Abstract
Subtilase cytotoxin (SubAB) was first isolated from a Shiga toxigenic Escherichia coli (STEC) strain that was responsible for an outbreak of hemolytic-uremic syndrome and is the prototype of a new family of AB5 cytotoxins. SubAB is a subtilase-like serine protease, and upon uptake by host cells, it is trafficked to the endoplasmic reticulum (ER), where it cleaves the essential ER chaperone BiP (GRP78) with high specificity. Previous work has shown that BiP cleavage by SubAB initiates ER stress-signaling pathways in host cells that eventuate in cell death associated with DNA fragmentation, a hallmark of apoptosis. The present study has investigated the role of the Bcl-2 protein family, which has been shown to regulate ER stress-induced apoptosis in other model systems. Examination of the cytotoxicity of SubAB for wild-type and bax−/−/bak−/− mouse embryonic fibroblasts and comparison of apoptotic markers in these cells revealed that SubAB cytotoxicity can be predominantly attributed to the activation of apoptotic pathways activated by Bax/Bak. The results of the present study further our understanding of the molecular mechanism whereby SubAB kills eukaryotic cells and contributes to STEC pathogenesis, in addition to consolidating the roles of Bcl-2 family members in the regulation of ER stress-induced apoptosis.
Shiga toxigenic Escherichia coli (STEC) is responsible for severe gastrointestinal disease in humans (23). Complications arising from STEC infection include hemorrhagic colitis (HC) and hemolytic-uremic syndrome (HUS), a life-threatening condition characterized by microangiopathic hemolytic anemia, thrombocytopenia, and renal failure (23). The cytotoxic activity of Shiga toxins (Stxs) expressed by STEC strains has long been considered to be predominantly responsible for the pathologies observed in HUS (23). However, recently, a novel subtilase cytotoxin (SubAB) was isolated from an STEC strain that was responsible for an outbreak of HUS and that is the prototype of a new family of AB5 cytotoxins (22). In vitro studies have demonstrated that SubAB is more toxic for Vero cells than Stxs, and intraperitoneal injection of mice with purified SubAB is lethal and induces pathological features resembling Stx-induced HUS (22, 31). Importantly, SubAB caused extensive microvascular thrombosis and other histological damage in the brain, kidneys, liver, and spleen that coincided with toxin-induced apoptosis, evident by in situ detection of DNA fragmentation by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) (31).
The A subunit (SubA) is a subtilase-like serine protease (22), and mutagenesis of the critical active-site Ser272 residue in SubA abolishes SubAB toxicity (22, 30). Upon uptake by host cells, SubAB is trafficked to the endoplasmic reticulum (ER), where SubA cleaves the essential ER chaperone BiP (GRP78) with high specificity (3, 21). BiP is a master regulator of ER homeostasis, and during ER stress BiP initiates a signaling pathway known as the unfolded protein response (UPR). This implements a series of changes in cellular activity in an attempt to alleviate ER stress (6, 10), including targeted upregulation of ER chaperones (including BiP), proteasome-dependent ER-associated degradation of unfolded proteins, global inhibition of protein translation, and cell-cycle arrest (2). BiP regulates the UPR via its interaction with three distinct ER membrane-spanning signaling molecules, protein kinase R (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) (2, 6, 10). Each of these proteins interacts with BiP via the luminal domain, and accumulation of unfolded proteins in the ER lumen is thought to titrate BiP away, initiating UPR signaling (2, 6, 10). Ultimately, the UPR is designed so that the cell is able to adapt and survive or is eliminated via apoptosis (2). The induction of ER-stress-induced apoptosis has been shown in many model systems to be regulated by the Bcl-2 family of proteins (9, 26, 29).
The Bcl-2 family of proteins comprises up to four Bc1-2 homology domains (BH1 to BH4), and the proteins are classified into two major groups (34): the antiapoptotic members (e.g., Bcl-2 and Bcl-X) and the proapoptotic members, which include both multidomain proteins (e.g., Bak and Bax) and BH3-only proteins (e.g., Bim and Puma) (34). According to current models, upon activation, Bax and Bak homo-oligomerize into protein-permeable channels in organelle membranes (including mitochondrial and ER membranes), facilitating membrane permeabilization and the release of apoptogenic factors into the cytoplasm that activate initiator caspases, thereby triggering the caspase cascade (1, 8, 27, 32). Although the mechanism for the activation of Bax and Bak is highly controversial, with several models being proposed, their activation appears to be regulated via their interactions with antiapoptotic and proapoptotic Bcl-2 family members, such that prosurvival family members bind Bax and Bak, preventing oligomerization, while BH3-only proteins activate Bax/Bak either directly, by binding to them, or indirectly, by inhibiting prosurvival members (4, 5, 7, 14, 34). The UPR signaling pathways have been shown to link to cell death pathways mediated by the Bcl-2 family via various mechanisms (17). For example, activated IRE1 has been shown to directly facilitate Bax/Bak oligomerization at the ER membrane; and CHOP, a transcription factor that is upregulated during the UPR by the ATF6 and PERK pathways, has been implicated in the transcriptional regulation of both Bcl-2 and Bim (11, 19, 24).
