Abstract
Whipple's disease is a chronic multisystemic infection caused by Tropheryma whipplei that is characterized by arthritis, weight loss, and diarrhea. The immunological defects in the duodenal mucosa, the site of major replication of the agent underlying the pathogenesis of Whipple's disease, are poorly understood. Mucosal immunoglobulins are essential for the defense against intestinal pathogens; therefore, we analyzed the B-cell response in duodenal specimens and sera of Whipple's disease patients. Whereas systemic immunoglobulin production was affected only marginally, duodenal biopsy specimens of Whipple's disease patients contained reduced numbers of immunoglobulin-positive plasma cells and secreted less immunoglobulin compared to healthy controls but showed a weak secretory IgA response toward T. whipplei. This T. whipplei-specific intestinal immune response was not observed in controls. Thus, we were able to demonstrate that general mucosal immunoglobulin production in Whipple's disease patients is impaired. However, this deficiency does not completely abolish T. whipplei-specific secretory IgA production that nonetheless does not protect from chronic infection.
Whipple's disease is a chronic multisystemic infection caused by Tropheryma whipplei (15, 18). The major clinical manifestations of Whipple's disease are arthritis, weight loss, and diarrhea that resolve upon antimicrobial treatment (15).
Immunoglobulins, especially IgA, are a major defense mechanism against infections at mucosal surfaces. IgA is secreted into the gut lumen, where it binds infectious agents, thereby blocking their adhesion and entry into the mucosa (9). Moreover, IgA is able to eliminate pathogens from the lamina propria and even binds viral proteins before assembly in the epithelial cells (8).
The local immunological defects underlying the pathogenesis of Whipple's disease are poorly understood, and data on mucosal humoral immunity are lacking. We have shown that in the duodenum of Whipple's disease patients the numbers of T cells and unspecific and T. whipplei-specific Th1 reactivity are reduced compared to healthy controls, and that macrophages are alternatively activated (3, 10, 13, 14). Thus, secretory immunity may be also impaired, since Th1 cells play a crucial role in mucosal B-cell differentiation (9). In addition, T. whipplei acquires a glycoproteic biofilm that might inhibit the specific humoral immune response (1). Indeed, in serum samples of Whipple's disease patients, reduced total IgG2 (11) and T. whipplei-specific IgG and IgM have been detected, whereas T. whipplei-specific IgA is enhanced compared to healthy persons (2).
However, nothing is known about the quantitative production of immunoglobulins and the specificity of secretory IgA in the duodenal mucosa of Whipple's disease patients, the site of major replication of the pathogen. Thus, we quantified systemic and duodenal production of immunoglobulins and determined T. whipplei-specific secretory IgA in the supernatants of short-term cultured duodenal biopsy specimens to characterize mucosal B-cell immunity in Whipple's disease.
MATERIALS AND METHODS
Patients.
We studied specimens from a total of 82 Whipple's disease patients (17 women, 65 men; median age, 57.0 years [range, 41 to 83 years]). Most of the patients (n = 77) were treated for 14 days with intravenous ceftriaxone or meropenem, followed by oral sulfamethoxazole/trimethoprim; only five patients were treated with other regimens (two with cotrim only, one with doxycycline only, one with doxycycline followed by ceftriaxone and cotrim, and one with various antimicrobials finally followed by recombinant gamma interferon). Sera were collected from 69 patients (15 women, 54 men; median age, 57.0 years [range, 41 to 83 years]) and stored at −70°C until assay. Biopsy specimens were collected from 27 patients (1 woman, 26 men; median age, 59.0 years [range, 41 to 83 years]) undergoing upper endoscopy for diagnosis or therapy control. Sera were collected from 32 healthy subjects (13 women, 19 men; median age, 49.0 years [range, 27 to 75 years]). Biopsy specimens were obtained by endoscopy from 31 control subjects (6 women, 25 men; median age, 51.5 years [range, 32 to 81 years]) who had no visible abnormalities at histological examination. This study was approved by the local ethics committee, and all participants gave written consent.
Immunohistochemistry.
Immunostaining on paraffin sections was performed as previously described in duodenal biopsy specimens from 21 Whipple's disease patients (2 untreated and 13 patients during/after treatment and 6 patients matched before and during/after treatment) and 10 healthy subjects (14). The primary antibodies were mouse anti-human-plasma cell (clone MUM1p; Dako, Hamburg, Germany) and rabbit anti-human-IgA, IgG, and IgM (all from Biozol, Eiching, Germany). Stains were visualized by using donkey anti-mouse or donkey anti-rabbit biotin (Dianova, Hamburg, Germany), streptavidin-alkaline phosphatase (Dako), and Fast red (Dako). Counts of plasma cells and immunoglobulin-positive cells were determined from three biopsy specimens per sampling as the mean cell count of 10 high-power fields (hpf) of 0.237 mm2 each.
