Abstract
Mycoplasma bovis is a small, cell wall-less bacterium that contributes to a number of chronic inflammatory diseases in both dairy and feedlot cattle, including mastitis and bronchopneumonia. Numerous reports have implicated M. bovis in the activation of the immune system, while at the same time inhibiting immune cell proliferation. However, it is unknown whether the specific immune-cell population M. bovis is capable of attaching to and potentially invading. Here, we demonstrate that incubation of M. bovis Mb1 with bovine peripheral blood mononuclear cells (PBMC) resulted in a significant reduction in their proliferative responses while still remaining viable and capable of gamma interferon secretion. Furthermore, we show that M. bovis Mb1 can be found intracellularly (suggesting a role for either phagocytosis or attachment/invasion) in a number of select bovine PBMC populations (T cells, B cells, monocytes, γδ T cells, dendritic cells, NK cells, cytotoxic T cells, and T-helper cells), as well as red blood cells, albeit it at a significantly lower proportion. M. bovis Mb1 appeared to display three main patterns of intracellular staining: diffuse staining, an association with the intracellular side of the cell membrane, and punctate/vacuole-like staining. The invasion of circulating immune cells and erythrocytes could play an important role in disease pathogenesis by aiding the transport of M. bovis from the lungs to other sites.
Mycoplasma bovis is a small, pleomorphic cell wall-less bacterium that is known to be a major contributing factor in the development of chronic pneumonia in feedlot cattle and mastitis in dairy cows. In addition to these two diseases, M. bovis has been linked to the development of otitis, keratoconjunctivitis, and arthritis (12). These diseases have large economic impacts, resulting in losses to both beef and dairy industries in Europe, Canada, and the United States (20). Furthermore, since M. bovis lacks a cell wall, the use of antibiotics to combat infections is limited, and the development of resistance to available antibiotics (tetracyclines and spectinomycin) has been observed (20). Interestingly, infection with M. bovis has been implicated in the potential exacerbation and enhancement of respiratory disease to other pathogens since coinfections with Histophilus somnus, bovine viral diarrhea virus, Mannheimia haemolytica, bovine respiratory syncytial virus, bovine parainfluenza virus type 3 have been observed (3, 4, 16, 25). These findings suggest an important synergism in the development of disease during the coinfection of animals involving M. bovis and other pathogens.
A number of factors appear to play an important role in the virulence and development of disease during M. bovis infection, although the specific mechanisms involved in these processes are still incompletely understood. M. bovis lacks a specialized organelle for attachment, as seen in M. pneumoniae and M. genitalium (1, 6), but instead expresses variable surface proteins (Vsps) that play a critical role in its attachment (24). These membrane surface proteins undergo substantial antigenic variation involving high-frequency phenotypic switching, resulting in an increased ability of M. bovis to evade the host's immune system (13, 14, 21). Furthermore, M. bovis can suppress the immune system via a secreted 26-amino-acid peptide that is 84% homologous to the C-terminal end of the VspL protein (33). This peptide appears to take part in the downregulation of lymphocyte proliferation and thereby ameliorates an appropriate immune response by the host. Another mechanism of immune evasion may involve the ability of M. bovis to inhibit neutrophil oxidative burst by a mechanism that appears to involve protein kinase C signaling (29). M. bovis is also capable of surviving in the environment for an extended period of time via the production of a biofilm, although this biofilm does not appear to enhance its resistance to antibiotics but rather protects it from temperature changes and desiccation (17). Other factors that are believed to play an important role in virulence include the production of hydrogen peroxide and an inflammatory toxin that can result in an increase in vascular permeability and the activation of complement (8, 31, 34).
Numerous reports have examined both in vivo and in vitro infections with M. bovis; however, the mechanisms involved during an M. bovis infection have not been fully examined and still remain controversial. Some in vivo research suggests that M. bovis typically adheres to bronchiolar epithelial cell surfaces, localizing between the cells, but does not appear to migrate intracellularly (30). On the other hand, some studies suggest not only that M. bovis attaches to various cell types but also that it is found intracellularly in neutrophils, macrophages, and hepatocytes, whereas bronchiolar epithelial cells displayed positive staining during an M. bovis infection (7, 15, 26). Whether this occurs via an active process in neutrophils and macrophages involving M. bovis itself or a mechanism involving phagocytosis remains to be examined. Studies of other mycoplasmas such as M. gallisepticum and M. suis have demonstrated that they are capable of invading erythrocytes (9, 36), thereby evading the immune system. These studies, along with those of M. bovis, further suggest that mycoplasmas may spread systemically via invasion of peripheral blood mononuclear cells (PBMC) and erythrocytes, while at the same time evading immune responses.
Some studies suggest a role for M. bovis-induced activation of various T-cell populations (CD4+, CD8+, and γδ T cells) and the production of specific cytokines (gamma interferon [IFN-γ] and interleukin-4 [IL-4]) (34), tumor necrosis factor alpha, and nitric oxide from bovine macrophages (11). This is not surprising since various reports, including those referred to above, have implicated M. bovis in the modulation of immune responses in vivo and in vitro (28, 29). We demonstrate here that M. bovis Mb1 attaches to and invades bovine PBMC, inhibiting their proliferation, but does not appear to alter functional responses in terms of cytokine production, including IFN-γ in particular. M. bovis invades all of the PBMC types in a relatively short period of time, which could then potentially contribute to an overall suppression of lymphocyte proliferation and possibly spread from the lungs to other organs of the host.
MATERIALS AND METHODS
Bacterial strains and media.
M. bovis Mb1 was used for all experiments and was previously isolated from synovial joint fluid of a calf showing signs of arthritis (23). Cultures containing M. bovis Mb1 were grown in modified Hayflick medium at 37°C in a 5% CO2 atmosphere. The growth rate of the strain was monitored by viable counts of serial dilutions of liquid cultures plated on Hayflick medium containing 1.5% agar, followed by incubation at 37°C in a 5% CO2 atmosphere. Aliquots of the culture taken at the exponential phase of growth were collected, and bacterial cells were separated by centrifugation (5,500 × g for 15 min) and washed with minimum essential medium (MEM; Invitrogen, Burlington, Ontario, Canada). The cells were suspended in MEM containing 30% glycerol to a cell density of 108 CFU/ml and stored at −70°C until needed. Tissue culture medium (MEM containing 10% fetal bovine serum [FBS], 0.05 mM 2-mercaptoethanol, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids (NEAA), and 10 mM HEPES buffer) was used to incubate purified PBMC and erythrocytes (RBCs) for the invasion and intracellular survival assays.
Purification of blood cells.
Blood samples (20 ml from each animal) from 12 clinically healthy animals with no history of M. bovis were collected in Vacutainer tubes containing sodium EDTA. The cells were separated by centrifugation (2,500 × g for 20 min), and the area containing the PBMC (buffy coat) was removed and transferred to Ficoll gradients (GE Healthcare, Mississauga, Ontario, Canada). The PBMC were collected from the gradient, washed three times with PBSA (137 mM NaCl, 2.7 mM KCl, 7 mM Na3PO4, and 1.5 mM KH2PO4) containing EDTA and suspended in tissue culture medium (MEM) to 107 cells/ml. The bovine erythrocytes were purified by centrifugation (1,000 × g for 15 min), and the plasma and buffy coat were removed. The cells were washed three times with phosphate-buffered saline (PBS), followed by centrifugation (800 × g for 5 min). The cells were suspended in tissue culture medium to 107 cells/ml.