Previous results have demonstrated that SubAB induces activation of ATF6 as well as rapid induction of the PERK and IRE1 pathways (33). The speed of activation of the last two pathways suggested that BiP cleavage by SubAB may trigger the immediate dissociation of BiP from its cognate signaling molecules, without the necessity for accumulation of unfolded proteins in the ER lumen, as is observed in other models of ER stress. BiP cleavage by SubAB ultimately leads to cell death, associated with cytochrome c release, caspase activation, and DNA fragmentation, all of which are hallmarks of apoptosis (18, 20, 33). However, the host factors regulating SubAB-induced apoptosis have yet to be identified, and the extent to which apoptosis plays a role in SubAB-induced cell death has not been examined. The present study has investigated the consequences of activation of ER stress-signaling pathways in SubAB-treated cells with the aim of identifying host cell determinants of toxin-induced apoptosis. In particular, we have examined the role of Bcl-2 family proteins, which have been shown to regulate apoptosis not only in other models of ER stress but also in response to a range of cytotoxic agents (9, 29).
MATERIALS AND METHODS
Toxin purification.
SubAB and its nontoxic derivative SubAA272B were purified as described previously (22, 30).
Cell culture and toxin treatment.
Vero (African Green monkey kidney) cells and mouse embryonic fibroblasts (MEFs) were routinely grown in Dulbecco modified Eagle medium with 5% fetal calf serum, 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate at 37°C. Cells were seeded into appropriately sized tissue culture plates, and confluent monolayers were exposed to either SubAB or SubAA272B at the indicated concentrations. Mouse embryonic fibroblasts (wild type [wt], bax−/−/bak−/−, and bim−/−/puma−/−) were obtained from Andreas Strasser and David Huang (Walter and Eliza Hall Institute, Melbourne, Australia).
Western immunoblotting.
After treatment, the culture medium was aspirated, and cells were washed with cold phosphate-buffered saline (PBS) and then lysed directly in boiling sample buffer (50 mM Tris-HCl, pH 6.8, 50 mM dithiothreitol, 1% SDS, 0.005% bromophenol blue, 10% glycerol) and immediately boiled for 5 min. Cell extracts were separated by SDS-PAGE, electroblotted onto an Immobilon PVDF-SQ membrane (Millipore), and incubated overnight with primary antibodies. Detection involved using appropriate horseradish peroxidase-linked secondary antibodies and chemiluminescent substrate (Sigma). The membranes were then reprobed with loading control antibodies, as necessary. Anti-BiP C-20 (catalog no. SC-1051) was obtained from Santa Cruz Biotechnology. Anti-Bak (catalog no. 1542-1), anti-Bax (catalog no. 1063-1), and anti-Bcl-2 (catalog no. 1017-1) were obtained from Epitomics. Anti-Bim (C34C5) (catalog no. 2933), anti-poly-(ADP-ribose) polymerase (anti-PARP; catalog no. 9542), mouse antisurvivin (6E4) (catalog no. 2802) (used for Vero cells), and rabbit antisurvivin (71G4B7) (catalog no. 2808) (used for MEFs) were from Cell Signaling. Anti-Puma (catalog no. P-4618) and anti-β-actin (AC-74) (catalog no. A-5316) were from Sigma. Images were captured using a Kodak 4000MM image station (Carestream Molecular Imaging), and net band intensities (mean pixel intensity by number of pixels) were determined using MI imaging software (Carestream Molecular Imaging). Net band intensities were normalized to those for the loading controls, and fold changes in protein levels were determined by comparison to the level for the untreated controls.
PS detection.
Translocation of phosphatidylserine (PS) from the inner to the outer leaflet of the plasma membrane, an early marker of apoptosis, was detected using a Vybrant apoptosis assay kit (Invitrogen). PS on the surface of apoptotic cells was labeled with annexin V conjugated to fluorescent Alexa 488 dye and measured by flow cytometry. Propidium iodide, which is unable to permeate live cells and early apoptotic cells, was used concurrently to distinguish late apoptotic/necrotic cells. Annexin V labeling was measured using a FACScanto flow cytometer (Becton Dickinson). A total of 50,000 events were examined in two independent experiments.