Short-term culture of intestinal biopsy specimens.
Culture supernatants of duodenal biopsy specimens were prepared as described below and stored at −70°C until assay (14, 16). Briefly, biopsy specimens were immediately placed in phosphate-buffered saline (PBS), washed, weighed, and incubated in RPMI 1640 medium (Gibco-BRL, Berlin, Germany) containing 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2.5 μg of amphotericin/ml (all from Seromed Biochrom KG, Berlin, Germany) without further additives for 48 h at 37°C in a humidified 5% CO2-70% O2 air atmosphere. Supernatants were stored at −70°C until assay.
Quantification of immunoglobulins.
IgG1, IgG2, IgG4, IgA, and IgM were quantified with a cytometric bead array (BD, San Diego, CA) according to the manufacturer's protocol from biopsy specimen supernatants from 22 healthy subjects and 23 Whipple's disease patients (17 during/after treatment and 6 matched before and during/after treatment) and sera from 32 healthy subjects and 69 Whipple's disease patients (27 before treatment, 26 during/after treatment, and 16 matched before and during/after treatment).
Western blot analysis.
T. whipplei-specific secretory IgA was determined by using Western blot analyses from biopsy specimen supernatants of nine treated Whipple's disease patients and eight healthy subjects. The total levels of T. whipplei-specific IgA and IgG from sera and biopsy specimen supernatants were determined and served as a positive control for the Western blots. Protein separation and blotting of native T. whipplei lysates was performed as described previously (5). Blots were blocked overnight at 4°C with PBS containing 0.1% Tween (PBST) and 5% dry milk, followed by 1 h of incubation at room temperature with biopsy specimen supernatants or sera. IgG and IgA was detected with peroxidase-coupled mouse anti-human IgA (clone GA112) and IgG (clone HP6017), respectively (both from Invitrogen, Karlsruhe, Germany). Secretory IgA was detected with mouse anti-human secretory IgA (clone SC-05; Acris, Herford, Germany) and goat anti-mouse peroxidase (Dianova). Blots were developed for 5 min at room temperature with ECL substrate (Sigma-Aldrich, Germany) and exposed to films. Supernatants and antibodies were diluted with PBST containing 2% dry milk, and blots were washed with PBST.
Statistical analysis.
Quantitative parameters are presented as medians and standard deviations and as individual data points with medians and interquartile ranges, respectively. The data were analyzed with the GraphPad Prism4 software package by means of the Mann-Whitney test or Fisher test. P values of < 0.05 were considered significant.
RESULTS
Immunoglobulins in sera.
In sera, the concentrations of IgA, IgG1, and IgG4 were similar in treated and untreated Whipple's disease patients and controls (data not shown). IgG2 was similar in the sera of treated Whipple's disease patients (46.6 ± 11.5 ng/ml) and healthy subjects (51.2 ± 9.2 ng/ml) and reduced in untreated patients (44.4 ± 9.9 ng/ml) compared to healthy subjects (P = 0.0009 [Mann-Whitney test]). The concentration of IgM was 9.5 ± 5.0 ng/ml for healthy subjects and was enhanced in both untreated (14.4 ± 6.3 ng/ml, P = 0.0003 [Mann-Whitney test]) and treated (12.7 ± 7.8 ng/ml, P = 0.032 [Mann-Whitney test]) Whipple's disease patients.
Plasma cells in duodenal biopsy specimens.
The total numbers of plasma cells were 81.9 × 10.5 cells/hpf in biopsy specimens of the duodenal mucosa of healthy subjects. The total numbers of plasma cells were reduced in the biopsy specimens of untreated (54.5 ± 21.4 cells/hpf, P = 0.0001 [Mann-Whitney test]) and comparable to healthy controls in treated Whipple's disease patients (78.2 ± 20.5 cells/hpf). The total numbers of IgA+ (Fig. 1), IgG+ (data not shown), and IgM+ (data not shown) plasma cells were reduced in the duodenal mucosa of untreated and treated Whipple's disease patients compared to healthy controls.
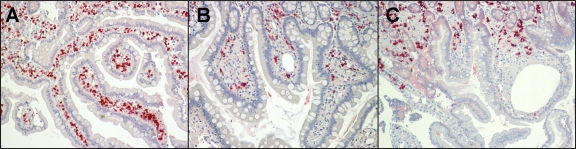
FIG. 1.
