Abstract
Overwhelming bacteremia is a leading cause of death. To understand the mechanisms involved in protective antibody and pathological inflammatory responses during bacteremia, we have been studying the murine model of Borrelia hermsii infection. Toll-like receptor (TLR) signaling plays an important role in generating the rapid anti-B. hermsii antibody responses required for the resolution of bacteremia. Using NF-κB reporter assays, we found that B. hermsii activates TLR2 and TLR9. However, TLR2−/− TLR9−/− mice exhibited an impairment in anti-B. hermsii antibody responses similar to that of TLR2−/− mice. Moreover, the impairment in the antibody responses of TLR2−/− mice or TLR2−/− TLR9−/− mice coincides with an order-of-magnitude-higher bacteremia, and death results from septic shock, as evidenced by a dysregulated systemic cytokine response and characteristic organ pathology. Since TLR2 appears to be the major extracellular sensor stimulated by B. hermsii, we hypothesized that during elevated bacteremia the activation of intracellular sensors of bacteria triggers dysregulated inflammation in TLR2−/− mice. Indeed, blocking the internalization of B. hermsii prevented the induction of inflammatory cytokine responses in TLR2-deficient cells. Furthermore, we found that B. hermsii activates the cytoplasmic sensor nucleotide-binding oligomerization domain 2 (NOD2). Macrophages deficient in both TLR2 and NOD2 have impaired cytokine responses to B. hermsii compared to cells lacking TLR2 alone, and B. hermsii-infected TLR2−/− NOD2−/− mice exhibited improved survival compared to TLR2−/− mice. These data demonstrate that TLR2 is critical for protective immunity and suggest that, during heightened bacteremia, recognition of bacterial components by intracellular sensors can lead to pathological inflammatory responses.
Overwhelming bacteremia is one of the leading causes of death by infectious disease. Understanding how the body clears bacterial infections in the systemic circulation would facilitate development of more effective intervention strategies. The bacterium Borrelia hermsii is a causative agent of relapsing fever in humans and is adapted for colonizing the vascular compartment (59). The murine model of B. hermsii infection recapitulates a number of clinical manifestations of the human disease and serves as an excellent experimental system to study the mechanisms of the control of bacteremia (8, 17, 25). Relapsing fever is characterized by recurrent episodes of bacteremia, and each episode is associated with bacterial populations expressing an antigenically distinct surface protein called variable major protein (13). A number of studies have shown that B-cell responses, in particular those that produce specific IgM antibodies, are critical for controlling B. hermsii bacteremia (5, 14, 19).
The IgM response to B. hermsii is T cell independent (TI), since mice lacking T cells are able to mount an effective antibody response and clear the infection (5, 14). Both B-cell receptor (BCR) and Toll-like receptor (TLR) signaling are required for initiating a rapid TI antibody response against B. hermsii (4). TLR2-deficient mice exhibit an impairment in IgM production, suffer heightened bacterial burden, and die during the resolution phase of bacteremia (4). The death of TLR2-deficient mice is puzzling, however, since mice lacking mature B cells (e.g., Rag1−/−) are impaired in controlling the infection, develop similarly high bacteremia, and become moribund but do not die rapidly (5). This suggests that high bacteremia alone does not cause the death observed in TLR2−/− mice and that the immune response mounted during the heightened bacteremia is a critical contributor for the induction of death.
Septic shock is a potentially lethal condition precipitated by a dysregulated systemic inflammatory response to infection (54). It accounts for 215,000 deaths in the United States each year, and the incidence of septic shock has been increasing by ∼10% annually (9). The initial inflammation has been shown to induce pathological changes in organ systems throughout the body (54), and understanding the early immunological events that precipitate the downstream organ dysfunction may provide new treatment strategies for controlling the detrimental immune responses and improving survival. A wide range of microbial pathogens can cause septic shock and these pathogens express an array of immunostimulatory molecules referred to as pathogen-associated molecular patterns (PAMPs) (45, 61). A variety of cells, including those of the immune system, use specific pattern recognition receptors (PRRs) such as TLRs and NOD-like receptors (NLRs) to detect the PAMPs and initiate an immune response (36). Stimulation of these sensors mediates interactions with specific adaptor molecules, e.g., MyD88 or RIPK, which create a platform to a cascade of kinases and transacting factors such as NF-κB, and events that ultimately result in the activation of various components of the immune system, e.g., cytokine responses and upregulation of costimulatory molecules. These responses not only play an important role in protective immunity but can also lead to pathological inflammation.
In the murine model of B. hermsii bacteremia, we have found that TLR2 is the primary sensor involved in the protective antibody responses to B. hermsii and that mice deficient in TLR2 succumb to sepsis. Characterization of the B. hermsii-induced inflammatory responses in the TLR2-deficient system indicates a role for intracellular sensors, including NOD2, a cytoplasmic sensor of bacterial peptidoglycan.
MATERIALS AND METHODS
Mice and infections.
Mice housed in microisolator cages with free access to food and water were maintained in a specific-pathogen-free facility of Thomas Jefferson University. The studies have been reviewed and approved by the Institutional Animal Care and Use Committee. C57BL/6/J and B6.129S1-Nod2tm1Flv/J (NOD2−/−) mice were purchased from Jackson Laboratories (Bar Harbor, ME). TLR2−/−, TLR9−/−, and TLR2−/− 9−/− mice on a C57BL/6 background were provided by Shizuo Akira, Osaka University, Osaka, Japan.
Six- to eight-week-old mice were infected intravenously via the tail vein with 5 × 104 bacteria of a fully virulent B. hermsii strain DAH-1 (58) or a partially attenuated strain DAH-p19, generated by serial passage of DAH-p1 in vitro 18 times (7), and the bacteremia was monitored by dark-field microscopy (7). Mice that become moribund do not appear to recover, and moribund mice were therefore euthanized.
Generation of double- and triple-knockout mice.
TLR2−/− mice were intercrossed to NOD2−/− mice to generate TLR2×NOD2 double-knockout mice. The TLR2 genotype was determined by PCR using the primers TLR2F (5′-TGG CAT GCC TCC ATC ATA GTT AAC C-3′), TLR2R (5′-GTC AGA AAC AAC CAC CAC CAT GC-3′), and NeoR (5′-ATC GCC TTC TAT CGC CTT CTT GAC G-3′) to yield wild-type and knockout products of about 550 and 750 bp, respectively. NOD2 and TLR9 genotypes were also determined by PCR using the following primers. For the wild-type NOD2 allele, IMR4112 (5′-ACA GAG ATG CCG ACA CCA TAC TG-3′) and IMR4113 (5′-TGG AGA AGG TTG AAG AGC AGA GTC-3′) were used to yield a 370-bp product. For the mutant NOD2 allele, IMR4114 (5′-TGA CTG TGG CTA ATG TCC TTT GTG-3′) and IMR4115 (5′-TTC TAT CGC CTT CTT GAC GAG TTC-3′) were used to yield a 1,000-bp product. For the wild-type TLR9 allele, TLR9F (5′-GAA GGT TCT GGG CTC AAT GGT CAT GTG-3′) and TLR9R (5′-GCA ATG GAA AGG ACT GTC CAC TTT GTG-3′) were used to yield a 1.200-bp product. For the mutant TLR9 allele, TLR9R and TLR9-Neo (5′-ATC GCC TTC TAT CGC CTT CTT GAC GAG-3′) were used to yield a 1,200-bp product. Mice deficient in TLR2, TLR9, and NOD2 (triple-knockout mice) were generated by intercrossing TLR2−/− TLR9−/− mice and TLR2−/− NOD2−/− mice.