DNA fragmentation analysis.
For quantitative, in situ detection of DNA fragmentation, cells were fixed sequentially with 1% formaldehyde and then 70% ethanol, followed by TUNEL using an APO-BrdU kit (Invitrogen), as described by the manufacturer. Incorporated bromodeoxyuridine (BrdU) was labeled with anti-BrdU-Alexa 488 dye and measured using a FACScanto flow cytometer. A total of 20,000 events were examined in two independent experiments.
Cytotoxicity assay.
Cytotoxicity was measured using a colorimetric crystal violet retention assay, performed as described by Kueng et al. (15), with minor modifications. Briefly, cells were grown to 80% confluence in 96-well trays. After treatment, cell monolayers were then washed in PBS (to remove dead cells) and fixed with 70% ethanol. Cells were stained with 0.4% crystal violet and then washed with PBS. Crystal violet was solubilized in 33% acetic acid, and the absorbance at 570 nm measured using a spectrophotometer. Each sample was assayed in quadruplicate.
RESULTS
Cellular levels of proapoptotic Bcl-2 family members in response to SubAB.
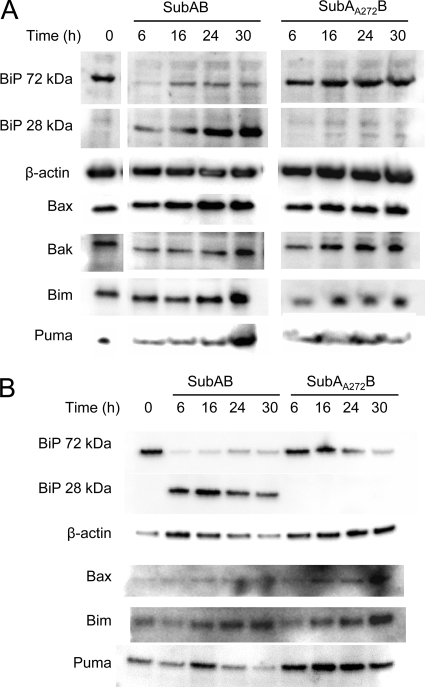
A diverse range of ER stress-inducing stimuli has been shown to induce expression of proapoptotic Bcl-2 family members while decreasing expression of antiapoptotic members (9). Accordingly, we treated Vero cells with SubAB (100 ng/ml) or the active-site mutant SubAA272B for up to 30 h and examined the cellular levels of various Bcl-2 family members by Western immunoblotting of whole-cell lysates (Fig. 1A). SubAB-mediated BiP cleavage was clearly evident after 6 h, with the appearance of a 28-kDa cleavage product and a corresponding substantial decrease in the intact 72-kDa species. Indeed, in previous studies we have shown that at the dose of toxin used here, BiP cleavage is almost complete within 1 h (21). However, from 16 to 30 h posttreatment, steady increases in the levels of both the 28-kDa and 72-kDa species were observed in the present study, consistent with upregulation of BiP transcription accompanied by continued SubAB-mediated cleavage, as previously reported (33). There was no decrease in intact 72-kDa BiP in cells treated with SubAA272B relative to the amount in untreated controls, nor was any cleaved 28-kDa species detectable (Fig. 1A). Quantitative analysis of the band intensities of the proapoptotic protein Bax (normalized to the β-actin band intensity) from two independent experiments did not reveal statistically significant increases in expression in SubAB-treated cells relative to that in untreated cells (mean ratios ± standard deviations [SDs], 1.5 ± 0.6, 2.0 ± 1.2, and 1.55 ± 1.1 at 16, 24, and 30 h, respectively) (Fig. 1A). A similar result was obtained for the BH3-only proapoptotic protein Puma (expression ratios in SubAB-treated versus untreated cells ranged from 2.2 ± 1.2 at 6 h to 1.6 ± 0.8 at 30 h, and none of these were statistically significant). Furthermore, for both proteins, there was no statistically significant difference between the expression levels observed for SubAB-treated cells and those observed for SubAA272B-treated cells at the comparable time points (maximal relative expression levels in the latter at 30 h, 1.4 ± 0.4 for Bax and 2.25 ± 2.0 for Puma). Quantitative analysis of the levels of the BH3-only protein Bim and the proapoptotic protein Bak indicated that there was no significant induction of either protein in either SubAB- or SubAA272B-treated cells at any time point (mean expression ratios relative to those for untreated cells were within the ranges of 0.9 to 1.15 and 0.7 to 1.15, respectively). Thus, there were no significant changes in the level of any of the Bcl-2 proteins examined that could be attributed to SubAB-dependent BiP cleavage and ER stress.