In vitro proliferation assays.
The proliferation of PBMC after stimulation with concanavalin A (ConA; Sigma-Aldrich, Oakville, Ontario, Canada) and/or M. bovis Mb1 was determined by seeding 96-well tissue culture plates at a concentration of 3 × 105 PBMC/well. Cells were incubated at 37°C in 5% CO2 in the presence of 1 μg of ConA/ml and/or M. bovis Mb1 (both live and heat-killed) at a multiplicity of infection (MOI) of 5:1 for 72 h in triplicate. A solution containing 0.4 μCi of [3H]thymidine (GE Healthcare)/well was added, and the cells were incubated for 18 h. The cells were harvested (Filtermate Harvester; Packard), the amount of incorporated [3H]thymidine was determined in a scintillation counter (Top Count NXT; Packard), and the stimulation index was determined by dividing the treated cell counts by the medium counts.
Apoptosis assays.
The role of M. bovis Mb1 in PBMC apoptosis was determined by the use of two commercially available apoptosis assay kits. The first kit examined annexin V staining (ApoTarget; Invitrogen) and was used to assess early apoptotic events. Briefly, PBMC were incubated with M. bovis Mb1 for 24 h (MOI of 5:1). Controls included untreated PBMC and PBMC treated with staurosporine (5 μM, 45 min). The cells were washed twice with PBS and suspended at 3 × 106 cells/ml in 1× annexin V binding buffer. The cells were then divided in 100-μl aliquots; then, 5-μl portions of the annexin V-fluorescein isothiocyanate (FITC) were added to each tube, and the tubes were kept in the dark at room temperature for 15 min. Finally, 400 μl of the annexin V binding buffer was added to each tube, and the cells were analyzed by flow cytometry within 1 h of staining. The second kit examined DNA fragmentation (FlowTACS; R&D Systems, Minneapolis, MN) and was used to assess late apoptotic events. Briefly, PBMC were incubated with M. bovis Mb1 for 72 h (MOI of 5:1). Controls included untreated PBMC and PBMC treated with staurosporine (5 μM, 45 min). PBMC were suspended at 106 cells/ml in 3.7% formaldehyde and fixed at room temperature for 10 min. The cells were collected by centrifugation at 1,500 × g for 5 min, the fixative was removed, and the pellet was suspended in 100 μl of cytonin and left at room temperature for 30 min. The cells were collected by centrifugation, and the cytonin was removed and washed with labeling buffer. The labeling reaction mixture (25 μl, TdT-deoxynucleoside triphosphate mix, 1× Mn2+, TdT, and 1× TdT labeling buffer) was added to each tube, followed by incubation at 37°C for 1 h. The reaction was stopped by using 1 ml of stop buffer, the cells were collected by centrifugation, and the supernatant was removed. Strep-fluorescein (25 ml) was added to each tube, and the cells were incubated for 10 min at room temperature, collected by centrifugation, and suspended in 500 μl of PBS. The cells were then analyzed by flow cytometry within 2 h of staining. In some cases propidium iodide (PI) was also added to the cells to further distinguish early and late apoptotic events.
Determination of IFN-γ secretion.
To determine the effect of M. bovis Mb1 on the ability of PBMC to produce IFN-γ (a general PBMC functionality test), enzyme-linked immunospot (ELISPOT) assays were conducted in triplicate. ELISPOT plates (Millipore HA plate) were coated overnight (24 h) at 4°C with an anti-bovine IFN-γ mouse monoclonal antibody (1:3,000 in sterile coating buffer). The plates were washed four times with sterile PBSA and blocked at 37°C for 2 h by using 1% albumin in PBSA. A suspension of purified PBMC (0.1 ml of 107 PBMC/ml) was incubated with 0.1 ml of an exponential-phase culture of Mb1 at Mb1/PBMC ratios of 0.1:1 and 1:1. Control wells included medium alone, ConA at 1 μg/ml, or ConA plus M. bovis Mb1. The PBMC-Mb1 mixes were incubated overnight at 37°C, and the plates washed with double-distilled H2O to lyse the cells. After these washes, the plates were incubated at room temperature for 2 h with a rabbit anti-bovine IFN-γ antibody (1:3,000 in 1% albumin-PBSA). After washes with PBST (0.1 M PBS plus 0.1% Tween 20), an alkaline phosphatase-conjugated goat anti-rabbit IgG antibody (1:1,500 in 1% albumin-PBSA) was added, followed by incubation for 2 h at room temperature. After this incubation, the plates were washed with PBST, the BCIP (5-bromo-4-chloro-3-indolylphosphate)/NBT substrate (Sigma-Aldrich) was added, and the cells secreting IFN-γ (spots) were developed for 30 min. The spots were then counted under a light microscope.
Cell invasion assays.
Aliquots of frozen M. bovis Mb1 (108 CFU/ml) were thawed on ice and coincubated with PBMC or erythrocytes (in MEM containing 10% FBS, 0.05 mM 2-mercaptoethanol, 1 mM sodium pyruvate, 0.1 mM NEAA, and 10 mM HEPES buffer) at an MOI of 5:1 (mycoplasma-PBMC) for 1, 2, 3, or 24 h. After these incubations, aliquots of the cultures were collected, serially diluted in PBS, and plated on Hayflick agar plates to determine the total number of bacteria. Aliquots were also collected to determine the viability of PBMC by a trypan-blue exclusion assay following counting on a Coulter counter. The rest of the cells in the cultures were collected by centrifugation at 800 × g for 5 min and washed with tissue culture medium. Extracellular M. bovis Mb1 was killed by incubation with medium containing 400 μg of gentamicin/ml for 3 h. This concentration was determined to be optimal for killing 100% of M. bovis Mb1 in this time frame. After the gentamicin treatment, cells were collected by centrifugation (800 × g for 5 min), washed, and suspended in culture medium, and aliquots were plated to detect the number of intracellular bacteria. Since we did not lyse the PBMC or RBCs before plating to recover M. bovis (since this would also lyse the bacterium), we were looking at the percent infected PBMC or RBCs and not at the percent Mb1 present inside the cells. Thus, in this assay, one colony on the plate represents one infected PBMC or RBCs. Numbers greater than 100% could be due to some PBMC or RBC cell lysis during the washing and plating steps.
Immunofluorescence.