Organ harvesting and histology.
TLR2−/− and wild-type mice were infected as described, and each day postinfection mice were sacrificed and the hearts, kidneys, livers, and spleens were harvested and placed in buffered Formalde-Fresh solution (Fisher Scientific). Lungs were inflated with buffered Formalde-Fresh solution and tied off at the trachea to fix while preserving the lung architecture. Organs were processed and embedded in paraffin wax, hematoxylin-eosin-stained slides were examined by a trained histologist, and photomicrographs were obtained by using an Olympus VANOX-TAH-2 microscope and a Fujix digital camera HC300z.
ELISA.
IgM levels were measured with enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (Bethyl Laboratories, Montgomery, TX). B. hermsii-specific IgM was determined by coating 96-well plates (ICN Biomedicals, Inc., Aurora, OH) with in vivo-grown B. hermsii DAH-vmp2 (105 wet bacteria/well) (18). The levels of interleukin-6 (IL-6) and IL-10 in B. hermsii-infected mouse serum was measured by using Duoset ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
ALT and BUN measurement.
Serum alanine aminotransferase (ALT) levels in the serum were measured by using Infinity ALT (GPT) reagent according to the manufacturer's instructions (Thermo Electron Corp., Waltham, MA). Blood urea nitrogen (BUN) levels were measured from plasma by using a QuantiChrom urea assay kit according to the manufacturer's instructions (BioAssay Systems, Hayward, CA).
Cell lines and cell culture.
Human embryonic kidney-293 (HEK293) cells (i) stably transfected with a murine TLR2 gene (293-mTLR2 cells, clone 6), (ii) stably transfected with murine TLR4, MD-2, and CD-14 genes (293-mTLR4/MD2-CD14, clone 6), or (iii) stably transfected with the pUNO-mcs vector alone (293-TLR-Null cells, clone 6) were purchased from InvivoGen, San Diego, CA. HEK293 cells (CRL-1573) were purchased from the American Type Culture Collection, Manassas, VA. Cells stably transfected with murine TLR9 (293-mTLR9 cells) were generated (C. F. Knetter and E. Lien, unpublished data).
For HEK293 cell culture, Dulbecco minimal essential medium (DMEM) with 2 mM l-glutamine was purchased from Cellgro/Mediatech, Herndon, VA. DMEM was supplemented with 10% heat-inactivated fetal calf serum (HyClone, Logan, UT) and appropriate antibiotics based on the expression vector and cell-line used. Penicillin and streptomycin were purchased from Cellgro/Mediatech, blasticidin S and hygromycin B (HygroGold) were purchased from InvivoGen, and G418 was purchased from Gibco-BRL.
For in vitro work with peritoneal exudate cells, RPMI 1640 with l-glutamine and 25 mM HEPES was purchased from Cellgro/Mediatech. RPMI 1640 was supplemented with 2% heat-inactivated fetal calf serum (HyClone), and penicillin and streptomycin (Cellgro/Mediatech). For in vitro work with bone marrow-derived macrophages, RPMI 1640 with l-glutamine and 25 mM HEPES was purchased from Cellgro/Mediatech. RPMI 1640 was supplemented with 10% heat-inactivated fetal calf serum (HyClone) and penicillin and streptomycin (Cellgro/Mediatech).
Plasmids, reagents, and bacterial products.
An expression vector containing the mouse NOD1 or NOD2 open reading frame (pUNO-mNOD1 or pUNO-mNOD2) and an NF-κB inducible firefly luciferase reporter plasmid (pNiFty2-Luc) were purchased from InvivoGen. A plasmid containing the Renilla luciferase gene under cytomegalovirus promoter control (pRL-CMV) was purchased from Promega, Madison, WI.
Synthetic lipopeptide Pam3Cys-SKKKK (Pam3CysK4) was purchased from EMC Microcollections, Tübingen, Germany. Soluble sonicated peptidoglycan from Escherichia coli K-12 (PGN-Ecndss Ultrapure) was purchased from InvivoGen. Soluble lipopolysaccharide (LPS) from E. coli O55:B5 was purchased from Sigma-Aldrich, St. Louis, MO. Synthetic oligodeoxynucleotides (Non-CpG Control ODN 2138) or immunostimulatory synthetic oligodeoxynucleotides containing CpG dinucleotide motifs (CpG ODN 1826) on a fully phosphorothioate backbone were purchased from Coley Pharmaceuticals, Wellesley, MA.
B. hermsii extracts were prepared from strain DAH-p1 harvested from the blood of infected mice and washed with phosphate-buffered saline (PBS; pH 7.2). The bacterial density was adjusted to 5 × 108/ml, followed by sonication. This preparation was solubilized by mixing in 0.05% Nonidet P-40, a nonionic detergent. Mock extract was prepared by subjecting PBS to same sonication and solubilization procedures to control potential contamination of immunostimulatory components such as peptidoglycan and LPS.
Whole B. hermsii were prepared from strain DAH-p1 harvested from blood of infected mice. Bacterial density was adjusted to 106/μl, and bacteria were killed without altering morphology by placing them at 42°C for 1 h.
NF-κB activation assays.
Studies examining the activation of NF-κB by B. hermsii, peptidoglycan, Pam3Cys, LPS, CpG DNA, or related compounds in cells either stably expressing TLRs or transiently expressing NOD1 or NOD2 were carried out as described previously (27). For NOD1- or NOD2-mediated NF-κB activation by B. hermsii, 96-well microtiter plates were seeded with 2.5 × 104 HEK293 cells/well and 24 h later, when the wells reached 50 to 60% confluence, were transfected with 0.33 ng of pUNO-mNOD1 or pUNO-mNOD2, 50 ng of pNifty-2-Luc, and 20 ng of pRL-CMV by using GeneJuice transfection reagent (Novagen, Madison, WI). At the same time, the peptidoglycan or B. hermsii extracts were added to facilitate intracellular uptake of potential NOD1 or NOD2 ligands. NF-κB-dependent luciferase activation was then measured after 24 h of coincubation.
For TLR-mediated NF-κB activation by B. hermsii, 96-well microtiter plates were seeded with 2.5 × 104 HEK293 cells/well and 24 h later, when the wells reached 50 to 60% confluence, were transfected with 50 ng of pNifty-2-Luc and 20 ng of pRL-CMV using GeneJuice transfection reagent. For TLR9-mediated NF-κB activation, B. hermsii extracts or appropriate control agonists were added at the time of transfection to facilitate the intracellular uptake of potential ligands and NF-κB-dependent luciferase activation was measured after 24 h of coincubation. For TLR2- or TLR4-mediated NF-κB activation, various concentrations of B. hermsii, mock extracts, or appropriate control TLR agonists were added 18 h posttransfection, and the NF-κB-dependent luciferase activation was measured after a 6-h coincubation.
Stimulation of BMDMs and peritoneal exudate cells.
Peritoneal exudate cells were harvested from the peritoneal cavity. Macrophages were derived from mouse bone marrow over 7 days in medium composed of 70% D-20 medium (DMEM [with 4.5 g of glucose, l-glutamine, and sodium pyruvate/liter; Mediatech, Inc.], 20% heat-inactivated fetal calf serum, 55 μM 2-mercaptoethanol, and penicillin-streptomycin) and 30% 0.22-μm-pore-size-filtered culture supernatants from L929 cells. A total of 5 × 105 cells/well were plated in a tissue culture-treated, nonpyrogenic, polystyrene 24-well plate, and whole, heat-killed B. hermsii or control agonists were added at the indicated concentrations. Plates were spun at 800 × g for 5 min to facilitate interaction of cells with bacteria. At 24 h after stimulation, the supernatant was collected, and the cytokine levels were measured by ELISA. For experiments blocking internalization, cells underwent 2 h of preincubation with 1 μM cytochalasin D for peritoneal exudates cells or with 3 μM cytochalasin D for bone marrow-derived macrophages (BMDMs; Tocris Bioscience, Ellisville, MO) prior to the addition of agonists.