FIG. 1.
Effect of SubAB on the levels of Bcl-2 protein family members. Vero cells (A) or MEFs (B) were treated with either SubAB (100 ng/ml) or SubAA272B (100 ng/ml) for the indicated times, and lysates were analyzed by Western blotting. β-Actin was used as an internal loading control, and BiP cleavage was used as a control for toxin activity. Data are representative of those from two independent experiments.
SubAB-induced apoptosis requires Bax/Bak but not BH3-only proteins Bim and Puma.
Of course, the absence of SubAB-dependent changes in the total levels of expression of Bcl-2 family members does not eliminate a central role for these proteins in toxin-mediated apoptosis. To examine this definitively, we sought to compare the induction of apoptosis in wt, bax−/−/bak−/−, and bim−/−/puma−/− cells. These knockouts were not available in the Vero cell background, and so we used MEFs, which have similar susceptibility to SubAB as Vero cells (33). Treatment of wt MEFs with SubAB or SubAA272B yielded results, in terms of toxin-dependent degradation of BiP, similar to those observed for Vero cells (Fig. 1B). Also, there were no clear effects on the total levels of the Bcl-2 family members that were tested (Fig. 1B).
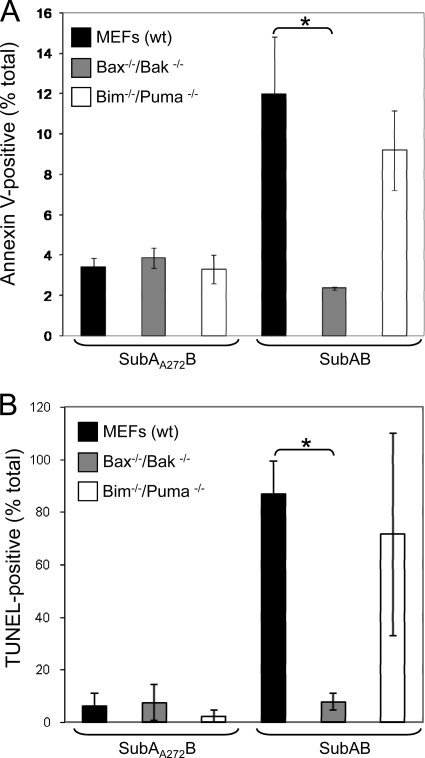
wt, bax−/−/bak−/−, and bim−/−/puma−/− MEFs were treated with SubAB, and the relative level of apoptosis in each cell line was measured using quantitative flow cytometric analysis of either surface-exposed phosphatidylserine (by annexin V labeling) at 24 h or in situ DNA fragmentation (using the TUNEL assay) at 30 h. Different time points were selected, because annexin V and TUNEL detect early and late apoptosis, respectively. At 24 h, 12.0% ± 2.8% of SubAB-treated wt MEFs were annexin V positive, whereas 3.4% ± 0.4% of SubAA272B-treated cells were annexin V positive (Fig. 2A). Notably, bax−/−/bak−/− cells that were treated with SubAB showed a significant reduction in annexin V labeling compared to the level for wt MEFs, with only 2.4% ± 0.1% being positive (P < 0.05). In contrast, there was no significant difference in the extent of annexin V labeling between wt and bim−/−/ puma−/− MEFs. As expected, there was no difference in annexin V labeling between any of the cell lines following treatment with SubAA272B. At 30 h posttreatment with SubAB, DNA fragmentation was clearly evident in wt MEFs, with 86.8% ± 12.6% of cells being TUNEL positive but only 6.05% ± 4.9% of wt cells treated with SubAA272B being positive (Fig. 2B). In accordance with the annexin V data, bax−/−/ bak−/− cells also demonstrated a significant reduction in TUNEL compared to that for wt MEFs, with only 7.7% ± 3.3% of cells being TUNEL positive (P < 0.05), which was comparable to that for cells treated with SubAA272B. The level of TUNEL in SubAB-treated bim−/−/puma−/− cells was not significantly different from that in wt MEFs. Thus, SubAB-induced apoptosis is dependent on the proteolytic activity of the toxin, as well as host proteins Bax/Bak (but not Bim/Puma).
FIG. 2.
Quantitative analysis of SubAB-induced apoptosis in wt, bax−/−/bak−/−, and bim−/−/puma−/− MEFs. (A) In situ labeling of phosphatidylserine on the surface of apoptotic cells. Cells were treated with either SubAB (100 ng/ml) or SubAA272B (100 ng/ml) for 24 h. Phosphatidylserine was labeled with annexin V conjugated to Alexa 488 dye. (B) In situ TUNEL of DNA fragmentation in MEFs that were treated with either SubAB (100 ng/ml) or SubAA272B (100 ng/ml) for 30 h. Data represent the means ± SDs from two independent experiments. *, P < 0.05, Student's unpaired, two-tailed t test.
