis and maintenance.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jay Bangs, Roberto Docampo, and Terry Pearson for the gifts of antisera directed against trypanosome BiP, acidocalcisomal H+ pyrophosphatase VP1, and procyclin, respectively. We also thank Nichelle Kunecke and Jennifer Wierman for technical assistance and Sunil Laxman for the plasmid pLEW79-1V5(PAC).
This study was supported by grant NIH R01AI31077.
The authors are solely responsible for the contents of this article.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 10 September 2010.
REFERENCES
- 1.Alibu V. P., Storm L., Haile S., Clayton C., Horn D. 2005. A doubly inducible system for RNA interference and rapid RNAi plasmid construction in Trypanosoma brucei. Mol. Biochem. Parasitol. 139:75–82 [DOI] [PubMed] [Google Scholar]
- 2.Allen C. L., Goulding D., Field M. C. 2003. Clathrin-mediated endocytosis is essential in Trypanosoma brucei. EMBO J. 22:4991–5002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athenstaedt K., Zweytick D., Jandrositz A., Kohlwein S. D., Daum G. 1999. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 181:6441–6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangs J. D., Uyetake L., Brickman M. J., Balber A. E., Boothroyd J. C. 1993. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei: divergent ER retention signals in a lower eukaryote. J. Cell Sci. 105:1101–1113 [DOI] [PubMed] [Google Scholar]
- 5.Beller M., Riedel D., Jansch L., Dieterich G., Wehland J., Jackle H., Kuhnlein R. P. 2006. Characterization of the Drosophila lipid droplet subproteome. Mol. Cell. Proteomics 5:1082–1094 [DOI] [PubMed] [Google Scholar]
- 6.Beller M., Sztalryd C., Southall N., Bell M., Jackle H., Auld D. S., Oliver B. 2008. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 6:e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besteiro S., Barrett M. P., Riviere L., Bringaud F. 2005. Energy generation in insect stages of Trypanosoma brucei: metabolism in flux. Trends Parasitol. 21:185–191 [DOI] [PubMed] [Google Scholar]
- 8.Blanchette-Mackie E. J., Dwyer N. K., Barber T., Coxey R. A., Takeda T., Rondinone C. M., Theodorakis J. L., Greenberg A. S., Londos C. 1995. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J. Lipid Res. 36:1211–1226 [PubMed] [Google Scholar]
- 9.Bordier C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604–1607 [PubMed] [Google Scholar]
- 10.Bougneres L., Helft J., Tiwari S., Vargas P., Chang B. H., Chan L., Campisi L., Lauvau G., Hugues S., Kumar P., Kamphorst A. O., Dumenil A. M., Nussenzweig M., MacMicking J. D., Amigorena S., Guermonprez P. 2009. A role for lipid bodies in the cross-presentation of phagocytosed antigens by MHC class I in dendritic cells. Immunity 31:232–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brasaemle D. L., Dolios G., Shapiro L., Wang R. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279:46835–46842 [DOI] [PubMed] [Google Scholar]
- 12.Cermelli S., Guo Y., Gross S. P., Welte M. A. 2006. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr. Biol. 16:1783–1795 [DOI] [PubMed] [Google Scholar]
- 13.Cornell R. B., Taneva S. G. 2006. Amphipathic helices as mediators of the membrane interaction of amphitropic proteins, and as modulators of bilayer physical properties. Curr. Protein Peptide Sci. 7:539–552 [DOI] [PubMed] [Google Scholar]
- 14.Cox J. S., Shamu C. E., Walter P. 1993. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73:1197–1206 [DOI] [PubMed] [Google Scholar]
- 15.Das A., Park J.-H., Hagen C. B., Parsons M. 1998. Distinct domains of a nucleolar protein mediate protein kinase binding, interaction with nucleic acids and nucleolar localization. J. Cell Sci. 111:2615–2623 [DOI] [PubMed] [Google Scholar]
