Abstract
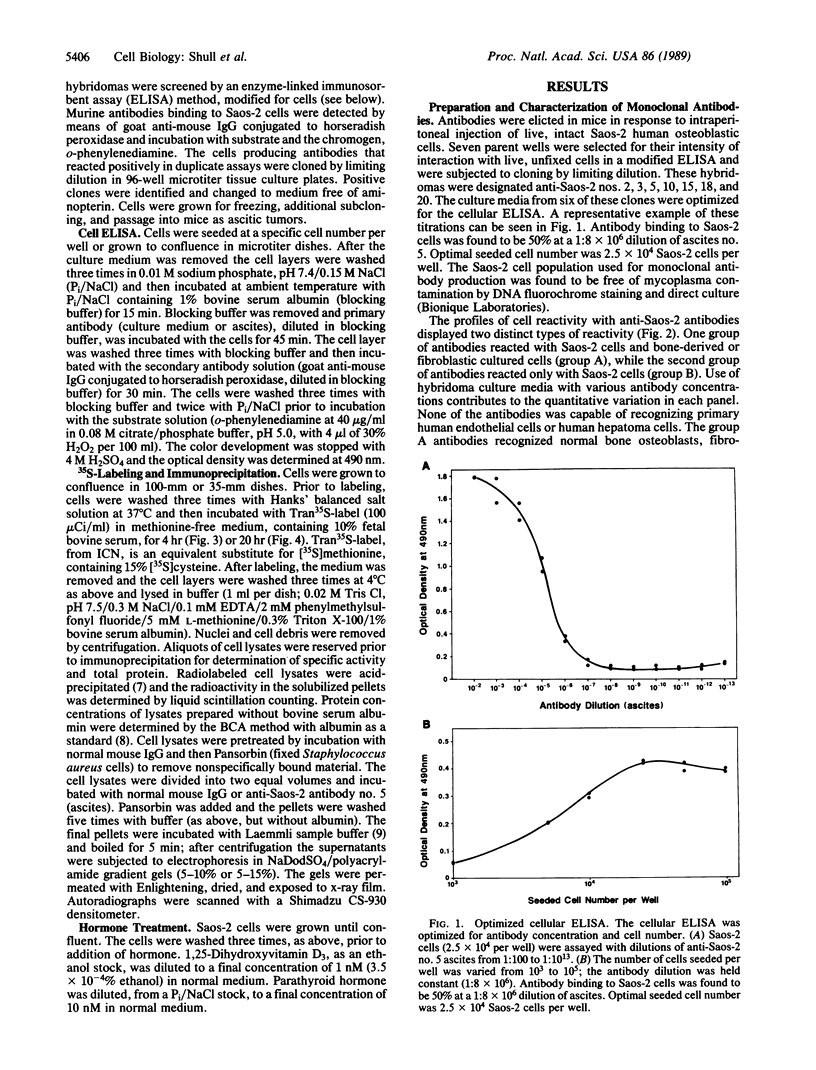
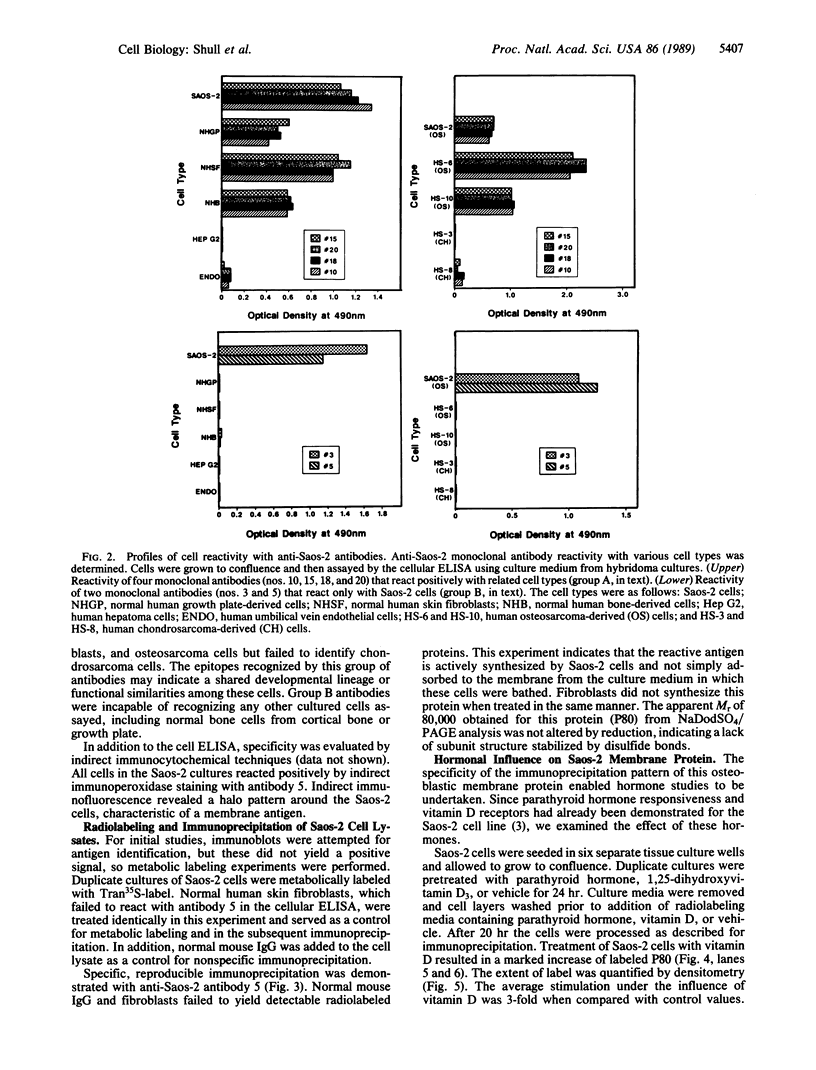
Monoclonal antibodies were elicited to membrane constituents of the osteoblastic human osteosarcoma cell line Saos-2. Two types of antibody reactivities were characterized: one group of antibodies identified fibroblastic and osteoblastic cultured cells, whereas the other group was specific for the parent cell line, Saos-2. Primary endothelial cells and hepatoma cells were not recognized by either group of antibodies. Through indirect immunofluorescent microscopy, the Saos-2-specific antigen was demonstrated to reside on the surface of these osteosarcoma cells. Metabolic radiolabeling of cultured Saos-2 cells and subsequent immunoprecipitation, electrophoretic separation, and autoradiography revealed this protein to have a Mr of 80,000. Similar experiments in the presence of hormones showed that the expression of this cell surface protein was influenced in an opposing fashion by the bone-regulating hormones parathyroid hormone and vitamin D. Vitamin D stimulated expression by 300%, whereas parathyroid hormone depressed expression by 50%. Thus, Saos-2 human osteoblastic cells demonstrate hormonal regulation through an apparently specific membrane protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruland O. S., Fodstad O., Stenwig A. E., Pihl A. Expression and characteristics of a novel human osteosarcoma-associated cell surface antigen. Cancer Res. 1988 Sep 15;48(18):5302–5309. [PubMed] [Google Scholar]
- Chen T. L., Hirst M. A., Feldman D. A receptor-like binding macromolecule for 1 alpha, 25-dihydroxycholecalciferol in cultured mouse bone cells. J Biol Chem. 1979 Aug 25;254(16):7491–7494. [PubMed] [Google Scholar]