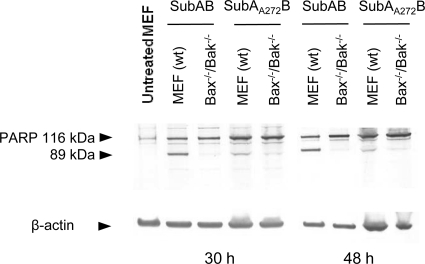
SubAB induces Bax/Bak-dependent PARP cleavage in MEFs.
PARP is a 116-kDa nuclear enzyme that is cleaved during apoptosis into 89- and 24-kDa fragments in a caspase-dependent process. We therefore examined PARP cleavage as a further measure of apoptosis, in addition to annexin V labeling and TUNEL. Cells were treated with SubAB or SubAA272B for 30 or 48 h, and whole-cell lysates were examined by SDS-PAGE and Western immunoblotting with anti-PARP antibodies. An increase in the amount of the 89-kDa PARP fragment was observed in SubAB-treated wt MEFs compared to the amount in the SubAA272B control, indicative of caspase-dependent processing of full-length PARP (Fig. 3). Significantly, PARP cleavage at 30 h also correlated with DNA fragmentation (Fig. 2B), both of which are caspase-dependent processes. Notably, the proteolytic profile of PARP in bax−/−/bak−/− MEFs differed from that of PARP in the wt, with no detectable increase in the 89-kDa fragment upon treatment with SubAB being noted, consistent with the absence of caspase-mediated cleavage. These data indicate that Bax/Bak lie upstream of caspase activation in the apoptotic pathway. There was a small amount of cleaved (89-kDa) PARP detectable at both 30 and 48 h in SubAA272B-treated wt MEFs which was not seen in untreated wt MEFs (Fig. 3). The active-site mutation has previously been shown to reduce the proteolytic activity and cytotoxicity of SubAB by >99.9%, but it does not completely abolish it (22). Thus, although cleavage of BiP was not detected in mutant toxin-treated MEFs (Fig. 1B), vestigial cytotoxicity may have contributed to the baseline (also Bax/Bak-dependent) PARP cleavage.
FIG. 3.
PARP processing in wt and bax−/−/bak−/− MEFs treated with SubAB. Cells were treated with either SubAB (100 ng/ml) or SubAA272B (100 ng/ml) for 30 h or 48 h, and lysates were analyzed by Western blotting with anti-PARP antibodies. β-Actin was used as an internal loading control. The mobilities of intact (116-kDa) and cleaved (89-kDa) PARP are indicated. Data are representative of those from two independent experiments.
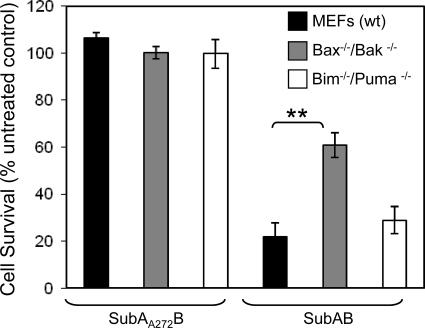
SubAB cytotoxicity is predominantly dependent on apoptotic pathways regulated by Bax/Bak.
In order to evaluate the extent to which Bax/Bak-dependent apoptosis contributes to SubAB-induced cell death, we examined the relative cytotoxicity of SubAB for wt MEFs and bax−/−/bak−/− MEFs. Cytotoxicity was measured using a colorimetric crystal violet retention assay. Upon treatment with SubAB for 48 h, bax−/−/bak−/− MEFs demonstrated a significant increase in the rate of survival compared to the rate for wt MEFs (61% and 22%, respectively; P < 0.001) (Fig. 4), whereas the rates of cell survival were comparable between bim−/−/puma−/− and wt MEFs. These data suggest that at least 50% of SubAB cytotoxicity is attributable to Bax/Bak-dependent apoptotic pathways.
FIG. 4.
Relative cytotoxicity of SubAB for wt, bax−/−/bak−/−, and bim−/−/puma−/− MEFs. Cells were treated with either SubAB (100 ng/ml) or SubAA272B (100 ng/ml) for 48 h, and cytotoxicity was measured using crystal violet staining. Data are expressed as percent cell survival compared to the rate of survival for untreated control cells. Data represent the means ± standard errors of the means from four independent experiments, **, P < 0.01, Student's unpaired, two-tailed t test.