Statistical analysis.
Statistics were performed by using GraphPad Prism version 5.0.
RESULTS
TLR2 is the primary innate sensor involved in protective responses during B. hermsii infection.
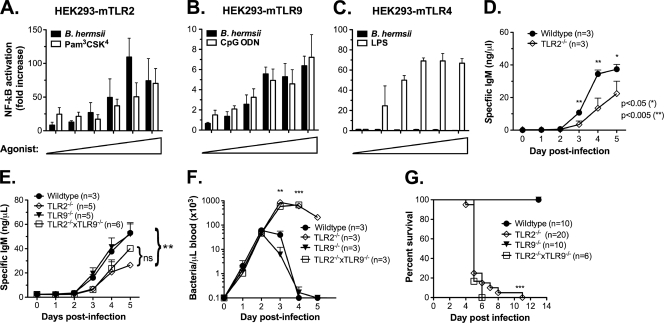
A specific IgM response against B. hermsii is required for protective immunity. Using the murine infection model, it has been previously demonstrated that MyD88-dependent TLR signaling plays an important role in generating rapid anti-B. hermsii antibody responses. Since a given bacterial species is expected to activate multiple members of the TLR family, in vivo experiments utilizing mice deficient in a single TLR may not allow determination of the role of each family member due to functional redundancy. To examine the ability of B. hermsii to stimulate a given TLR family member, we utilized an in vitro system consisting of HEK 293 cells stably expressing individual TLRs, with an NF-κB reporter system as a readout for TLR activation (48). Since the outer membrane of B. hermsii is predominantly composed of lipoproteins, we expected that B. hermsii would activate TLR2. Indeed, NF-κB reporter assays revealed that B. hermsii is sensed by TLR2 in a dose-dependent manner (Fig. 1 A). TLR9 detects unmethylated DNA, which is abundant in the genome of bacteria (61), and we were able to demonstrate that B. hermsii can also induce TLR9-dependent NF-κB activation (Fig. 1B). Although TLR4 is known as a receptor involved in detecting bacterial LPS, it is capable of detecting other microbial ligands (2, 40, 43). For this reason, we also examined TLR4 and found that B. hermsii does not induce TLR4-dependent NF-κB activation (Fig. 1C). B. hermsii does contain flagellin, but it lacks the consensus sequences necessary for TLR5 recognition (unpublished data); this discouraged us from pursuing a role for TLR5 in the B. hermsii system. TLR11 is a receptor that recognizes an unknown component of uropathogenic E. coli and is expressed mainly in the kidney and bladder (65). For this reason, we did not consider TLR11 as a potential sensor of B. hermsii. Collectively, these data indicate that TLR2 and TLR9 are the primary TLR family members involved in the detection of B. hermsii.
FIG. 1.
TLR2 is critical for protective responses during B. hermsii infection. Human embryonic kidney (HEK293) cells expressing either murine TLR2 (A), murine TLR9 (B), or murine TLR4 (C) and luciferase under NF-κB-promoter control were stimulated for 6 h with increasing concentrations of B. hermsii sonicate (0.5 to 16%; 0.5% B. hermsii sonicate corresponds to 1.25 × 104 spirochetes) or specific control agonists (1 to 50 ng/ml for Pam3CSK4 and LPS; 0.3 to 10 ng/ml for CpG DNA). Luciferase assays were performed to determine NF-κB activation relative to mock-stimulated controls, and all values were normalized for transfection efficiency. (D) Wild-type and TLR2−/− mice were injected with 2.5 × 107 heat-killed B. hermsii DAH-p1, and blood was collected daily. B. hermsii-specific IgM levels were determined by ELISA. (E to G) Wild-type, TLR2−/−, TLR9−/−, or TLR2−/− TLR9−/− mice were infected intravenously with B. hermsii DAH-p1, and blood was sampled daily. B. hermsii-specific IgM levels were determined by ELISA (E), bacteremia was determined by microscopic counting (F), and survival was monitored (G). The antibody responses (see panel E) on days 3 and 4 postinfection were compared by using Bonferroni post-test (**, P < 0.01). Bacteremia data, presented as means ± the standard deviation (SD), were compared by using an unpaired Student t test (**, P < 0.01; ***, P < 0.001). Survival curves were compared by the log-rank test (***, P < 0.001). ns, Not significant.
We have previously shown that TLR9−/− mice produce anti-B. hermsii IgM as rapidly as do wild-type mice (4), whereas TLR2−/− mice show reduced circulating B. hermsii-specific IgM during an active infection (4, 16). Since TLR2−/− mice exhibit an order-of-magnitude-higher bacterial burden compared to that observed in wild-type mice, the lower IgM levels could be attributed to sequestration of circulating IgM by bacteria. To address this, we immunized wild-type and TLR2−/− mice with an equal number of heat-killed B. hermsii DAH-p1. We found that, under these conditions, TLR2−/− mice exhibited an impaired IgM response (Fig. 1D), similar to that seen after active infection (Fig. 1E), indicating a role for TLR2 in anti-B. hermsii responses. TLR9 has been shown to activate antigen-specific B cells (41), as well as memory B cells (15), indicating its role in the generation of rapid as well as long-lasting IgM responses to B. hermsii. Since TLR2 and TLR9 may be working in a cooperative manner to promote a robust anti-B. hermsii IgM response, we infected TLR2−/− TLR9−/− mice with B. hermsii strain DAH-p1 and found a similar reduction in antibody responses compared to TLR2−/− mice (Fig. 1E). This impaired anti-B. hermsii antibody response correlated with more severe bacteremia in TLR2−/− mice or TLR2−/− TLR9−/− mice compared to wild-type or TLR9−/− mice (Fig. 1F). Wild-type mice infected with DAH-p1 suffer a peak bacterial burden of 50 to 100,000/μl of blood during the primary episode and clear the bacteria in 2 to 3 days (Fig. 1F), with a resolution of all bacteremic episodes by 4 weeks postinfection (5). In contrast, TLR2−/− mice and TLR2−/− TLR9−/− mice not only develop a 10-fold-higher bacteremia than that seen in either wild-type or TLR9−/− mice (Fig. 1F) but also die during or after clearance of the primary episode (Fig. 1G). Collectively, these data suggest that whereas TLR9 may be able to detect components of B. hermsii, TLR2 is the primary innate sensor involved in protective responses during B. hermsii infection.
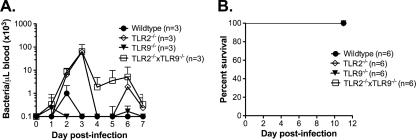
TLR2−/− mice suffer heightened bacteremia after infection with partially attenuated DAH-p19 but do not die.