Immunofluorescence assays were conducted to determine the specific cell type in the PBMC population that M. bovis Mb1 attaches to and/or invades. M. bovis Mb1 was labeled by using octadecyl rhodamine B chloride (R18; Sigma-Aldrich) as previously described (27). Briefly, M. bovis Mb1 from a 2-day culture was collected by centrifugation and incubated with 600 μg of R18/ml for 15 min in the dark. After a wash with MEM, the labeled M. bovis Mb1 were suspended in tissue culture medium, followed by incubation with PBMC (MOI of 0.5) for 2 and 24 h in four-well Lab-Tek Permanox chamber slides (VWR, Edmonton, Alberta, Canada). After the 2- and 24-h incubations, the medium was removed, and the cells were briefly washed with PBS. The cells were then fixed using 2% formaldehyde for 10 min on ice. The formaldehyde was removed, and the cells washed with PBS and blocked using 0.2% gelatin in PBS for 1 h at room temperature. After an overnight incubation at 4°C with mouse monoclonal antibodies (1:100 in PBS) against CD1b (dendritic cells), CD3 (T cells), CD4 (T helper cells), CD8 (cytotoxic T cells), CD14 (monocytes), CD21 (B cells), CD335 (NK cells), or TcR1-N24 (γδ T cells) (VMRD, Pullman, WA), the cells were washed with PBS and incubated at room temperature for 3 h with an FITC-conjugated goat anti-mouse secondary antibody (1:100 in PBS). The various secondary antibodies included FITC-conjugated anti-IgG1 (CD3, CD14, CD8, and CD335), anti-IgG2a (CD4, CD1b), anti-IgG2b (TcR1-N24), or anti-IgM (CD21) (Invitrogen). Controls consisted of using no primary antibody and an isotype control for each respective primary antibody. In some experiments, a third label was included to examine intracellular IFN-γ staining. The cells were permeabilized using a saponin-based buffer (Cytoperm) and incubated with a rabbit anti-bovine IFN-γ antibody for 3 h (1:100 in Cytoperm wash buffer). The cells were washed with the Cytoperm wash buffer and incubated with an Alexa 405-conjugated secondary antibody for 3 h (1:100 in Cytoperm wash buffer). The slides were then washed, and coverslips were mounted by using FluorSave (VWR) and allowed to dry overnight before imaging the next day using a confocal Leica TCS SP5 microscope.
Statistics.
Data are expressed as the median of the values, and the statistical analyses were conducted by using GraphPad Prism version 5 and Microsoft Office Excel 2007 software. Comparisons of two groups were made by using a Student t test for unpaired data. Comparisons of more than two groups were made by using a two-way analysis of analysis of variance with a post hoc Tukey test.
RESULTS
Invasion of bovine PBMC.
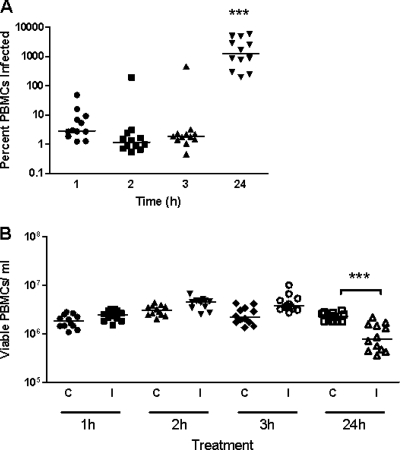
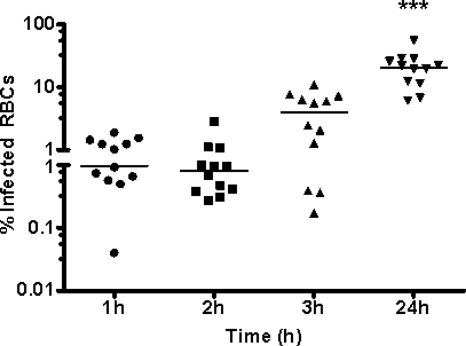
A number of previous studies have demonstrated an important role for M. bovis in the downregulation of proliferation of bovine PBMC in response to mitogens (5, 28, 33), although the specific subpopulation of PBMC affected was not identified. In addition, M. bovis appears to attach and in some cases to migrate intracellularly (or show very close association with the cell) by an unknown mechanism (7, 15). In neutrophils and macrophages, however, the process could potentially solely involve phagocytosis. To investigate whether the mechanism of inhibition of proliferative responses involves attachment or invasion of PBMC by the bacterium, we performed assays to determine whether all or a particular PBMC subpopulation is affected by M. bovis. Bovine PBMC from 12 animals were isolated as described in Materials and Methods and incubated with M. bovis Mb1 for different times, and the results are shown in Fig. 1. Within the first 1 h, M. bovis Mb1 began to invade PBMC, although the majority of the M. bovis Mb1 at this stage was still extracellular (Fig. 1A). After 24 h of incubation, the amount of intracellular M. bovis Mb1 increased significantly with respect to the 3-h values (Fig. 1A). However, in phagocytic cells (neutrophils and those of monocytic lineage, for example) it is unknown whether M. bovis Mb1 is actively taken up (phagocytosed) or whether the bacteria invade these particular cells by a mechanism independent of phagocytosis. To determine whether these PBMC were still viable, a trypan blue exclusion assay was conducted at various time points. The results indicated that the number of viable PBMC did not appear to be altered in control and infected cultures after 3 h of incubation, although at the 24-h time point there were significantly fewer viable M. bovis Mb1-infected PBMC compared to controls (Fig. 1B).
FIG. 1.
Invasion of bovine PBMC by M. bovis Mb1. Bovine PBMC from 12 beef cattle were incubated for various times (1, 2, 3, and 24 h) with M. bovis Mb1, and the percentage of M. bovis-infected PBMC was determined by a gentamicin resistance assay. (A) Viable M. bovis recovered. At 1, 2, and 3 h, M. bovis demonstrates a low-level invasion/infection of PBMC (between 5 and 10% of PBMC are infected). At 24 h, all PBMC are infected, and those with >100% infection are representative of more than 1 M. bovis organism per PBMC. The bars show the median of the values. After 24 h of incubation, the number of intracellular M. bovis was significantly higher (***, P < 0.001) than the 1-, 2-, and 3-h time points. (B) Viable bovine PBMC after incubation with M. bovis. C, control PBMC; I, PBMC infected with M. bovis. The bars show the median of the values. After 24 h of incubation with M. bovis, there was a significant decrease (***, P < 0.001) in PBMC viability.
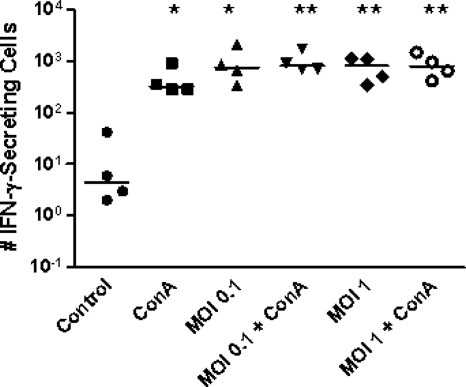
We also measured the ability of PBMC to secrete cytokines, an indication of their functional status, by detecting IFN-γ production by M. bovis Mb1-infected PBMC using an ELISPOT assay. The results are shown in Fig. 2. Incubation of PBMC for 24 h with M. bovis Mb1 at various Mb1/PBMC ratios (0.1:1 and 1:1) resulted in the significant upregulation of IFN-γ-secreting cells compared to the controls (Fig. 2). Interestingly, the addition of ConA (1 μg/ml) did not further enhance this response, suggesting that this particular response is neither synergistic nor additive (Fig. 2). As expected, incubation of bovine PBMC with ConA alone for 24 h resulted in an increase in the number of IFN-γ-secreting cells (Fig. 2).