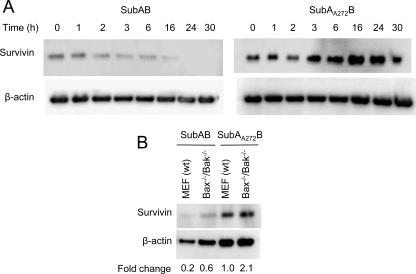
SubAB-induced decreases in cellular levels of survivin.
SubAB has been shown to trigger cell-cycle arrest at G1 phase in Vero cells in a manner dependent on the host factors PERK and cyclin D1 (20). The mitotic regulator survivin is essential for proper chromosome segregation and cytokinesis and has previously been implicated in ER stress-induced apoptosis (16, 28). In order to determine if survivin may also play a role in SubAB-induced cell-cycle arrest, survivin protein levels in SubAB-treated Vero cells were examined over a time course of 30 h. A marked reduction in survivin levels was apparent at 2 to 3 h in cells treated with SubAB compared to the levels in those treated with SubAA272B. The survivin levels in SubAB-treated cells continued to decrease thereafter, and the protein was undetectable at 24 to 30 h (Fig. 5). Both wt and bax−/−/bak−/− MEFs also displayed a marked SubAB-dependent decrease in survivin protein levels at 48 h (densitometric analysis indicated 80% and 72% reductions, respectively).
FIG. 5.
Effect of SubAB on survivin protein levels. (A) Vero cells were treated with either SubAB (100 ng/ml) or SubAA272B (100 ng/ml) for 0 to 30 h, and lysates were analyzed by Western blotting with the appropriate antisurvivin antibody (see Materials and Methods). β-Actin was used as an internal loading control. (B) wt and bax−/−/bak−/− MEFs were treated with either SubAB (100 ng/ml) or SubAA272B (100 ng/ml) for 48 h, and lysates were analyzed by Western blotting with the appropriate antisurvivin antibody. β-Actin was used as an internal loading control. The fold change in survivin levels relative to the level in SubAA272B-treated wt MEFs was calculated from relative band intensities normalized to the band intensity for the β-actin loading controls (see Materials and Methods).
DISCUSSION
In a step toward characterizing the molecular basis for SubAB-induced cell death, we examined the role of the Bcl-2 protein family, the members of which have been shown to regulate ER stress-induced apoptosis in other model systems (9, 24, 26, 29). Although the mechanism whereby BH3-only proteins of the Bcl-2 family regulate the initiation of apoptosis is highly contentious, experimental evidence suggests that they can interact with antiapoptotic members to prevent them from inhibiting Bax/Bak oligomerization (5). Unlike other BH3-only members that exhibit specificity toward particular prosurvival Bcl-2 family members, Bim and Puma have been shown to interact with all prosurvival members, making them potent activators of apoptosis (7). Consequently, we examined the effect of SubAB on bim−/−/puma−/− MEFs to determine if these proteins play a role in SubAB-induced apoptosis, but we observed no significant reduction in the level of apoptotic response or cytotoxicity. These data cannot exclude the possibility that other BH3-only proteins may be compensating for the absence of Bim and Puma in our system. Nevertheless, our results indicate that although Bim and Puma may contribute to SubAB-induced cell death, they are not essential.
On the other hand, our data clearly demonstrate the importance of Bax/Bak in apoptotic cell death mediated by the SubAB toxin. A deficiency in these proteins reduced the presentation of both early and late apoptotic markers. Moreover, this decrease in SubAB-induced apoptosis corresponded to a significant (50%) decrease in overall cytotoxicity. This suggests that a major proportion of the cell death induced by SubAB can be attributed to the induction of Bax/Bak-dependent apoptotic pathways. In a previous study, BiP upregulation was shown to be necessary to inhibit Bax activation and chemotherapeutic drug resistance (25). Therefore, it is tempting to speculate that SubAB-mediated decreases in BiP levels directly predispose cells to Bax-dependent apoptosis.
We also examined the cellular levels of the Bim, Puma, Bax, and Bak proteins in response to SubAB treatment, since proapoptotic Bcl-2 family members have been shown to be upregulated in response to ER stress in some systems (9). However, none of the proteins that we examined were significantly induced, suggesting that in our system other factors (e.g., posttranslational modifications) are more important than expression levels for regulating the activities of these proteins. Although we have clearly shown that Bax and Bak are critical for SubAB-induced apoptosis, we have yet to determine how Bax/Bak are regulated in our system. Indeed, to date, a definitive and unequivocal mechanism for their activation in any system remains elusive.