The fact that TLR2−/− mice suffer elevated bacteremia and subsequently die during or after bacterial clearance suggests that bacterial burden is a critical parameter in triggering the pathological events leading to their death. To test this possibility, we infected wild-type and TLR2−/− mice with DAH-p19, a derivative strain of DAH-p1 whose virulence has been partially attenuated by serial passage in vitro (7) but which retains the ability to induce a robust inflammatory cytokine response from BMDMs in vitro (data not shown). Compared to DAH-p1, the peak bacteremia of strain DAH-p19 in wild-type mice is more than an order of magnitude lower (Fig. 1F versus Fig. 2 A). Although DAH-p19 grows to a higher density in TLR2−/− mice than in wild-type mice and TLR2−/− mice display recurrent episodes of bacteremia, all TLR2−/− mice eventually clear this attenuated strain and survive the infection (Fig. 2).
FIG. 2.
TLR2−/− mice suffer heightened bacteremia following infection with partially attenuated B. hermsii strain DAH-p19 but do not die. Wild-type, TLR9−/−, TLR2−/−, and TLR2−/− TLR9−/− mice were infected intravenously with B. hermsii DAH-p19. (A) Blood was sampled daily, and the bacterial count was determined by microscopy. (B) Survival was monitored.
Recently, it has been shown that TLRs and other PRRs can regulate the expression or activity of one another (62, 63). This raised the possibility that TLR2 could act as a negative regulator of other PRRs and that the loss of TLR2 could result in exaggerated inflammatory responses to bacterial insult. However, DAH-p19-infected TLR2−/− mice survive B. hermsii infection, and TLR2−/− BMDMs or peritoneal exudate cells demonstrate no increased responsiveness to stimulation by other PAMPs such as CpG, poly(I:C), and MDP in vitro compared to wild-type cells (data not shown). This suggests that the TLR2−/− mice infected with DAH-p1 are not dying due to a dysregulated activation of other PRRs, but rather that the mice are dying, in part, due to the substantially higher bacterial load.
Exaggerated systemic cytokine responses in infected TLR2−/− mice.
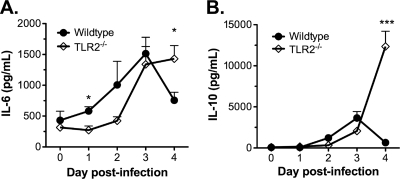
Septic shock is a condition that can arise when a poorly controlled infection induces a hyperactive immune response. This dysregulated immune response can cause pathological physiological changes throughout the body, resulting in the dysfunction of multiple organ systems (1, 54). Immunodeficient mice (e.g., Rag1−/− or scid mice) suffer persistently elevated B. hermsii bacteremia and become moribund but do not die rapidly like TLR2−/− mice (5), suggesting that high bacteremia alone is not sufficient to cause death. Considering the rapid death observed in TLR2−/− mice and the likelihood that it is driven by an excessive immune response due to an increased bacterial load, septic shock seemed a plausible cause. Since systemic inflammation is a critical attribute of septic shock, we sought to determine the presence of a dysregulated systemic immune response in wild-type and TLR2−/− mice during the course of DAH-p1 infection by measuring the systemic levels of several cytokines such as tumor necrosis factor alpha (TNF-α), IL-1β, IL-6, and IL-10 daily. Mouse-to-mouse variability prevented us from drawing conclusions regarding TNF-α and IL-1β levels, but we found that TLR2−/− mice exhibit a prolonged IL-6 response (Fig. 3 A) as reported earlier (16) and dramatically elevated IL-10 (Fig. 3B) by 4 days postinfection. By comparison, TLR2−/− mice infected with DAH-p19 showed no elevated cytokines compared to wild-type mice at any time after infection (data not shown). These data are consistent with a dysregulated systemic inflammatory response in DAH-p1-infected TLR2−/− mice, since IL-6 and IL-10 are considered to be markers of systemic inflammation in sepsis (51).
FIG. 3.
Exaggerated systemic cytokine response in B. hermsii-infected TLR2−/− mice. Wild-type (n = 5) or TLR2−/− (n = 5) mice were infected with B. hermsii DAH-p1, blood was collected on each day postinfection, and the IL-6 (A) and IL-10 (B) levels in the blood were determined by ELISA. The data, presented as means ± the SD, were compared by using an unpaired Student t test (*, P < 0.05; ***, P < 0.001).
TLR2−/− mice exhibit organ damage and dysfunction that is characteristic of septic shock.
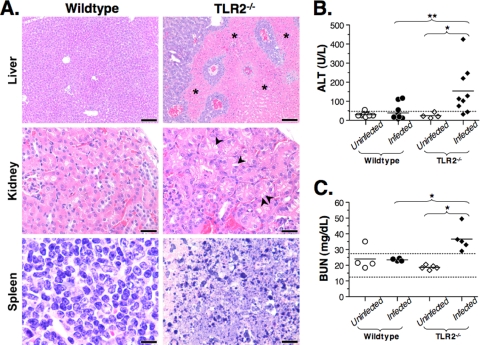
To better determine the cause of death of TLR2−/− mice after DAH-p1 infection, organs of wild-type and TLR2−/− mice on each day postinfection were examined. Histological evaluation of livers from TLR2−/− mice on 4 and 5 days postinfection revealed extensive areas of hypoxia spanning several lobules but sparing the areas most proximal to large blood vessels (Fig. 4 A). To confirm the histopathology, we measured ALT levels, an enzyme released after liver damage. We found that by 4 days postinfection, TLR2−/− mice showed significantly elevated ALT levels compared to either uninfected TLR2−/− controls or wild-type mice at 4 days postinfection (Fig. 4B). Collectively, the histopathology and serum ALT data indicate hypoxia-induced liver damage in TLR2−/− mice by 4 days postinfection, consistent with liver damage observed during septic shock (20).
FIG. 4.
B. hermsii-infected TLR2−/− mice develop organ damage and dysfunction characteristic of septic shock. (A) Histopathological analysis was performed on hematoxylin-eosin-stained organ sections of wild-type and TLR2−/− mice at 5 days postinfection. Livers showed hypoxic foci in TLR2−/− mice (indicated with asterisks). Kidneys of TLR2−/− mice showed vacuolar degeneration (indicated with arrowheads), and the splenic white pulp showed extensive apoptosis in TLR2−/− mice. This panel reflects observations from two independent experiments involving at least five mice per group. (B and C) Serum ALT (B) or BUN (C) levels were measured in wild-type and TLR2−/− mice prior to infection and then at 4 days postinfection. The dashed line in panel B indicates the upper limit of healthy ALT levels. Dashed lines in panel C indicate the upper and lower limits of healthy mouse BUN values. These data are representative of two independent experiments. Statistically significant differences, indicated by “*” (P < 0.05) or “**” (P < 0.01) were determined by the Mann-Whitney test.
Examination of kidneys from wild-type mice did not reveal any significant changes over the course of infection. However, by 4 or 5 days postinfection, TLR2−/− mice developed tubular vacuolation and epithelial cell swelling in the renal cortex (Fig. 4A). These changes are also observed after the induction of septic peritonitis in experimental mouse models of septic shock and strongly correlate with kidney dysfunction (20, 47, 64). To directly assay kidney function in infected mice, we measured BUN on each day postinfection. We found that unlike wild-type mice, infected TLR2−/− mice showed significantly elevated BUN levels (Fig. 4C), an observation consistent with the histopathology observed.
Examination of the spleens from B. hermsii-infected TLR2−/− mice revealed extensive lymphocyte apoptosis predominantly in the white pulp. Such changes were not observed in wild-type mice at any time point during the infection (Fig. 4A). This is consistent with the current understanding of septic shock, since significant lymphocyte apoptosis in the white pulp of the spleen has been reported in animal models for septic shock (21, 31, 32). In addition, studies of septic patients have demonstrated apoptotic lymphocytes in the circulation and postmortem examinations have also revealed lymphocyte apoptosis in the spleens of septic shock patients (33, 34). The lungs of infected TLR2−/− mice showed increased leukocyte trafficking and margination compared to wild-type mice, but no edema or atelectasis was observed (data not shown).