Immunoglobulin production in duodenal biopsy specimens of Whipple's disease patients compared to healthy subjects, as evidenced by exemplary immunostaining of IgA (colored in red). (A) High number of IgA+ plasma cells in a healthy control; (B and C) low number of IgA+ plasma cells in a Whipple's disease patient before the start of treatment (B) and in the same Whipple's disease patient after 6 months of antimicrobial treatment (C).
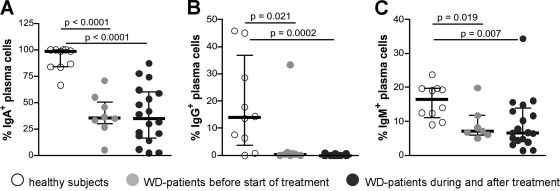
The relative frequencies of immunoglobulin-positive plasma cells were determined to exclude the possibility that the reduced absolute number of plasma cells positive for the respective immunoglobulins just reflects a diminished total number of all plasma cells. This also illustrated that the relative frequency of IgA+, IgG+, and IgM+ cells among all plasma cells was reduced in untreated and treated Whipple's disease patients compared to healthy subjects (Fig. 2).
FIG. 2.
Percentage of immunoglobulin-producing plasma cells among all plasma cells in duodenal biopsy specimens from Whipple's disease (WD) patients and healthy controls as determined by immunohistochemistry. (A) Percentage of IgA+ plasma cells; (B) percentage of IgG+ plasma cells; (C) percentage of IgM+ plasma cells. Individual values, medians, and interquartile ranges are shown, and P values were determined by using the Mann-Whitney test.
Immunoglobulins in biopsy specimen supernatants.
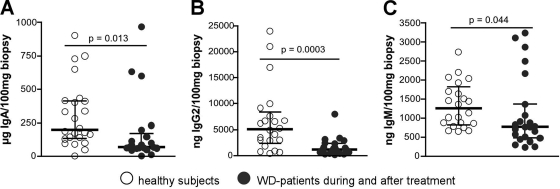
The concentration of IgA, IgG2, and IgM was reduced in biopsy specimen supernatants of treated Whipple's disease patients (Fig. 3); the concentration of IgG4 was also reduced (data not shown), while the concentration of IgG1 was similar compared to healthy subjects (data not shown).
FIG. 3.
Immunoglobulin concentrations in supernatants of duodenal biopsy specimens obtained from healthy subjects and Whipple's disease patients during or after antimicrobial treatment. (A) IgA; (B) IgG2; (C) IgM. Individual values, medians, and interquartile ranges are shown, and P values were determined by using the Mann-Whitney test. WD, Whipple's disease.
Data on supernatants of duodenal biopsy specimens are only presented for treated Whipple's disease patients. The data of untreated Whipple's disease patients were similar compared to treated patients and healthy controls. However, this may be due to the low number of samples from untreated patients available (n = 6).
T. whipplei-specific secretory IgA in biopsy specimen supernatants.
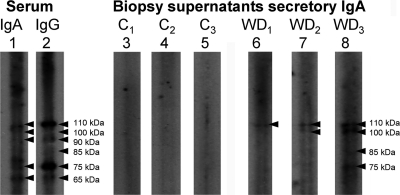
Secretory specific IgA against native T. whipplei was only detected in the supernatants of duodenal biopsy specimens in one of eight healthy subjects but was detected in all of nine Whipple's disease patients tested (P = 0.0004 [Fisher exact test]) (Fig. 4). In six patients only a 110-kDa T. whipplei antigen was reactive with the supernatant; in three patients additional bands of 100, 85, and 75 kDa, respectively, were detected. Overall, the immune reaction of biopsy specimen supernatants was quite weak compared to the reactivity of sera and not easy to detect (Fig. 4).
FIG. 4.
Western blot for T. whipplei-specific immunoglobulins. Exemplary Western blots of T. whipplei-specific IgA (lane 1) and IgG (lane 2) in the serum from a Whipple's disease (WD) patient (positive control), secretory IgA in biopsy specimen supernatants from three healthy controls (lanes 3 to 5), and secretory IgA in biopsy specimen supernatants from three Whipple's disease patients (lanes 6 to 8). Arrowheads indicate T. whipplei-antigens recognized by the respective supernatants and sera used as positive controls. C1 to C3, healthy subjects; WD1 to WD3, Whipple's disease patients.