It is important to note that even though cell survival after SubAB treatment was significantly greater in Bax/Bak-knockout MEFs than in wt MEFs, survival was still only about 60% of that in untreated or SubAA272B-treated cells. This indicates the existence of alternative (Bax/Bak-independent) mechanisms of cell death. In this instance, the absence of any apoptotic markers suggests that nonapoptotic pathways of cell death are likely involved. Indeed, a recent study demonstrated that the ER stress-inducing chemical thapsigargin induces necrotic cell death in Bax/Bak-deficient MEFs (12). Similar to the findings in this study for SubAB, thapsigargin was shown to elicit PARP cleavage in wt MEFs but not in Bak/Bax-deficient MEFs (12). In that study, activated intact PARP was shown to mediate depletion of energetic pools of ATP, resulting in cellular dysfunction and subsequent necrosis during ER stress (12). It is possible that a similar PARP-mediated, necrotic cell death pathway may also be compensating for the absence of Bax/Bak in our system. In support of this hypothesis, we also observed a similar reduction in intact PARP in wt but not Bax/Bak-deficient cells.
Previous work has shown that SubAB induces cell-cycle arrest in G1 phase as a result of downregulation of cyclin D1 (20), and we have now shown that SubAB treatment also results in decreased expression of the mitotic regulator survivin. Emerging data indicate that cell-cycle control is intimately linked to the regulation of cell death. Moreover, there is clear evidence to suggest that Bcl-2 family proteins are involved in regulating the interplay between these pathways (13, 35). Further investigation of the role of Bcl-2 family members in SubAB-induced apoptosis and cell-cycle arrest will provide invaluable insight into the interaction between these pathways.
Acknowledgments
We are grateful to Andreas Strasser and David Huang for providing wt and gene-knockout mouse embryonic fibroblast lines.
This research was supported by program grant 565526 from the National Health and Medical Research Council (NHMRC) of Australia and RO1 grant AI-068715 from the National Institutes of Health. J.C.P. is an NHMRC Australia Fellow.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 16 August 2010.
REFERENCES
- 1.Antonsson, B., S. Montessuit, B. Sanchez, and J. C. Martinou. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276:11615-11623. [DOI] [PubMed] [Google Scholar]
- 2.Boyce, M., and J. Yuan. 2006. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 13:363-373. [DOI] [PubMed] [Google Scholar]
- 3.Chong, D. C., J. C. Paton, C. M. Thorpe, and A. W. Paton. 2008. Clathrin-dependent trafficking of subtilase cytotoxin, a novel AB5 toxin that targets the ER chaperone BiP. Cell. Microbiol. 10:795-806. [DOI] [PubMed] [Google Scholar]
- 4.Czabotar, P. E., P. M. Colman, and D. C. Huang. 2009. Bax activation by Bim? Cell Death Differ. 16:1187-1191. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher, J. I., and D. C. Huang. 2008. Controlling the cell death mediators Bax and Bak: puzzles and conundrums. Cell Cycle 7:39-44. [DOI] [PubMed] [Google Scholar]
- 6.Gething, M. J. 1999. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 10:465-472. [DOI] [PubMed] [Google Scholar]
- 7.Giam, M., D. C. Huang, and P. Bouillet. 2008. BH3-only proteins and their roles in programmed cell death. Oncogene 27:S128-S136. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths, G. J., L. Dubrez, C. P. Morgan, N. A. Jones, J. Whitehouse, B. M. Corfe, C. Dive, and J. A. Hickman. 1999. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 144:903-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath-Engel, H. M., N. C. Chang, and G. C. Shore. 2008. The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene 27:6419-6433. [DOI] [PubMed] [Google Scholar]
- 10.Hendershot, L. M. 2004. The ER function BiP is a master regulator of ER function. Mt. Sinai J. Med. 71:289-297. [PubMed] [Google Scholar]
- 11.Hetz, C., P. Bernasconi, J. Fisher, A. H. Lee, M. C. Bassik, B. Antonsson, G. S. Brandt, N. N. Iwakoshi, A. Schinzel, L. H. Glimcher, and S. J. Korsmeyer. 2006. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312:572-576. [DOI] [PubMed] [Google Scholar]
- 12.Janssen, K., S. Horn, M. T. Niemann, P. T. Daniel, K. Schulze-Osthoff, and U. Fischer. 2009. Inhibition of the ER Ca2+ pump forces multidrug-resistant cells deficient in Bak and Bax into necrosis. J. Cell Sci. 122:4481-4491. [DOI] [PubMed] [Google Scholar]