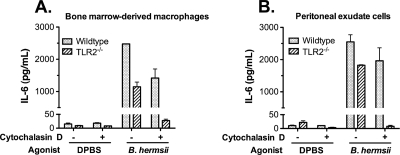
Bacterial internalization is required for cellular responses to B. hermsii in the absence of TLR2.
Since TLR2 appears to be the primary surface sensor capable of detecting B. hermsii, we sought to determine whether intracellular sensors of bacteria are responsible for the pathological inflammation observed in TLR2−/− mice. To test this possibility, we used cytochalasin D, a pharmacological inhibitor of endocytosis. LPS, which is recognized by an extracellular sensor, TLR4, induced robust IL-6 responses in the presence or absence of cytochalasin D (data not shown), indicating that blocking internalization by cytochalasin D does not impair responses induced by extracellular TLRs. We found that BMDMs from both wild-type and TLR2−/− mice were able to respond to B. hermsii when pretreated with only the dimethyl sulfoxide vehicle. After inhibition of endocytosis with cytochalasin D, B. hermsii was able to induce a robust IL-6 response in wild-type BMDMs, but TLR2−/− BMDMs lost their ability to respond (Fig. 5 A). Since we do not yet know which type of cell(s) is responsible for inducing inflammation in the mice, we also used all cells harvested from peritoneal cavity, giving us a diverse cell population including macrophages, neutrophils and B cells. As with BMDMs, wild-type mouse peritoneal exudate cells could produce a robust IL-6 response to B. hermsii when bacterial internalization was inhibited but TLR2−/− mouse peritoneal exudate cells could not (Fig. 5B). These data suggest that, in the absence of TLR2, intracellular sensors of bacteria are necessary for the detection of B. hermsii and the induction of pathological inflammation in infected TLR2−/− mice. These data also provide additional evidence that TLR2 is the major extracellular sensor of B. hermsii.
FIG. 5.
Bacterial internalization is required to generate a cytokine response to B. hermsii in the absence of TLR2. Bone marrow-derived macrophages (BMDMs) (A) or peritoneal exudate cells (B) of either wild-type or TLR2−/− mice were stimulated for 24 h with Dulbecco's phosphate-buffered saline (DPBS) or B. hermsii at a multiplicity of infection of 10 (A) or 1 (B) with or without preincubation with cytochalasin D, an actin polymerization inhibitor that blocks endocytosis (3 μM [A]; 1 μM [B]). IL-6 levels in the culture supernatant were measured by ELISA.
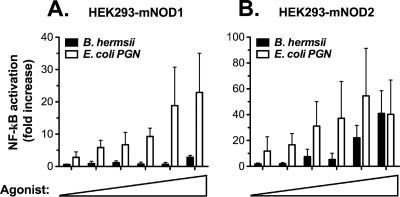
B. hermsii induces NOD2-mediated NF-κB activation.
Although TLR9, an intracellular sensor, can be activated by B. hermsii, the fact that TLR2−/− TLR9−/− mice died similarly to TLR2−/− mice suggests that activation of other intracellular PRRs may be contributing to the pathological inflammatory response in infected TLR2−/− mice. NOD1 and NOD2, cytoplasmic sensors of bacterial peptidoglycan, have been shown to play an important role in responding to a wide variety of bacterial species (23). NOD1 detects diaminopimelic acid, a component of Gram-negative bacterial peptidoglycan, whereas NOD2 detects muramyl dipeptide, a component found in all types of bacterial peptidoglycan. Previous work has determined that while B. hermsii is neither a Gram-positive nor a Gram-negative bacterium, its peptidoglycan contains ornithine but not diaminopimelic acid (38). Due to this fact, we did not expect NOD1-mediated NF-κB activation in response to B. hermsii, and our reporter assay indeed confirmed that B. hermsii does not activate NOD1 (Fig. 6 A). Since ornithine-containing peptidoglycan is known to be a NOD2 agonist (28), and B. hermsii peptidoglycan contains both ornithine and muramyl dipeptide, we expected that B. hermsii activates NOD2. Indeed, using the same NF-κB reporter assay, we demonstrated that B. hermsii induces robust activation of NOD2 (Fig. 6B).
FIG. 6.
NOD2 induces NF-κB activation in response to B. hermsii. Human embryonic kidney (HEK293) cells expressing (A) murine NOD1 or (B) murine NOD2 and luciferase under NF-κB promoter control were stimulated for 24 h with increasing concentrations of B. hermsii sonicate (0.8 to 25%; 0.5% B. hermsii sonicate corresponds to 1.25 × 104 spirochetes) or specific control agonists (E. coli peptidoglycan [PGN]; 0.16 to 50 μg/ml). Luciferase assays were performed to determine NF-κB activation relative to mock-stimulated controls, and all values were normalized for transfection efficiency.
NOD2 contributes to the death of TLR2−/− mice.
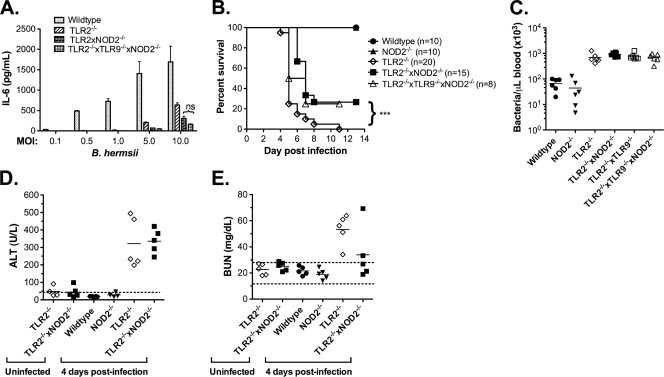
Since both NOD2 and TLR9 are activated by B. hermsii, we sought to determine whether these sensors play a significant role in cellular immune responses to B. hermsii in vitro. We exposed wild-type, TLR2−/−, TLR2−/− NOD2−/−, and TLR2−/− TLR9−/− NOD2−/− BMDMs to increasing numbers of whole B. hermsii and measured IL-6 responses after 24 h. As expected, cells lacking TLR2 showed significantly impaired responses to B. hermsii compared to wild-type cells in vitro (Fig. 7 A). Although it seems paradoxical that the loss of a sensor important for the induction of inflammation could result in excessive inflammation in vivo, it is important to note that the TLR2−/− mice experience a bacterial burden that is an order of magnitude higher. Therefore, it is possible that under conditions of elevated bacteremia, activation of other sensors, such as NOD2, could compensate for the impaired overall cellular responsiveness to B. hermsii in infected TLR2−/− mice. Indeed, we found that the loss of NOD2, in addition to TLR2, further impaired cellular responses to B. hermsii (Fig. 7A), suggesting that NOD2 may play a role in the pathological inflammation observed in infected TLR2−/− mice. We considered that TLR9 may compensate for NOD2 deficiency; however, the loss of TLR9 in addition to TLR2 and NOD2 showed no significant decrease in cellular responses to B. hermsii (Fig. 7A), suggesting that TLR9 is not involved in the pathological inflammation observed in infected TLR2−/− mice.
FIG. 7.