FIG. 2.
M. bovis Mb1-induced IFN-γ secretion. Bovine PBMC were incubated with M. bovis Mb1 (MOIs of 0.1 and 1), and the number of IFN-γ-secreting cells was determined by an ELISPOT assay. M. bovis incubation with PBMC from four beef cattle resulted in a significant increase in the number of IFN-γ-secreting cells compared to the control. However, the response was not significantly enhanced by the combination of M. bovis and ConA. The bars show the median values. *, P < 0.05; **, P < 0.01.
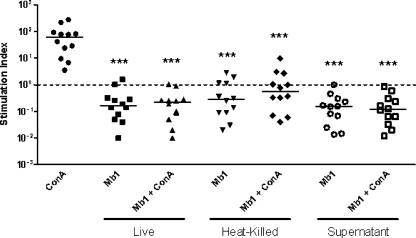
Incubation with live or heat-killed M. bovis Mb1 for 72 h did not induce PBMC proliferation and, in contrast, resulted in the significant attenuation of PBMC proliferative responses to ConA (Fig. 3). These findings suggest an important role for either live or dead M. bovis Mb1 in an enhanced impairment of PBMC proliferative responses. The incubation of M. bovis Mb1 supernatants with bovine PBMC also significantly reduced their proliferative responses compared to ConA, suggesting an important role for a secreted factor(s) (Fig. 3). As expected, treatment of PBMC for 72 h with ConA alone resulted in the significant upregulation of PBMC proliferation (Fig. 3). The invasion assays, together with the IFN-γ secretion and proliferation tests, indicate that M. bovis invades PBMC without affecting their viability in the first few hours postinfection, although at 24 h PBMC viability does appear to decrease slightly. These results also suggest that the intracellular presence of M. bovis Mb1 contributes to the impairment of PBMC proliferation.
FIG. 3.
M. bovis Mb1 inhibition of bovine PBMC proliferation. Compared to ConA, incubation of live, heat-killed, or culture supernatant of M. bovis Mb1 with bovine PBMC from 12 beef cattle with or without the addition of ConA resulted in the significant attenuation (***, P < 0.001) of PBMC proliferation. The dotted line shows the baseline level, while the bars show the median values.
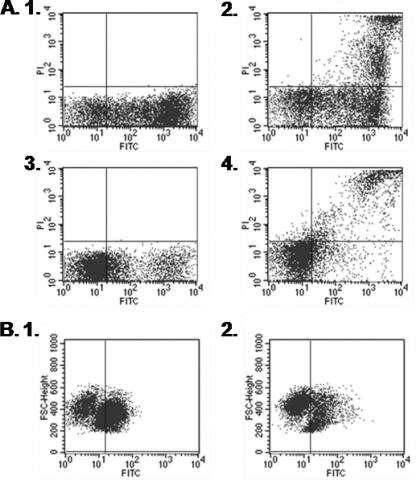
Based on our earlier findings of M. bovis Mb1 inhibiting bovine PBMC proliferative responses, we wanted to determine whether M. bovis Mb1 played a potential role in bovine PBMC apoptosis. To do this, we conducted experiments examining both early and late apoptotic events as outlined in Materials and Methods. We found that at both the 24- and 72-h time points M. bovis Mb1 did not increase apoptosis in bovine PBMC (Fig. 4). We observed no increase in annexin V labeling of phosphatidylserine on the cell membrane or increases in DNA fragmentation. It is interesting, however, that in general the PBMC population already displayed apoptotic events at both 24 and 72 h without the addition of M. bovis Mb1 or the apoptosis inducer staurosporine (5 μM for 45 min) (Fig. 4). These findings would suggest that some of the cell populations are already going through natural apoptotic events at these time points that are independent of M. bovis Mb1.
FIG. 4.
M. bovis and apoptosis of bovine PBMC. Bovine PBMC were incubated with or without M. bovis Mb1 for either 24 h (A) or 72 h (B). Both early and late apoptotic events were monitored by flow cytometry. (A1) annexin V-FITC labeling of early apoptotic cells (81%) in bovine PBMC at 24 h under baseline conditions. (A2) Annexin V FITC and PI labeling of apoptotic cells displaying both late apoptotic and necrotic events (22%) in bovine PBMC at 24 h under baseline conditions. (A3) Annexin V-FITC labeling of early apoptotic cells (34%) in bovine PBMC incubated with M. bovis for 24 h. (A4) Annexin V-FITC and PI labeling of apoptotic cells displaying both late apoptotic and necrotic events (13%) in bovine PBMC incubated with M. bovis for 24 h. (B1) FlowTACS (DNA fragmentation) analysis of late apoptotic events (57%) in bovine PBMC at 72 h under baseline conditions. (B2) FlowTACS (DNA fragmentation) analysis of late apoptotic events (31%) in bovine PBMC incubated with M. bovis for 72 h.
Identification of the PBMC population containing M. bovis Mb1.
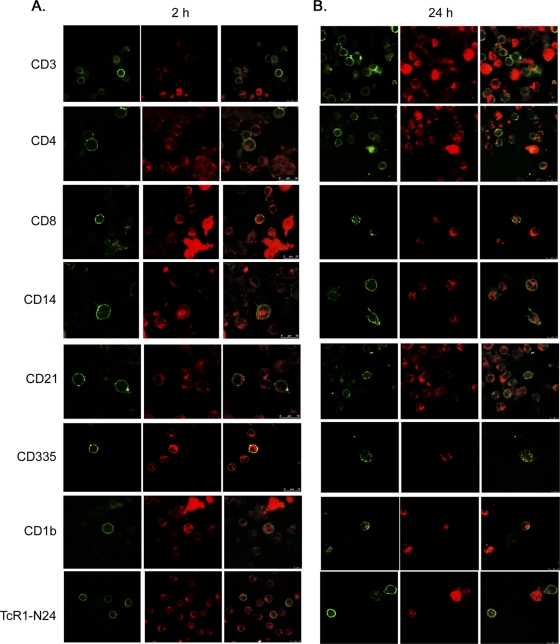
To determine which specific cell populations M. bovis Mb1 was attaching to and invading, immunofluorescence studies with specific cell type markers were conducted as described in Materials and Methods, and the results are shown in Fig. 5. Labeled M. bovis Mb1 was found to be substantially associated with all PBMC populations at both 2 and 24 h, including T cells (CD3), T helper cells (CD4), cytotoxic T cells (CD8), monocytes (CD14), B cells (CD21), NK cells (CD335), dendritic cells (CD1b), and γδ T cells (TcR1-N24). However, the degree and relative internalization of M. bovis Mb1 did vary between 2 and 24 h for each of the cell specific markers. In T cells (CD3), M. bovis Mb1 was found to be intracellular within 2 h and shared a close association with the cell membrane (Fig. 5A), whereas at 24 h it appeared to display diffuse staining in some T cells, while in others it appears to be localized to a particular pole of the cell (Fig. 5B). In T helper cells (CD4), M. bovis Mb1 did not appear to readily associate with the cell membrane at either 2 or 24 h but rather displayed a diffuse intracellular staining pattern (Fig. 5). In cytotoxic T cells (CD8), M. bovis Mb1 shared a close association with the cell membrane at 2 h but at 24 h appeared to migrate further intracellularly to form a centralized structure (Fig. 5). In monocytes (CD14), M. bovis Mb1 displayed intracellular diffuse staining at both 2 and 24 h while still maintaining an association with the cell membrane in some cases (Fig. 5). In B cells (CD21), M. bovis Mb1 displayed a close association with the cell membrane at both 2 and 24 h although at 24 h it appeared to localize primarily to one pole of the cell (Fig. 5). In NK cells (CD335), M. bovis Mb1 displayed diffuse and close membrane-associated staining (Fig. 5A). However, at 24 h it appeared to be located primarily in vacuole-like structures just beneath the NK cell membrane (Fig. 5B). In dendritic cells (CD1b), M. bovis Mb1 displayed a diffuse staining pattern at both 2 and 24 h (Fig. 5), although at 24 h it did appear to be localized primarily at one pole of the cell (Fig. 5B). Finally, in γδ T cells, M. bovis Mb1 displayed a close intracellular association with the cell membrane at 2 h (Fig. 5A); however, at 24 h it appeared to be centrally located in one large cluster (Fig. 5B).