- 16.El-Sayed N. M., Myler P. J., Bartholomeu D. C., Nilsson D., Aggarwal G., Tran A. N., Ghedin E., Worthey E. A., Delcher A. L., Blandin G., Westenberger S. J., Caler E., Cerqueira G. C., Branche C., Haas B., Anupama A., Arner E., Aslund L., Attipoe P., Bontempi E., Bringaud F., Burton P., Cadag E., Campbell D. A., Carrington M., Crabtree J., Darban H., Da Silveira J. F., de Jong P., Edwards K., Englund P. T., Fazelina G., Feldblyum T., Ferella M., Frasch A. C., Gull K., Horn D., Hou L., Huang Y., Kindlund E., Klingbeil M., Kluge S., Koo H., Lacerda D., Levin M. J., Lorenzi H., Louie T., Machado C. R., McCulloch R., McKenna A., Mizuno Y., Mottram J. C., Nelson S., Ochaya S., Osoegawa K., Pai G., Parsons M., Pentony M., Pettersson U., Pop M., Ramirez J. L., Rinta J., Robertson L., Salzberg S. L., Sanchez D. O., Seyler A., Sharma R., Shetty J., Simpson A. J., Sisk E., Tammi M. T., Tarleton R., Teixeira S., Van Aken S., Vogt C., Ward P. N., Wickstead B., Wortman J., White O., Fraser C. M., Stuart K. D., Andersson B. 2005. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309:409–415 [DOI] [PubMed] [Google Scholar]
- 17.Fang J., Rohloff P., Miranda K., Docampo R. 2007. Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect. Biochem. J. 407:161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farese R. V., Jr., Walther T. C. 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139:855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field M. C., Carrington M. 2004. Intracellular membrane transport systems in Trypanosoma brucei. Traffic 5:905–913 [DOI] [PubMed] [Google Scholar]
- 20.Fridberg A., Olson C. L., Nakayasu E. S., Tyler K. M., Almeida I. C., Engman D. M. 2008. Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. J. Cell Sci. 121:522–535 [DOI] [PubMed] [Google Scholar]
- 21.Friesen J. A., Campbell H. A., Kent C. 1999. Enzymatic and cellular characterization of a catalytic fragment of CTP:phosphocholine cytidylyltransferase alpha. J. Biol. Chem. 274:13384–13389 [DOI] [PubMed] [Google Scholar]
- 22.Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. 1982. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J. Cell Biol. 61:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujimoto Y., Itabe H., Sakai J., Makita M., Noda J., Mori M., Higashi Y., Kojima S., Takano T. 2004. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim. Biophys. Acta 1644:47–59 [DOI] [PubMed] [Google Scholar]
- 24.Fukumoto S., Fujimoto T. 2002. Deformation of lipid droplets in fixed samples. Histochem. Cell Biol. 118:423–428 [DOI] [PubMed] [Google Scholar]
- 25.Guo Y., Walther C., Rao M., Stuurman N., Goshima G., Terayama K., Wong J. S., Vale R. D., Walter P., Farese R. V. 2008. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453:657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanks S. K., Hunter T. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576–596 [PubMed] [Google Scholar]
- 27.Harding H. P., Zhang Y., Ron D. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274 [DOI] [PubMed] [Google Scholar]
- 28.Ikeda M., Kida Y., Ikushiro S., Sakaguchi M. 2005. Manipulation of membrane protein topology on the endoplasmic reticulum by a specific ligand in living cells. J. Biochem. 138:631–637 [DOI] [PubMed] [Google Scholar]
- 29.Ingelmo-Torres M., Gonzalez-Moreno E., Kassan A., Hanzal-Bayer M., Tebar F., Herms A., Grewal T., Hancock J. F., Enrich C., Bosch M., Gross S. P., Parton R. G., Pol A. 2009. Hydrophobic and basic domains target proteins to lipid droplets. Traffic 10:1785–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen B. C., Brekken D. L., Randall A. C., Kifer C. T., Parsons M. 2005. Species specificity in ribosome biogenesis: a nonconserved phosphoprotein is required for formation of the large ribosomal subunit in Trypanosoma brucei. Eukaryot. Cell 4:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen B. C., Sivam D., Kifer C. T., Myler P. J., Parsons M. 2009. Widespread variation in transcript abundance within and across developmental stages of Trypanosoma brucei. BMC Genomics 10:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen B. C., Wang Q., Kifer C. T., Parsons M. 2003. The NOG1 GTP-binding protein is required for biogenesis of the 60S ribosomal subunit. J. Biol. Chem. 278:32204–32211 [DOI] [PubMed] [Google Scholar]
- 33.Kamisaka Y., Noda N., Yamaoka M. 2004. Appearance of smaller lipid bodies and protein kinase activation in the lipid body fraction are induced by an increase in the nitrogen source in the Mortierella fungus. J. Biochem. 135:269–276 [DOI] [PubMed] [Google Scholar]
- 34.Lee S. H., Stephens J. L., Paul K. S., Englund P. T. 2006. Fatty acid synthesis by elongases in trypanosomes. Cell 126:691–699 [DOI] [PubMed] [Google Scholar]