NOD2 contributes to pathological inflammation in the TLR2−/− system. (A) Bone marrow-derived macrophages from wild-type, TLR2−/−, TLR2−/− NOD2−/−, or TLR2−/− TLR9−/− NOD2−/− mice were stimulated with whole B. hermsii DAH-p1 at indicated multiplicity of infection, and IL-6 levels in the supernatants collected after 24 h of stimulation were measured by ELISA. (B) Wild-type, NOD2−/−, TLR2−/−, TLR2−/− NOD2−/−, and TLR2−/− TLR9−/− NOD2−/− mice were infected with B. hermsii DAH-p1, blood was sampled daily, and survival was monitored. (C) Bacterial counts were determined by microscopy. Compared to TLR2−/− mice, TLR2−/− NOD2−/− mice and TLR2−/− TLR9−/− NOD2−/− mice showed significantly improved survival, as determined by log-rank test (***, P < 0.001). Serum ALT (D) and BUN (E) levels were measured in the indicated mice prior to infection or at 4 days postinfection with B. hermsii DAH-p1. The dashed line in panel D indicates the upper limit of healthy ALT levels. The dashed lines in panel E indicate the upper and lower limits of healthy mouse BUN values.
To assess the role of NOD2 and TLR9 in vivo, we infected wild-type, TLR2−/−, TLR2−/− NOD2−/−, and TLR2−/− TLR9−/− NOD2−/− mice with DAH-p1 and monitored the bacteremia, cytokine levels, and survival. Since our in vitro work implicated NOD2-mediated inflammation in the TLR2−/− system, we hypothesized that the loss of NOD2 in the TLR2-deficient mouse system would result in a less severe inflammatory response, which could result in delayed death and improved survival. In fact, we found that mice lacking both TLR2 and NOD2 had a significant delay in death and improved survival of the initial wave of bacteremia compared to TLR2−/− mice (Fig. 7B). However, the surviving mice eventually become moribund and must be euthanized 2 to 3 weeks postinfection. Importantly, improved survival of initial bacteremia occurs despite displaying no difference in peak bacteremia (Fig. 7C). Although the differences are not statistically significant (P < 0.057; data not shown), compared to TLR2−/− mice, TLR2−/− NOD2−/− mice showed lower levels of IL-10, which correlates to decreased sepsis severity and improved survival in other animal models of septic shock (51). Interestingly, whereas the ALT levels in TLR2−/− NOD2−/− mice were roughly the same as those seen in the TLR2−/− mice at 4 days postinfection, the BUN levels in TLR2−/− NOD2−/− mice were somewhat lower compared to those seen in TLR2−/− mice (Fig. 7D and E). In fact, three out of five infected TLR2−/− NOD2−/− mice exhibited normal levels of BUN (Fig. 7E). Collectively, these data are consistent with our finding that TLR2−/− NOD2−/− mice have improved survival of the initial wave of bacteremia compared to TLR2−/− mice.
The loss of TLR9 in addition to TLR2 and NOD2 had no effect on survival, providing support to our in vitro findings. Collectively, these data indicate that NOD2, but not TLR9, is involved in the pathological inflammatory response observed during B. hermsii infection of TLR2−/− mice.
DISCUSSION
The ability of the immune system to generate a rapid antibody response during bacterial infection can prevent serious pathological consequences. In the murine model of B. hermsii infection, MyD88- and Btk-mediated signaling play essential roles in the generation of specific IgM required for the rapid resolution of bacteremia. In the present study, we show that among the MyD88-dependent members of the TLR family, TLR2 and TLR9 are activated by B. hermsii, but TLR2-mediated signaling alone plays a role in generating a rapid IgM response and is essential for survival during bacteremia. Moreover, our data indicate that in the absence of TLR2-mediated signaling, the activation of other intracellular sensors, such as NOD2, can result in a dysregulated inflammatory response leading to septic shock.
Several possible mechanisms could account for the impaired antibody responses during B. hermsii infection of TLR2−/− mice. First, it is important to note that among the B-cell subsets, B1b cells play an important role in controlling B. hermsii infection (3, 6). Dynamic movement of B cells is important for antibody responses (49), and TLR signaling has been shown to govern various aspects of B1 cell migration (30). For example, TLR2 stimulation of B1 cells alters the expression of integrins involved in adhesion and migration (30), and TLR2 signaling induces the secretion of Cxcl13 (56), a chemokine critical for B1 cell migration (10). Since Borrelia infection induces not only Cxcl13 production (26, 56) but also the migration of B1b cells (3), it is possible that in the absence of TLR2 signaling, impairment in B1b cell migration could result in a delay in the B. hermsii-specific antibody responses. In addition to their role in B1-cell migration, TLR stimulation can induce B1 cell differentiation into plasmablasts within 48 h (12, 44), a process that is critical for the development of a rapid antibody response upon antigen encounter. Furthermore, B-cell-intrinsic TLR signaling is critical for optimal B-cell proliferation (41). Since B1 cells express several TLRs, including TLR2 (29), it is possible that, during B. hermsii infection, antigen-specific B1 cells deficient in TLR2 are inherently impaired in their proliferative responses, as well as their ability to rapidly differentiation into antibody-secreting cells.
Although the pathogens responsible for septic shock vary, the characteristics of the dysregulated inflammation and the resulting organ pathology are surprisingly conserved (33). The cecal ligation and puncture (CLP) model of septic peritonitis is the most widely studied model for septic shock, and the CLP model recapitulates the organ pathology seen in human patients. Unfortunately, immune-based intervention strategies developed using the CLP model have failed to translate to success in human patients (22, 50, 53) and in one case actually increased mortality in the treatment group (42). This raises the possibility that while CLP can replicate the downstream organ damage and dysfunction, it may not accurately reproduce the early immunological events that take place in most septic patients. Since septic shock is a condition that arises from many different types of infections, especially respiratory, urologic, bacteremic, and abdominal/gastrointestinal infections (11, 39, 46, 55), it is possible that many infection models will be required to fully understand the early immunological events that precipitate downstream organ damage and dysfunction in patients. For example, it has been shown that loss of TLR9 in the CLP model improves survival (52); however, in the B. hermsii infection system, it is a loss of NOD2, but not of TLR9, that results in improved survival. Although these two infection systems may both reproduce the pathophysiology of septic shock, differences in the bacterial composition, the routes of bacterial exposure, and the duration of the infections could all help explain the contrasting roles described for TLR9 in these two model systems. This highlights the potential vulnerability of using one infection system to study a condition as complex as septic shock. Therefore, alternative models of sepsis, such as the B. hermsii infection in TLR2−/− mice, may provide crucial insights into the pathological immune responses taking place in septic patients. The B. hermsii model system is a defined bacterial infection system, involves inflammation in response to PAMPs that are conserved across bacterial genera, and utilizes a microorganism that is specifically adapted for colonization of the vascular compartment, making it an excellent model for the study of immune responses during overwhelming bacteremia.
Considering that NOD2 has been shown to play a significant role in defense against a wide range of bacterial infections (23, 35) and has been shown to be important in augmenting TLR-mediated inflammation in response to bacterial ligands, particularly after the induction of TLR tolerance (37), it is understandable that the loss of NOD2 could significantly lessen inflammation in a bacterial infection system. Although TLR2−/− NOD2−/− cells are impaired in their ability to respond, they still exhibit a dose-dependent cytokine response to B. hermsii. In addition, while TLR2−/− NOD2−/− mice show improved survival of the initial wave of bacteremia and have lower BUN levels at 4 days postinfection compared to TLR2−/− mice, these mice become moribund by 2 to 3 weeks postinfection. These findings indicate that NOD2 is not the only intracellular sensor involved in the induction of sepsis in our TLR2−/− mouse model and suggest that other intracellular sensors play a significant role in the induction of pathophysiological changes in this model system. It has recently been shown that cytoplasmic sensors, such as the NALP1, NALP3, and AIM2 inflammasomes, can recognize and induce inflammation in response to widely conserved PAMPs, including bacterial nucleic acids and structural components of peptidoglycan (57). The relevance of these inflammasomes during immune responses in sepsis is still largely unknown.