FIG. 5.
Confocal z-scan of M. bovis Mb1 invasion of select bovine PBMC populations. All confocal images represent a scan (slice) through the cell, and therefore any staining within the cell membrane is considered internal. Bovine PBMC were incubated with R18-labeled M. bovis Mb1 (red color) for 2 h (A) and 24 h (B), respectively. Large red “structures” are indicative of a “clump” of M. bovis. Whether these specific structures are intracellular or external are unknown. The green color is indicative of select FITC-labeled PBMC populations. M. bovis was found intracellular in all cell types at 2 and 24 h, although the specific locale and degree of internalization varied both temporally and between the cell types themselves. In some cells, M. bovis appeared to primarily associate with the intracellular membrane (T cells; CD3, cytotoxic T cells; CD8, B cells; CD21). In others it displayed diffuse staining (T helper cells; CD4, monocytes; CD14), and in some cases it appeared to be contained within vacuolelike structures (cytotoxic T cells; CD8, NK cells; CD335). Each image is representative of four fields of view and three separate experiments.
We have demonstrated that M. bovis Mb1, although inhibiting proliferation of bovine PBMC, still induces an IFN-γ response. However, the specific cell types involved in M. bovis Mb1-induced IFN-γ production were unknown. We demonstrate here that M. bovis Mb1 results in IFN-γ production in T cells, T helper cells, cytotoxic T cells, NK cells, and γδ T cells (Fig. 6). We did not observe IFN-γ production in monocytes, dendritic cells, or B cells. Controls included comparisons made to cells with no M. bovis Mb1 added and no primary antibody, all of which were negative for IFN-γ fluorescence. It is interesting that both PBMC infected with M. bovis Mb1 or those void of the bacterium result in IFN-γ production (Fig. 6). These findings suggest an important role for multiple signaling events coupled to both M. bovis Mb1 cell invasion and PBMC cell-to-cell communication.
FIG. 6.
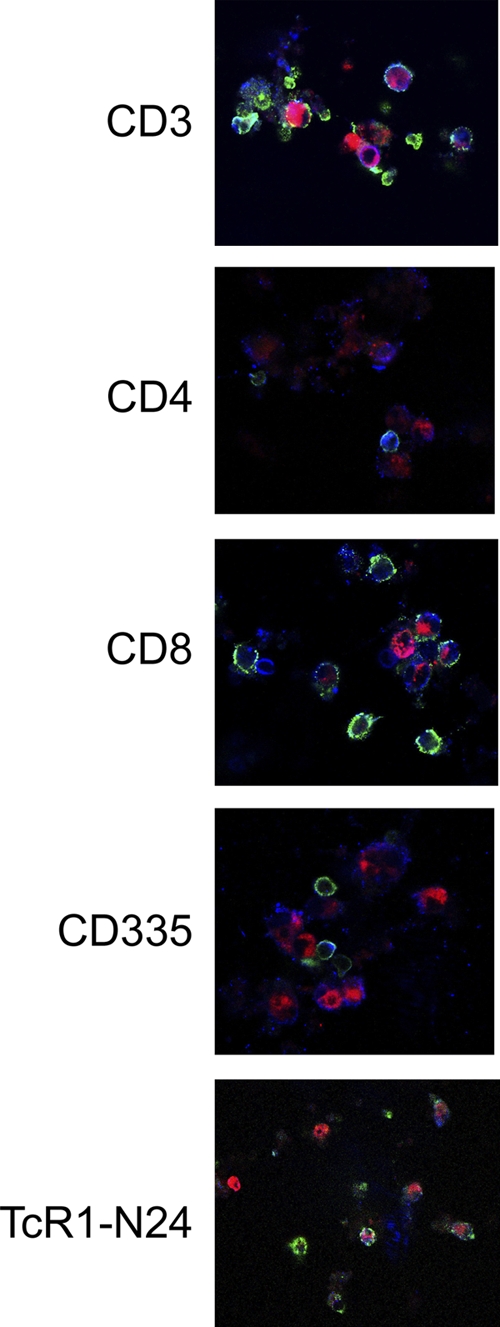
Confocal z-scan of M. bovis Mb1-induced IFN-γ production in select PBMC populations. Bovine PBMC were incubated with R18-labeled M. bovis Mb1 (red color) for 24 h, and the select PBMC type-induced IFN-γ production was determined. The green color is indicative of select FITC-labeled PBMC populations, and the blue (Alexa 405) color is indicative of IFN-γ. The pink coloring observed in some cells indicates colocalization of M. bovis and IFN-γ. M. bovis induced IFN-γ production in CD3 (T cells), CD4 (T-helper cells), CD8 (cytotoxic T cells), CD335 (NK cells), and TcR1-N24 cells (γδ T cells).
M. bovis invades bovine erythrocytes.
Since we found that M. bovis Mb1 was capable of invading PBMC (Fig. 1 and 5) and since other studies have suggested both cell-associated and intracellular M. bovis (although in macrophages and neutrophils the process may involve phagocytosis and not invasion) (7, 15, 26, 34), we sought to determine whether other blood cells, such as RBCs, could also be a target of M. bovis invasion. In other studies, it has been demonstrated that other mycoplasmas such as M. gallisepticum and M. suis are capable of invading erythrocytes (9, 36), and thus it is possible that M. bovis would have a similar effect. We performed invasion assays with purified RBCs, and the results are shown in Fig. 7. Incubation of RBCs with M. bovis Mb1 for various periods (1, 2, 3, and 24 h) resulted in a significant increase in intracellular M. bovis Mb1 (Fig. 7). During the first 3 h M. bovis Mb1 was found to be primarily extracellular; however, after 24 h of incubation the majority of M. bovis was found to be intracellular (Fig. 7).