- Franceschi R. T., James W. M., Zerlauth G. 1 alpha, 25-dihydroxyvitamin D3 specific regulation of growth, morphology, and fibronectin in a human osteosarcoma cell line. J Cell Physiol. 1985 Jun;123(3):401–409. doi: 10.1002/jcp.1041230316. [DOI] [PubMed] [Google Scholar]
- Franceschi R. T., Linson C. J., Peter T. C., Romano P. R. Regulation of cellular adhesion and fibronectin synthesis by 1 alpha,25-dihydroxyvitamin D3. J Biol Chem. 1987 Mar 25;262(9):4165–4171. [PubMed] [Google Scholar]
- Hirsch S., Gordon S. Surface antigens as markers of mouse macrophage differentiation. Int Rev Exp Pathol. 1983;25:51–75. [PubMed] [Google Scholar]
- Jilka R. L. Are osteoblastic cells required for the control of osteoclast activity by parathyroid hormone? Bone Miner. 1986 Sep;1(4):261–266. [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Jose M., Yamada S., DeLuca H. F. A specific high-affinity binding macromolecule for 1,25-dihydroxyvitamin D3 in fetal bone. Science. 1977 Sep 9;197(4308):1086–1088. doi: 10.1126/science.887939. [DOI] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Luben R. A., Wong G. L., Cohn D. V. Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology. 1976 Aug;99(2):526–534. doi: 10.1210/endo-99-2-526. [DOI] [PubMed] [Google Scholar]
- Majeska R. J., Rodan S. B., Rodan G. A. Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology. 1980 Nov;107(5):1494–1503. doi: 10.1210/endo-107-5-1494. [DOI] [PubMed] [Google Scholar]
- Manolagas S. C., Haussler M. R., Deftos L. J. 1,25-Dihydroxyvitamin D3 receptor-like macromolecule in rat osteogenic sarcoma cell lines. J Biol Chem. 1980 May 25;255(10):4414–4417. [PubMed] [Google Scholar]