In conclusion, the present study demonstrates that TLR2 is critical for protective immunity to B. hermsii and that B. hermsii infection in TLR2−/− mice represents a novel model of septic shock. Although a role for NOD2 has previously been described in defense against a wide range of bacterial infections, as well as inflammatory disorders such as Crohn's disease (24, 35, 45, 60), our work strongly indicates that NOD2 and other intracellular sensors can contribute to death in a nonperitonitis model of septic shock.
Acknowledgments
We thank Tim Manser for critical reading of the manuscript and David Horn for stimulating discussions. We thank Shizuo Akira for providing TLR2−/− and TLR9−/− mice.
This study was supported by the U.S. National Institutes of Health (RO1 AI065750 to K.R.A.).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Abraham, E., and M. Singer. 2007. Mechanisms of sepsis-induced organ dysfunction. Crit. Care Med. 35:2408-2416. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 3.Alugupalli, K. R. 2008. A distinct role for B1b lymphocytes in T-cell-independent immunity. Curr. Top. Microbiol. Immunol. 319:105-130. [DOI] [PubMed] [Google Scholar]
- 4.Alugupalli, K. R., S. Akira, E. Lien, and J. M. Leong. 2007. MyD88- and Bruton's tyrosine kinase-mediated signals are essential for T-cell-independent pathogen-specific IgM responses. J. Immunol. 178:3740-3749. [DOI] [PubMed] [Google Scholar]
- 5.Alugupalli, K. R., R. M. Gerstein, J. Chen, E. Szomolanyi-Tsuda, R. T. Woodland, and J. M. Leong. 2003. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 170:3819-3827. [DOI] [PubMed] [Google Scholar]
- 6.Alugupalli, K. R., J. M. Leong, R. T. Woodland, M. Muramatsu, T. Honjo, and R. M. Gerstein. 2004. B1b lymphocytes confer T-cell-independent long-lasting immunity. Immunity 21:379-390. [DOI] [PubMed] [Google Scholar]
- 7.Alugupalli, K. R., A. D. Michelson, M. R. Barnard, D. Robbins, J. Coburn, E. K. Baker, M. H. Ginsberg, T. G. Schwan, and J. M. Leong. 2001. Platelet activation by a relapsing fever spirochete results in enhanced bacterium-platelet interaction via integrin αIIbβ3 activation. Mol. Microbiol. 39:330-340. [DOI] [PubMed] [Google Scholar]
- 8.Alugupalli, K. R., A. D. Michelson, I. Joris, T. G. Schwan, K. Hodivala-Dilke, R. O. Hynes, and J. M. Leong. 2003. Spirochete-platelet attachment and thrombocytopenia in murine relapsing fever borreliosis. Blood 102:2843-2850. [DOI] [PubMed] [Google Scholar]
- 9.Angus, D. C., W. T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M. R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303-1310. [DOI] [PubMed] [Google Scholar]
- 10.Ansel, K. M., R. B. Harris, and J. G. Cyster. 2002. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16:67-76. [DOI] [PubMed] [Google Scholar]
- 11.Bagshaw, S. M., S. Lapinsky, S. Dial, Y. Arabi, P. Dodek, G. Wood, P. Ellis, J. Guzman, J. Marshall, J. E. Parrillo, Y. Skrobik, and A. Kumar. 2009. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 35:871-881. [DOI] [PubMed] [Google Scholar]
- 12.Balasz, M., F. Martin, T. Zhou, and J. F. Kearney. 2002. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17:341-352. [DOI] [PubMed] [Google Scholar]
- 13.Barbour, A. G. 1990. Antigenic variation of a relapsing fever Borrelia species. Annu. Rev. Microbiol. 44:155-171. [DOI] [PubMed] [Google Scholar]
- 14.Barbour, A. G., and V. Bundoc. 2001. In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype-specific immunoglobulin M antibodies. Infect. Immun. 69:1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernasconi, N. L., N. Onai, and A. Lanzavecchia. 2003. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood 101:4500-4504. [DOI] [PubMed] [Google Scholar]
- 16.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, J. H. Weis, T. G. Schwan, and J. J. Weis. 2006. Dual role of MyD88 in rapid clearance of relapsing fever Borrelia spp. Infect. Immun. 74:6750-6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadavid, D., and A. G. Barbour. 1998. Neuroborreliosis during relapsing fever: review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin. Infect. Dis. 26:151-164. [DOI] [PubMed] [Google Scholar]
- 18.Colombo, M. J., and K. R. Alugupalli. 2008. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J. Immunol. 180:4858-4864. [DOI] [PubMed] [Google Scholar]
- 19.Connolly, S. E., and J. L. Benach. 2005. The versatile roles of antibodies in Borrelia infections. Nat. Rev. Microbiol. 3:411-420. [DOI] [PubMed] [Google Scholar]
- 20.Dear, J. W., H. Yasuda, X. Hu, S. Hieny, P. S. Yuen, S. M. Hewitt, A. Sher, and R. A. Star. 2006. Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int. 69:832-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efron, P. A., K. Tinsley, D. J. Minnich, V. Monterroso, J. Wagner, P. Lainee, K. Lorre, P. E. Swanson, R. Hotchkiss, and L. L. Moldawer. 2004. Increased lymphoid tissue apoptosis in baboons with bacteremic shock. Shock 21:566-571. [DOI] [PubMed] [Google Scholar]
- 22.Fisher, C. J., Jr., J. F. Dhainaut, S. M. Opal, J. P. Pribble, R. A. Balk, G. J. Slotman, T. J. Iberti, E. C. Rackow, M. J. Shapiro, R. L. Greenman, et al. 1994. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, double-blind, placebo-controlled trial. JAMA 271:1836-1843. [PubMed] [Google Scholar]
- 23.Franchi, L., J. H. Park, M. H. Shaw, N. Marina-Garcia, G. Chen, Y. G. Kim, and G. Nuñez. 2008. Intracellular NOD-like receptors in innate immunity, infection, and disease. Cell Microbiol. 10:1-8. [DOI] [PubMed] [Google Scholar]
- 24.Fritz, J. H., R. L. Ferrero, D. J. Philpott, and S. E. Girardin. 2006. Nod-like proteins in immunity, inflammation, and disease. Nat. Immunol. 7:1250-1257. [DOI] [PubMed] [Google Scholar]
- 25.Gebbia, J. A., J. C. Monco, J. L. Degen, T. H. Bugge, and J. L. Benach. 1999. The plasminogen activation system enhances brain and heart invasion in murine relapsing fever borreliosis. J. Clin. Invest. 103:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelderblom, H., D. Londono, Y. Bai, E. S. Cabral, J. Quandt, R. Hornung, R. Martin, A. Marques, and D. Cadavid. 2007. High production of CXCL13 in blood and brain during persistent infection with the relapsing fever spirochete Borrelia turicatae. J. Neuropathol Exp. Neurol. 66:208-217. [DOI] [PubMed] [Google Scholar]
- 27.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zahringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 28.Girardin, S. E., L. H. Travassos, M. Herve, D. Blanot, I. G. Boneca, D. J. Philpott, P. J. Sansonetti, and D. Mengin-Lecreulx. 2003. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278:41702-41708. [DOI] [PubMed] [Google Scholar]