FIG. 7.
Invasion of bovine RBCs by M. bovis Mb1. Bovine RBCs from 12 beef cattle were incubated for various times (1, 2, 3, and 24 h) with M. bovis Mb1 (MOI of 5), and the percentage of M. bovis-infected PBMC was determined by a gentamicin resistance assay. The bars show the median values. During the first 3 h, M. bovis was found to be primarily extracellular, with some M. bovis-infected PBMC occurring at the 3-h time point. However, after 24 h of incubation, ca. 20% of the PBMC were infected with M. bovis.
DISCUSSION
M. bovis has emerged as a leading candidate in chronic unresponsive pneumonia in feedlot cattle and in veal calves with fatal bronchopneumonia. M. bovis pathogenesis involves a number of factors including Vsps, the inhibition of lymphocyte proliferation, alterations in cytokine production, and the induction of immune-cell apoptosis (8, 31, 34). These immunomodulatory effects, along with the potential of M. bovis to invade various immune and nonphagocytic cells, quite likely contribute to its evasion of the immune system. However, the specific mechanisms and functional responses of specific lymphocyte populations in response to M. bovis infection remain unknown. Previous studies (5, 28, 33) have demonstrated that M. bovis causes the downregulation of lymphocyte proliferative responses to various mitogens, possibly by a mechanism(s) involving a lymphoinhibitory peptide extracted from the cell surface (33). In the present study, we extend those studies and find that the proliferative response is also downregulated by a factor released by M. bovis. This is the first report of such a secreted factor, and we are currently investigating the nature of this product. Despite their inability to proliferate, the functional responses of the lymphocytes, i.e., IFN-γ secretion, appear to be unaffected (Fig. 2). These results raise an important point that, although these bovine lymphocytes are unable to proliferate, they are functional and capable of mounting an immune response via the production of cytokines. M. bovis therefore appears to downregulate immune responses not via the inhibition of immune modulators and cytokines but rather by preventing lymphocyte population increases. These effector responses likely play an important role in the establishment of M. bovis infection. Indeed, previous reports have suggested that M. bovis inhibition of lymphocyte proliferation may be an important factor in its pathogenesis (5, 28, 33).
M. bovis is potentially capable of attaching to and invading various cell populations, although a role for phagocytosis cannot be excluded, (7, 15, 26; this study). We wanted to determine whether PBMC were also a target for attachment and invasion. First, we demonstrated that M. bovis invasion of bovine PBMC exhibits a distinct time course. Within a few hours postinfection the majority of PBMC have M. bovis associated with their cell surface, and by 24 h postinfection 100% of the PBMC contain intracellular M. bovis Mb1 (Fig. 1A). Interestingly, M. bovis was also capable of invading erythrocytes (Fig. 7), albeit at a much lower percentage compared to PBMC (20% in erythrocytes compared to 100% in PBMC). These findings suggest an important difference between erythrocytes and the PBMC populations, i.e., the cell surface expression of putative receptor(s) or other potential antigen(s) required for M. bovis attachment and invasion likely varies between erythrocytes and PBMC. It is conceivable that erythrocytes express low amounts (or potentially not the “full suite”) of required, yet-to-be-identified, cell surface receptor(s) and/or other potential cell surface attachment antigen(s) for M. bovis to invade. Attachment to and invasion of erythrocytes has also been reported in M. suis and M. gallisepticum (9, 10, 36). Importantly, these erythrocytes do not appear “normal” and display a misshapen/malformed morphology (36). It is therefore possible that these erythrocytes may exhibit decreased viability. Furthermore, this same study found that M. gallisepticum had a rate of infection of <5% (36), and in another study Mycoplasma invasiveness appeared to play an important role in systemic spread (19). The invasion of erythrocytes could therefore potentially serve as a mechanism of systemic spread for M. bovis.
The invasion of PBMC by M. bovis does not appear to affect their viability, as determined by a trypan blue exclusion assay, although by 24 h postinfection there was a significant reduction in viable PBMC compared to controls (Fig. 1B). It is possible that the decrease in PBMC viability seen at 24 h occurs at an earlier time point between 3 and 24 h, although this remains unknown. A potential explanation could involve the possibility of either necrotic or apoptotic cell death. Previous studies have demonstrated that the endonuclease activity of various mycoplasmas can result in an increased sensitivity of PBMC to inducers of apoptosis (18, 22, 35). A study in 2002 by Vanden Bush and Rosenbusch demonstrated that M. bovis induced apoptosis of lymphocytes in vitro, as determined by annexin V staining and a TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay (35). In the present experiments involving both early and late apoptosis detection, we determined that M. bovis Mb1 did not further enhance the baseline apoptosis, nor did it decrease the viability observed in the PBMC population (Fig. 4). In fact, our data suggest that M. bovis Mb1 alleviates the proapoptotic signals and may act as a prosurvival factor (close to a 50% reduction in apoptosis is observed in M. bovis Mb1 treated cells at both 24 and 72 h). Whether this is a strain-dependent phenomenon remains to be determined. To date, there are no studies on the specific host-cell signaling pathways modulated by M. bovis, especially with regard to apoptosis. These may include changes in membrane structure and the activation/inactivation of caspase-dependent or -independent signaling events. Further studies will need to be conducted to assess the relationship between M. bovis and PBMC apoptosis before a clear conclusion can be made.