- McSheehy P. M., Chambers T. J. Osteoblastic cells mediate osteoclastic responsiveness to parathyroid hormone. Endocrinology. 1986 Feb;118(2):824–828. doi: 10.1210/endo-118-2-824. [DOI] [PubMed] [Google Scholar]
- Partridge N. C., Frampton R. J., Eisman J. A., Michelangeli V. P., Elms E., Bradley T. R., Martin T. J. Receptors for 1,25(OH)2-vitamin D3 enriched in cloned osteoblast-like rat osteogenic sarcoma cells. FEBS Lett. 1980 Jun 16;115(1):139–142. doi: 10.1016/0014-5793(80)80744-7. [DOI] [PubMed] [Google Scholar]
- Peck W. A., Burks J. K., Wilkins J., Rodan S. B., Rodan G. A. Evidence for preferential effects of parathyroid hormone, calcitonin and adenosine on bone and periosteum. Endocrinology. 1977 May;100(5):1357–1364. doi: 10.1210/endo-100-5-1357. [DOI] [PubMed] [Google Scholar]
- Price P. A., Baukol S. A. 1,25-Dihydroxyvitamin D3 increases synthesis of the vitamin K-dependent bone protein by osteosarcoma cells. J Biol Chem. 1980 Dec 25;255(24):11660–11663. [PubMed] [Google Scholar]
- RAISZ L. G. Stimulation of bone resorption by parathyroid hormone in tissue culture. Nature. 1963 Mar 9;197:1015–1016. doi: 10.1038/1971015a0. [DOI] [PubMed] [Google Scholar]
- Robey P. G., Termine J. D. Human bone cells in vitro. Calcif Tissue Int. 1985 Sep;37(5):453–460. [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Rodan S. B., Imai Y., Thiede M. A., Wesolowski G., Thompson D., Bar-Shavit Z., Shull S., Mann K., Rodan G. A. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987 Sep 15;47(18):4961–4966. [PubMed] [Google Scholar]
- Silve C. M., Hradek G. T., Jones A. L., Arnaud C. D. Parathyroid hormone receptor in intact embryonic chicken bone: characterization and cellular localization. J Cell Biol. 1982 Aug;94(2):379–386. doi: 10.1083/jcb.94.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]