- 29.Gururajan, M., J. Jacob, and B. Pulendran. 2007. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One 2:e863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ha, S. A., M. Tsuji, K. Suzuki, B. Meek, N. Yasuda, T. Kaisho, and S. Fagarasan. 2006. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 203:2541-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiramatsu, M., R. S. Hotchkiss, I. E. Karl, and T. G. Buchman. 1997. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock 7:247-253. [DOI] [PubMed] [Google Scholar]
- 32.Hotchkiss, R. S., P. E. Swanson, J. P. Cobb, A. Jacobson, T. G. Buchman, and I. E. Karl. 1997. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit. Care Med. 25:1298-1307. [DOI] [PubMed] [Google Scholar]
- 33.Hotchkiss, R. S., P. E. Swanson, B. D. Freeman, K. W. Tinsley, J. P. Cobb, G. M. Matuschak, T. G. Buchman, and I. E. Karl. 1999. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 27:1230-1251. [DOI] [PubMed] [Google Scholar]
- 34.Hotchkiss, R. S., K. W. Tinsley, P. E. Swanson, R. E. Schmieg, Jr., J. J. Hui, K. C. Chang, D. F. Osborne, B. D. Freeman, J. P. Cobb, T. G. Buchman, and I. E. Karl. 2001. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 166:6952-6963. [DOI] [PubMed] [Google Scholar]
- 35.Inohara, N., M. Chamaillard, C. McDonald, and G. Nuñez. 2005. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74:355-383. [DOI] [PubMed] [Google Scholar]
- 36.Kawai, T., and S. Akira. 2009. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21:317-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim, Y. G., J. H. Park, M. H. Shaw, L. Franchi, N. Inohara, and G. Nuñez. 2008. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity 28:246-257. [DOI] [PubMed] [Google Scholar]
- 38.Klaviter, E. C., and R. C. Johnson. 1979. Isolation of the outer envelope, chemical components, and ultrastructure of Borrelia hermsii grown in vitro. Acta Trop. 36:123-131. [PubMed] [Google Scholar]
- 39.Kumar, A., D. Roberts, K. E. Wood, B. Light, J. E. Parrillo, S. Sharma, R. Suppes, D. Feinstein, S. Zanotti, L. Taiberg, D. Gurka, A. Kumar, and M. Cheang. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34:1589-1596. [DOI] [PubMed] [Google Scholar]
- 40.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 41.Leadbetter, E. A., I. R. Rifkin, A. M. Hohlbaum, B. C. Beaudette, M. J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416:603-607. [DOI] [PubMed] [Google Scholar]
- 42.Lopez, A., J. A. Lorente, J. Steingrub, J. Bakker, A. McLuckie, S. Willatts, M. Brockway, A. Anzueto, L. Holzapfel, D. Breen, M. S. Silverman, J. Takala, J. Donaldson, C. Arneson, G. Grove, S. Grossman, and R. Grover. 2004. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit. Care Med. 32:21-30. [DOI] [PubMed] [Google Scholar]
- 43.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. U. S. A. 100:1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin, F., A. M. Oliver, and J. F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617-629. [DOI] [PubMed] [Google Scholar]
- 45.Meylan, E., J. Tschopp, and M. Karin. 2006. Intracellular pattern recognition receptors in the host response. Nature 442:39-44. [DOI] [PubMed] [Google Scholar]
- 46.Mikkelsen, M. E., A. N. Miltiades, D. F. Gaieski, M. Goyal, B. D. Fuchs, C. V. Shah, S. L. Bellamy, and J. D. Christie. 2009. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit. Care Med. 37:1670-1677. [DOI] [PubMed] [Google Scholar]
- 47.Miyaji, T., X. Hu, P. S. Yuen, Y. Muramatsu, S. Iyer, S. M. Hewitt, and R. A. Star. 2003. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 64:1620-1631. [DOI] [PubMed] [Google Scholar]
- 48.Nilsen, N., U. Nonstad, N. Khan, C. F. Knetter, S. Akira, A. Sundan, T. Espevik, and E. Lien. 2004. Lipopolysaccharide and double-stranded RNA up-regulate Toll-like receptor two independently of myeloid differentiation factor 88. J. Biol. Chem. 279:39727-39735. [DOI] [PubMed] [Google Scholar]
- 49.Okada, T., and J. G. Cyster. 2006. B-cell migration and interactions in the early phase of antibody responses. Curr. Opin. Immunol. 18:278-285. [DOI] [PubMed] [Google Scholar]
- 50.Opal, S. M., C. J. Fisher, Jr., J. F. Dhainaut, J. L. Vincent, R. Brase, S. F. Lowry, J. C. Sadoff, G. J. Slotman, H. Levy, R. A. Balk, M. P. Shelly, J. P. Pribble, J. F. LaBrecque, J. Lookabaugh, H. Donovan, H. Dubin, R. Baughman, J. Norman, E. DeMaria, K. Matzel, E. Abraham, and M. Seneff. 1997. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit. Care Med. 25:1115-1124. [DOI] [PubMed] [Google Scholar]
- 51.Osuchowski, M. F., K. Welch, J. Siddiqui, and D. G. Remick. 2006. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 177:1967-1974. [DOI] [PubMed] [Google Scholar]
- 52.Plitas, G., B. M. Burt, H. M. Nguyen, Z. M. Bamboat, and R. P. DeMatteo. 2008. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J. Exp. Med. 205:1277-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reinhart, K., and W. Karzai. 2001. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit. Care Med. 29:S121-125. [DOI] [PubMed] [Google Scholar]
- 54.Riedemann, N. C., R. F. Guo, and P. A. Ward. 2003. The enigma of sepsis. J. Clin. Invest. 112:460-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rivers, E., B. Nguyen, S. Havstad, J. Ressler, A. Muzzin, B. Knoblich, E. Peterson, and M. Tomlanovich. 2001. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 345:1368-1377. [DOI] [PubMed] [Google Scholar]
- 56.Rupprecht, T. A., C. J. Kirschning, B. Popp, S. Kastenbauer, V. Fingerle, H. W. Pfister, and U. Koedel. 2007. Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2. Infect. Immun. 75:4351-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroder, K., and J. Tschopp. The inflammasomes. Cell 140:821-832. [DOI] [PubMed]
- 58.Schwan, T. G., and B. J. Hinnebusch. 1998. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science 280:1938-1940. [DOI] [PubMed] [Google Scholar]
- 59.Southern, P. M., Jr., and J. P. Sanford. 1969. Relapsing fever: a clinical and microbiological review. Medicine 48:129-149. [Google Scholar]
- 60.Strober, W., P. J. Murray, A. Kitani, and T. Watanabe. 2006. Signaling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 6:9-20. [DOI] [PubMed] [Google Scholar]
- 61.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe, T., A. Kitani, P. J. Murray, and W. Strober. 2004. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat. Immunol. 5:800-808. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe, T., A. Kitani, P. J. Murray, Y. Wakatsuki, I. J. Fuss, and W. Strober. 2006. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity 25:473-485. [DOI] [PubMed] [Google Scholar]
- 64.Yasuda, H., P. S. Yuen, X. Hu, H. Zhou, and R. A. Star. 2006. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 69:1535-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang, D., G. Zhang, M. S. Hayden, M. B. Greenblatt, C. Bussey, R. A. Flavell, and S. Ghosh. 2004. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science 303:1522-1526. [DOI] [PubMed] [Google Scholar]