In the present study we have demonstrated that M. bovis is capable of invading bovine PBMC, inhibiting PBMC proliferation, although functional responses, specifically the production of IFN-γ, appear to remain intact (Fig. 1 to 3). Some reports suggest that M. bovis typically adheres to bronchiolar epithelial cell layer but does not migrate intracellularly (30). On the other hand, other studies have demonstrated the intracellular localization of M. bovis within neutrophils, macrophages, and potentially epithelial cells (7, 15, 26). M. bovis infection is also capable of activating specific subsets of T-cell populations (CD4+, CD8+, and γδ T cells), resulting in specific cytokine responses, but whether M. bovis invades these populations was unknown (34). It is likely that M. bovis invades specific cell types, potentially resulting in their activation directly; however, M. bovis attachment and/or invasion may also result in the release of cytokines/chemokines from these same cells, resulting in the stimulation of other lymphocytes. We demonstrate here that within 2 h of M. bovis infection, M. bovis is located on the cell surface of the majority of PBMC populations, including T cells (CD3+), T helper cells (CD4+), cytotoxic T cells (CD8+), monocytes (CD14+), B cells (CD21+), NK cells (CD335+), dendritic cells (CD1b), or γδ T cells (TcR1-N24) (Fig. 5A). Furthermore, the PBMC populations do appear to have intracellular M. bovis at this time point, although M. bovis still appears to share a close association with the cell membrane (Fig. 5A). At 24 h postinfection, we found M. bovis to display distinct invasion patterns (Fig. 5B). M. bovis invaded all PBMC populations and displayed specific cellular localizations, including close association with the intracellular side of the cell membrane, and diffuse and punctuate staining patterns depending on the subpopulation in question (Fig. 5B). It is likely that the expression of a yet-to-be-identified cell surface receptor(s) and/or antigen likely varies between different cell populations. Furthermore, M. bovis could also potentially elicit various signaling events, depending upon the cell type it attaches to, which may aid in its specific intracellular localization. Unfortunately, the identity of the specific host receptor(s) involved in M. bovis attachment and invasion remain unknown and further studies are needed to identify these. M. bovis appears to invade the NK cell population and displays a very distinct staining pattern. Upon invasion at 24 h, M. bovis is present within submembrane vesicular structures of NK cells (Fig. 5B), but whether this is a host cell defense mechanism in an attempt to kill M. bovis or whether this is a potential immune evasion mechanism by M. bovis remains to be elucidated. It is most likely that these vesicular structures are related to M. bovis invasion since NK cells typically do not phagocytose bacteria but rather target and kill bacterium-infected cells (2, 32). On the other hand, it is still unclear whether the presence of M. bovis inside phagocytic host cells (monocytes and dendritic cells) is due to the bacterium itself or whether this process involves phagocytosis of the microorganism. Studies from our laboratory have also demonstrated that M. bovis Mb1 displays species specific invasion properties. We found that M. bovis Mb1 was capable of an intracellular localization, although at a very low percentage, in both ovine and porcine PBMC (unpublished observation). It is quite likely, however, that this low percentage of intracellular M. bovis in these PBMC was actually due to phagocytosis. We have demonstrated that although M. bovis Mb1 inhibits PBMC proliferation, there is still a marked increase in IFN-γ production. We have further shown that M bovis Mb1 induces IFN-γ in a specific subset of bovine PBMC, including T cells, T helper cells, cytotoxic T cells, NK cells, and γδ T cells. It is important to note that IFN-γ is produced by both cells that have intracellular M. bovis Mb1 and those that have no associated M. bovis Mb1. These results suggest two important experimental outcomes. On one hand, M. bovis induces IFN-γ production in the same infected cell, suggesting that some of the immune cell functional response is unimpaired by its intracellular presence. Second, M. bovis results in the indirect activation and production of IFN-γ from immune cells. This pathway could involve cell-to-cell communication events between the immune cells themselves or potentially due to M. bovis-secreted factors. Furthermore, studies by Vanden Bush demonstrated that M. bovis coincubation with bovine PBMC from naive animals did not result in IFN-γ production (34). The difference likely lies in the use of heat-killed M. bovis in the Vander Bush study compared to the use of live M. bovis in ours. These findings suggest that live bacteria or potentially bacterial factors affected by heat treatment are required to elicit an IFN-γ response in bovine PBMC.
These findings raise important questions in regards to both the attachment and the invasion of M. bovis in bovine PBMC. M. pneumoniae and M. genitalium possess a specialized organelle comprised of specialized adhesins (P1 and P30) and accessory proteins (P40, P90, and HMW1 to HMW3) for attachment to host cells. M. bovis, on the other hand, does not possess this structure but rather appears to rely on its Vsps and potentially other yet-to-be-identified surface proteins. As an example of other mycoplasma proteins involved in attachment, the M. suis MSG1 (GAPDH) protein was found to be involved in attachment of the bacteria to erythrocytes (10). Whether the M. bovis GAPDH protein is involved in attachment to PBMC or erythrocytes remains to be determined.
The findings presented here suggest that, along with the invasion of circulating immune cells, the invasion of erythrocytes could potentially aid in a mechanism of transport of mycoplasmas to other tissues within the host. Identification of proteins involved in M. bovis attachment and invasion would help us to understand the mechanisms of pathogenesis, as well as increase the arsenal of potential targets for development of a subunit vaccine for the prevention of this economically important disease.
Acknowledgments
We thank Natasa Arsic for help with the flow cytometry in our apoptosis experiments.
This study received financial support from the Beef Cattle Research Council, Ontario Cattlemen's Association, the Alberta Livestock Development Fund, the Saskatchewan Agriculture Development Fund, and the Advancing Agriculture and Agri-Food Program.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 16 August 2010.
Published with the permission of the Director of VIDO as journal series 553.
REFERENCES
- 1.Baseman, J. B., S. P. Reddy, and S. F. Dallo. 1996. Interplay between mycoplasma surface proteins, airway cells, and the protean manifestations of mycoplasma-mediated human infections. Am. J. Respir. Crit. Care Med. 154:S137-S144. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac, A., P. B. Savage, and L. Teyton. 2007. The Biology of NKT Cells. Annu. Rev. Immunol. 25:297-336. [DOI] [PubMed] [Google Scholar]
- 3.Booker, C. W., S. M. Abutarbush, P. S. Morley, G. K. Jim, T. J. Pittman, O. C. Schunicht, T. Perrett, B. K. Wildman, R. K. Fenton, P. T. Guichon, and E. D. Janzen. 2008. Microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in Western Canada. Can. Vet. J. 49:473-481. [PMC free article] [PubMed] [Google Scholar]