- 13.Janumyan, Y., Q. Cui, L. Yan, C. G. Sansam, M. Valentin, and E. Yang. 2008. G0 function of BCL2 and BCL-xL requires BAX, BAK, and p27 phosphorylation by Mirk, revealing a novel role of BAX and BAK in quiescence regulation. J. Biol. Chem. 283:34108-34120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, H., H. C. Tu, D. Ren, O. Takeuchi, J. R. Jeffers, G. P. Zambetti, J. J. Hsieh, and E. H. Cheng. 2009. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell 36:487-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kueng, W., E. Silber, and U. Eppenberger. 1989. Quantification of cells cultured on 96-well plates. Anal. Biochem. 182:16-19. [DOI] [PubMed] [Google Scholar]
- 16.Lensa, S., G. Vadera, and R. Medemaa. 2006. The case for Survivin as mitotic regulator. Curr. Opin. Cell Biol. 18:616-622. [DOI] [PubMed] [Google Scholar]
- 17.Li, J., B. Lee, and A. S. Lee. 2006. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J. Biol. Chem. 281:7260-7270. [DOI] [PubMed] [Google Scholar]
- 18.Matsuura, G., N. Morinaga, K. Yahiro, R. Komine, J. Moss, H. Yoshida, and M. Noda. 2009. Novel subtilase cytotoxin produced by Shiga-toxigenic Escherichia coli induces apoptosis in Vero cells via mitochondrial membrane damage. Infect. Immun. 77:2919-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullough, K. D., J. L. Martindale, L. O. Klotz, T. Y. Aw, and N. J. Holbrook. 2001. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 21:1249-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morinaga, N., K. Yahiro, G. Matsuura, J. Moss, and M. Noda. 2008. Subtilase cytotoxin, produced by Shiga-toxigenic Escherichia coli, transiently inhibits protein synthesis of Vero cells via degradation of BiP and induces cell cycle arrest at G1 by downregulation of cyclin D1. Cell. Microbiol. 10:921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paton, A. W., T. Beddoe, C. M. Thorpe, J. C. Whisstock, M. C. Wilce, J. Rossjohn, U. M. Talbot, and J. C. Paton. 2006. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443:548-552. [DOI] [PubMed] [Google Scholar]
- 22.Paton, A. W., P. Srimanote, U. M. Talbot, H. Wang, and J. C. Paton. 2004. A new family of potent AB5 cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 200:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puthalakath, H., L. A. O'Reilly, P. Gunn, L. Lee, P. N. Kelly, N. D. Huntington, P. D. Hughes, E. M. Michalak, J. McKimm-Breschkin, N. Motoyama, T. Gotoh, S. Akira, P. Bouillet, and A. Strasser. 2007. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129:1337-1349. [DOI] [PubMed] [Google Scholar]
- 25.Ranganathan, A. C., L. Zhang, A. P. Adam, and J. A. Aguirre-Ghiso. 2006. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 66:1702-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao, R. V., H. M. Ellerby, and D. E. Bredesen. 2004. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 11:372-380. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-Vela, A., J. T. Opferman, E. H. Cheng, and S. J. Korsmeyer. 2005. Proapoptotic BAX and BAK control multiple initiator caspases. EMBO Rep. 6:379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohn, J., V. I. Khaoustov, Q. Xie, C. C. Chung, B. Krishnan, and B. Yoffe. 2003. The effect of ursodeoxycholic acid on the survivin in thapsigargin-induced apoptosis. Cancer Lett. 191:83-92. [DOI] [PubMed] [Google Scholar]
- 29.Szegezdi, E., S. E. Logue, A. M. Gorman, and A. Samali. 2006. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7:880-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talbot, U. M., J. C. Paton, and A. W. Paton. 2005. Protective immunization of mice with an active-site mutant of subtilase cytotoxin of Shiga toxin-producing Escherichia coli. Infect. Immun. 73:4432-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, H., J. C. Paton, and A. W. Paton. 2007. Pathological changes in mice induced by subtilase cytotoxin, an emerging Escherichia coli AB5 toxin that targets the endoplasmic reticulum. J. Infect. Dis. 196:1093-1101. [DOI] [PubMed] [Google Scholar]
- 32.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Pro-apoptotic Bax and Bak: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfson, J. J., K. L. May, C. M. Thorpe, D. M. Jandhyala, J. C. Paton, and A. W. Paton. 2008. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell. Microbiol. 10:1775-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youle, R. J., and A. Strasser. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9:47-59. [DOI] [PubMed] [Google Scholar]
- 35.Zinkel, S., A. Gross, and E. Yang. 2006. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 13:1351-1359. [DOI] [PubMed] [Google Scholar]