- 35.Lemercier G., Dutoya S., Luo S., Ruiz F. A., Rodrigues C. O., Baltz T., Docampo R., Bakalara N. 2002. A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J. Biol. Chem. 277:37369–37376 [DOI] [PubMed] [Google Scholar]
- 36.Listenberger L. L., Brown D. A. 2007. Fluorescent detection of lipid droplets and associated proteins. Curr. Protoc. Cell Biol. Chapter 24:Unit 24.2. [DOI] [PubMed] [Google Scholar]
- 37.Liu P., Ying Y., Zhao Y., Mundy D. I., Zhu M., Anderson R. G. 2004. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 279:3787–3792 [DOI] [PubMed] [Google Scholar]
- 38.Moraes M. C., Jesus T. C., Hashimoto N. N., Dey M., Schwartz K. J., Alves V. S., Avila C. C., Bangs J. D., Dever T. E., Schenkman S., Castilho B. A. 2007. A novel membrane-bound eIF2 alpha kinase in the flagellar pocket of Trypanosoma brucei. Eukaryot. Cell 6:1979–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morita Y. S., Paul K. S., Englund P. T. 2000. Specialized fatty acid synthesis in African trypanosomes: myristate for GPI anchors. Science 288:140–143 [DOI] [PubMed] [Google Scholar]
- 40.Murphy D. J. 2001. The biogenesis and functions of lipid bodies in animals, plants, and microorganisms. Prog. Lipid Res. 40:325–438 [DOI] [PubMed] [Google Scholar]
- 41.Ozeki S., Cheng J., Tauchi-Sato K., Hatano N., Taniguchi H., Fujimoto T. 2005. Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J. Cell Sci. 118:2601–2611 [DOI] [PubMed] [Google Scholar]
- 42.Park J. H., Brekken D. L., Randall A. C., Parsons M. 2002. Molecular cloning of Trypanosoma brucei CK2 catalytic subunits: the alpha isoform is nucleolar and phosphorylates the nucleolar protein Nopp44/46. Mol. Biochem. Parasitol. 119:97–106 [DOI] [PubMed] [Google Scholar]
- 43.Parker H. L., Hill T., Alexander K., Murphy N. B., Fish W. R., Parsons M. 1995. Three genes and two isozymes: gene conversion and the compartmentalization and expression of the phosphoglycerate kinases of Trypanosoma (Nannomonas) congolense. Mol. Biochem. Parasitol. 69:269–279 [DOI] [PubMed] [Google Scholar]
- 44.Parsons M., Worthey E. A., Ward P. N., Mottram J. C. 2005. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei, and Trypanosoma cruzi. BMC Genomics 6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson J. P., Beecroft R. P., Tolson D. L., Liu M. K., Pearson T. W. 1988. Procyclin: an unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Mol. Biochem. Parasitol. 31:203–216 [DOI] [PubMed] [Google Scholar]
- 46.Shalaby T., Liniger M., Seebeck T. 2001. The regulatory subunit of a cGMP-regulated protein kinase A of Trypanosoma brucei. Eur. J. Biochem. 268:6197–6206 [DOI] [PubMed] [Google Scholar]
- 47.Sutterwala S. S., Hsu F. F., Sevova E. S., Schwartz K. J., Zhang K., Key P., Turk J., Beverley S. M., Bangs J. D. 2008. Developmentally regulated sphingolipid synthesis in African trypanosomes. Mol. Microbiol. 70:281–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tauchi-Sato K., Ozeki S., Houjou T., Taguchi R., Fujimoto T. 2002. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 277:44507–44512 [DOI] [PubMed] [Google Scholar]
- 49.Van Hellemond J. J., Tielens A. G. 2006. Adaptations in the lipid metabolism of the protozoan parasite Trypanosoma brucei. FEBS Lett. 580:5552–5558 [DOI] [PubMed] [Google Scholar]
- 50.van Herpen R. E., Oude Ophuis R. J., Wijers M., Bennink M. B., van de Loo F. A., Fransen J., Wieringa B., Wansink D. G. 2005. Divergent mitochondrial and endoplasmic reticulum association of DMPK splice isoforms depends on unique sequence arrangements in tail anchors. Mol. Cell. Biol. 25:1402–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vickerman K. 1985. Developmental cycles and biology of pathogenic trypanosomes. Br. Med. Bull. 41:105–114 [DOI] [PubMed] [Google Scholar]
- 52.Wirtz E., Leal S., Ochatt C., Cross G. A. 1999. A tightly regulated inducible expression system for conditional gene knockouts and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 99:89–101 [DOI] [PubMed] [Google Scholar]
- 53.Yu W., Bozza P. T., Tzizik D. M., Gray J. P., Cassara J., Dvorak A. M., Weller P. F. 1998. Co-compartmentalization of MAP kinases and cytosolic phospholipase A2 at cytoplasmic arachidonate-rich lipid bodies. Am. J. Pathol. 152:759–769 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.