- 4.Booker, C. W., P. T. Guichon, G. K. Jim, O. C. Schunicht, R. J. Harland, and P. S. Morley. 1999. Seroepidemiology of undifferentiated fever in feedlot calves in western Canada. Can. Vet. J. 40:40-48. [PMC free article] [PubMed] [Google Scholar]
- 5.Boothby, J. T., D. E. Jasper, J. G. Zinkl, C. B. Thomas, and J. D. Dellinger. 1983. Prevalence of mycoplasmas and immune responses to Mycoplasma bovis in feedlot calves. Am. J. Vet. Res. 44:831-838. [PubMed] [Google Scholar]
- 6.Chaudhry, R., A. K. Varshney, and P. Malhotra. 2007. Adhesion proteins of Mycoplasma pneumoniae. Front. Biosci. 12:690-699. [DOI] [PubMed] [Google Scholar]
- 7.Dyer, N., L. Hansen-Lardy, D. Krogh, L. Schaan, and E. Schamber. 2008. An outbreak of chronic pneumonia and polyarthritis syndrome caused by Mycoplasma bovis in feedlot bison (Bison bison). J. Vet. Diagn. Invest. 20:369-371. [DOI] [PubMed] [Google Scholar]
- 8.Geary, S. J., M. E. Tourtellotte, and J. A. Cameron. 1981. Inflammatory toxin from Mycoplasma bovis: isolation and characterization. Science 212:1032-1033. [DOI] [PubMed] [Google Scholar]
- 9.Groebel, K., K. Hoelzle, M. M. Wittenbrink, U. Ziegler, and L. E. Hoelzle. 2009. Mycoplasma suis invades porcine erythrocytes. Infect. Immun. 77:576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoelzle, L. E., K. Hoelzle, M. Helbling, H. Aupperle, H. A. Schoon, M. Ritzmann, K. Heinritzi, K. M. Felder, and M. M. Wittenbrink. 2007. MSG1, a surface-localized protein of Mycoplasma suis is involved in the adhesion to erythrocytes. Microbes Infect. 9:466-474. [DOI] [PubMed] [Google Scholar]
- 11.Jungi, T. W., M. Krampe, M. Sileghem, C. Griot, and J. Nicolet. 1996. Differential and strain-specific triggering of bovine alveolar macrophage effector functions by mycoplasmas. Microb. Pathog. 21:487-498. [DOI] [PubMed] [Google Scholar]
- 12.Kirby, F. D., and R. A. Nicholas. 1996. Isolation of Mycoplasma bovis from bullocks’ eyes. Vet. Rec. 138:552. [PubMed] [Google Scholar]
- 13.Lysnyansky, I., R. Rosengarten, and D. Yogev. 1996. Phenotypic switching of variable surface lipoproteins in Mycoplasma bovis involves high-frequency chromosomal rearrangements. J. Bacteriol. 178:5395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lysnyansky, I., K. Sachse, R. Rosenbusch, S. Levisohn, and D. Yogev. 1999. The vsp locus of Mycoplasma bovis: gene organization and structural features. J. Bacteriol. 181:5734-5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda, T., T. Shibahara, K. Kimura, Y. Wada, K. Sato, Y. Imada, Y. Ishikawa, and K. Kadota. 2003. Mycoplasma bovis-associated suppurative otitis media and pneumonia in bull calves. J. Comp. Pathol. 129:100-110. [DOI] [PubMed] [Google Scholar]
- 16.Martin, S. W., K. G. Bateman, P. E. Shewen, S. Rosendal, and J. E. Bohac. 1989. The frequency, distribution and effects of antibodies, to seven putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Can. J. Vet. Res. 53:355-362. [PMC free article] [PubMed] [Google Scholar]
- 17.McAuliffe, L., R. J. Ellis, K. Miles, R. D. Ayling, and R. A. Nicholas. 2006. Biofilm formation by mycoplasma species and its role in environmental persistence and survival. Microbiology 152:913-922. [DOI] [PubMed] [Google Scholar]
- 18.Minion, F. C., K. J. Jarvill-Taylor, D. E. Billings, and E. Tigges. 1993. Membrane-associated nuclease activities in mycoplasmas. J. Bacteriol. 175:7842-7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Much, P., F. Winner, L. Stipkovits, R. Rosengarten, and C. Citti. 2002. Mycoplasma gallisepticum: influence of cell invasiveness on the outcome of experimental infection in chickens. FEMS Immunol. Med. Microbiol. 34:181-186. [DOI] [PubMed] [Google Scholar]
- 20.Nicholas, R. A., and R. D. Ayling. 2003. Mycoplasma bovis: disease, diagnosis, and control. Res. Vet. Sci. 74:105-112. [DOI] [PubMed] [Google Scholar]
- 21.Nussbaum, S., I. Lysnyansky, K. Sachse, S. Levisohn, and D. Yogev. 2002. Extended repertoire of genes encoding variable surface lipoproteins in Mycoplasma bovis strains. Infect. Immun. 70:2220-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paddenberg, R., A. Weber, S. Wulf, and H. G. Mannherz. 1998. Mycoplasma nucleases able to induce internucleosomal DNA degradation in cultured cells possess many characteristics of eukaryotic apoptotic nucleases. Cell Death Differ. 5:517-528. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Casal, J., and T. Prysliak. 2007. Detection of antibodies against the Mycoplasma bovis glyceraldehyde-3-phosphate dehydrogenase protein in beef cattle. Microb. Pathog. 43:189-197. [DOI] [PubMed] [Google Scholar]
- 24.Sachse, K., J. H. Helbig, I. Lysnyansky, C. Grajetzki, W. Muller, E. Jacobs, and D. Yogev. 2000. Epitope mapping of immunogenic and adhesive structures in repetitive domains of Mycoplasma bovis variable surface lipoproteins. Infect. Immun. 68:680-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahriar, F. M., E. G. Clark, E. Janzen, K. West, and G. Wobeser. 2002. Coinfection with bovine viral diarrhea virus and Mycoplasma bovis in feedlot cattle with chronic pneumonia. Can. Vet. J. 43:863-868. [PMC free article] [PubMed] [Google Scholar]
- 26.Srikumaran, S., C. L. Kelling, and A. Ambagala. 2007. Immune evasion by pathogens of bovine respiratory disease complex. Anim. Health Res. Rev. 8:215-229. [DOI] [PubMed] [Google Scholar]
- 27.Tarshis, M., M. Salman, and S. Rottem. 1991. Fusion of mycoplasmas: the formation of cell hybrids. FEMS Microbiol. Lett. 66:67-71. [DOI] [PubMed] [Google Scholar]
- 28.Thomas, C. B., J. Mettler, P. Sharp, J. Jensen-Kostenbader, and R. D. Schultz. 1990. Mycoplasma bovis suppression of bovine lymphocyte response to phytohemagglutinin. Vet. Immunol. Immunopathol. 26:143-155. [DOI] [PubMed] [Google Scholar]
- 29.Thomas, C. B., P. Van Ess, L. J. Wolfgram, J. Riebe, P. Sharp, and R. D. Schultz. 1991. Adherence to bovine neutrophils and suppression of neutrophil chemiluminescence by Mycoplasma bovis. Vet. Immunol. Immunopathol. 27:365-381. [DOI] [PubMed] [Google Scholar]
- 30.Thomas, L. H., C. J. Howard, K. R. Parsons, and H. S. Anger. 1987. Growth of Mycoplasma bovis in organ cultures of bovine fetal trachea and comparison with Mycoplasma dispar. Vet. Microbiol. 13:189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tryon, V. V., and J. B. Baseman. 1992. Pathogenic mechanisms and determinants, p. 447-451. In J. Maniloff (ed.), Mycoplasmas: molecular biology and pathogenesis. American Society for Microbiology, Washington, DC.
- 32.Tupin, E., Y. Kinjo, and M. Kronenberg. 2007. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5:405-417. [DOI] [PubMed] [Google Scholar]
- 33.Vanden Bush, T. J., and R. F. Rosenbusch. 2004. Characterization of a lympho-inhibitory peptide produced by Mycoplasma bovis. Biochem. Biophys. Res. Commun. 315:336-341. [DOI] [PubMed] [Google Scholar]
- 34.Vanden Bush, T. J., and R. F. Rosenbusch. 2003. Characterization of the immune response to Mycoplasma bovis lung infection. Vet. Immunol. Immunopathol. 94:23-33. [DOI] [PubMed] [Google Scholar]
- 35.Vanden Bush, T. J., and R. F. Rosenbusch. 2002. Mycoplasma bovis induces apoptosis of bovine lymphocytes. FEMS Immunol. Med. Microbiol. 32:97-103. [DOI] [PubMed] [Google Scholar]
- 36.Vogl, G., A. Plaickner, S. Szathmary, L. Stipkovits, R. Rosengarten, and M. P. Szostak. 2008. Mycoplasma gallisepticum invades chicken erythrocytes during Infection. Infect. Immun. 76:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]