DISCUSSION
Whipple's disease is characterized by a massive infiltration of T. whipplei in the duodenal mucosa. Despite the accumulation of the pathogen, evidence for duodenal inflammation is lacking (14), general Th1 reactivity is reduced (10), and T. whipplei-specific Th1 cells are absent in the peripheral blood and the duodenal mucosa (13). In contrast, a T. whipplei-specific systemic B-cell response can be detected (1, 2, 5). Whipple's disease is associated with the HLA-DRB1*13 and DQB1*06 genotypes (12) that preferentially might present antigenic epitopes to stimulate a humoral response instead of cellular immune reactions.
However, nothing is known of the specific secretory mucosal immune response, which is regarded more relevant for the defense against intestinal pathogens (8, 9). In the present study we found a discrete T. whipplei-specific secretory IgA response in supernatants of duodenal biopsy specimens from Whipple's disease patients that was not detected in healthy controls. Since no CD154 expression on CD4+ T cells can be detected in the peripheral blood following T. whipplei-specific stimulation (our unpublished data), not only T. whipplei-specific Th1 cells (13) but also Th cells specific for T. whipplei in general seem to be lacking in Whipple's disease patients (7). Thus, it remains unclear why a specific secretory immune response is possible despite the absence of T. whipplei-specific CD4+ Th cells in the peripheral blood. However, the secretory immune response was weak, and perhaps T. whipplei-specific T-cell help is provided in adjacent lymph nodes, at least in the initial phase of the infection, enabling the priming of T. whipplei-specific B cells so only the systemic spread of T. whipplei-specific Th cells is obviated. The presence of predominant Th2 cytokines (10) and of a clear systemic T. whipplei-specific humoral immunity (2, 5) support this idea. Alternatively, T. whipplei-specific T-cell help might be circumvented in Whipple's disease patients, resulting in only a sparse mucosal B-cell activation. Antigen presentation through HLA alleles associated with Whipple's disease (12) and alternatively activated macrophages might play a role (3, 14) and possibly, T. whipplei-specific secretory IgA can be produced independently of Th1 help as shown before for virus-specific secretory IgA in mice (6) and for salivary secretory IgA in children (17).
Immunoglobulins in the serum were marginally affected in Whipple's disease patients: the concentrations of only IgG2 were reduced, and the primary immunoglobulin IgM was enhanced in sera of both untreated and treated Whipple's disease patients compared to healthy controls. This indicates that initial humoral immune responses take place, whereas an impaired class switch might be a result of insufficient Th cell activity specific for T. whipplei or the cytokine milieu generated in the lymphoid tissues, where the priming of T. whipplei-specific B cells might occur.
In contrast to peripheral immunoglobulins, IgG2, IgG4, IgM, and IgA were secreted in reduced amounts by duodenal biopsy specimens of Whipple's disease patients compared to controls. This is in agreement with our immunohistological findings, which clearly show reduced absolute numbers and percentages of IgG+, IgM+, and IgA+ plasma cells in the duodena of Whipple's disease patients compared to healthy persons. This finding is also in accordance with a previous study, which showed reduced percentages of IgA+ plasma cells in duodenal biopsy specimens of Whipple's disease patients (4). Combined with the reduced amounts found for IgG2 in the sera of Whipple's disease patients, this is in line with the lack of T-cell help discussed above.
In conclusion, we were able to demonstrate that general mucosal immunoglobulin production in Whipple's disease patients is impaired but that this deficiency does not completely abolish T. whipplei-specific secretory IgA production. Nonetheless, secretory IgA production during Whipple's disease is not able to protect patients from chronic infection.
Acknowledgments
We thank Diana Bösel and Martina Seipel for technical assistance.
This study was supported by EU contract number QLG1-CT-2002-01049 and the German Research Foundation (DFG KFO104 and SFB633).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Bonhomme, C. J., P. Renesto, B. Desnues, E. Ghigo, H. Lepidi, P. Fourquet, F. Fenollar, B. Henrissat, J. L. Mege, and D. Raoult. 2009. Tropheryma whipplei glycosylation in the pathophysiologic profile of Whipple's disease. J. Infect. Dis. 199:1043-1052. [DOI] [PubMed] [Google Scholar]
- 2.Bonhomme, C. J., P. Renesto, S. Nandi, A. M. Lynn, and D. Raoult. 2008. Serological microarray for a paradoxical diagnostic of Whipple's disease. Eur. J. Clin. Microbiol. Infect. Dis. 27:959-968. [DOI] [PubMed] [Google Scholar]
- 3.Desnues, B., H. Lepidi, D. Raoult, and J. L. Mege. 2005. Whipple disease: intestinal infiltrating cells exhibit a transcriptional pattern of M2/alternatively activated macrophages. J. Infect. Dis. 192:1642-1646. [DOI] [PubMed] [Google Scholar]
- 4.Ectors, N., K. Geboes, R. De Vos, H. Heidbuchel, P. Rutgeerts, V. Desmet, and G. Vantrappen. 1992. Whipple's disease: a histological, immunocytochemical, and electron microscopic study of the immune response in the small intestinal mucosa. Histopathology 21:1-12. [DOI] [PubMed] [Google Scholar]
- 5.Fenollar, F., B. Amphoux, and D. Raoult. 2009. A paradoxical Tropheryma whipplei Western blot differentiates patients with Whipple disease from asymptomatic carriers. Clin. Infect. Dis. 49:717-723. [DOI] [PubMed] [Google Scholar]
- 6.Franco, M. A., and H. B. Greenberg. 1997. Immunity to rotavirus in T-cell-deficient mice. Virology 238:169-179. [DOI] [PubMed] [Google Scholar]
- 7.Frentsch, M., O. Arbach, D. Kirchhoff, B. Moewes, M. Worm, M. Rothe, A. Scheffold, and A. Thiel. 2005. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat. Med. 11:1118-1124. [DOI] [PubMed] [Google Scholar]
- 8.Lamm, M. E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51:311-340. [DOI] [PubMed] [Google Scholar]
- 9.Macpherson, A. J., K. D. McCoy, F. E. Johansen, and P. Brandtzaeg. 2008. The immune geography of IgA induction and function. Mucosal Immunol. 1:11-22. [DOI] [PubMed] [Google Scholar]
- 10.Marth, T., N. Kleen, A. Stallmach, S. Ring, S. Aziz, C. Schmidt, W. Strober, M. Zeitz, and T. Schneider. 2002. Dysregulated peripheral and mucosal Th1/Th2 response in Whipple's disease. Gastroenterology 123:1468-1477. [DOI] [PubMed] [Google Scholar]
- 11.Marth, T., M. Neurath, B. A. Cuccherini, and W. Strober. 1997. Defects of monocyte interleukin 12 production and humoral immunity in Whipple's disease. Gastroenterology 113:442-448. [DOI] [PubMed] [Google Scholar]
- 12.Martinetti, M., F. Biagi, C. Badulli, G. E. Feurle, C. Muller, V. Moos, T. Schneider, T. Marth, A. Marchese, L. Trotta, S. Sachetto, A. Pasi, A. De Silvestri, L. Salvaneschi, and G. R. Corazza. 2009. The HLA alleles DRB1*13 and DQB1*06 are associated to Whipple's disease. Gastroenterology 136:2289-2294. [DOI] [PubMed] [Google Scholar]
- 13.Moos, V., D. Kunkel, T. Marth, G. E. Feurle, B. LaScola, R. Ignatius, M. Zeitz, and T. Schneider. 2006. Reduced peripheral and mucosal Tropheryma whipplei-specific Th1 response in patients with Whipple's disease. J. Immunol. 177:2015-2022. [DOI] [PubMed] [Google Scholar]
- 14.Moos, V., C. Schmidt, A. Geelhaar, D. Kunkel, K. Allers, K. Schinnerling, C. Loddenkemper, F. Fenollar, A. Moter, D. Raoult, R. Ignatius, and T. Schneider. 2001. Impaired immune functions of monocytes and macrophages in Whipple's disease. Gastroenterology 138:210-220. [DOI] [PubMed] [Google Scholar]
- 15.Schneider, T., V. Moos, C. Loddenkemper, T. Marth, F. Fenollar, and D. Raoult. 2008. Whipple's disease: new aspects of pathogenesis and treatment. Lancet Infect. Dis. 8:179-190. [DOI] [PubMed] [Google Scholar]
- 16.Schneider, T., T. Zippel, W. Schmidt, G. Pauli, U. Wahnschaffe, S. Chakravarti, W. Heise, E. O. Riecken, M. Zeitz, and R. Ullrich. 1998. Increased immunoglobulin G production by short-term cultured duodenal biopsy samples from HIV-infected patients. Gut 42:357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinbrenner, M., R. Hafer, B. Gruhn, A. Muller, D. Fuchs, J. Hermann, and F. Zintl. 2005. T-cell independent production of salivary secretory IgA after hematopoietic stem cell transplantation in children. Oral Microbiol. Immunol. 20:282-288. [DOI] [PubMed] [Google Scholar]
- 18.Whipple, G. H. 1907. A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Bull. Johns Hopkins Hosp. 18:382-393. [Google Scholar]