Abstract
Leptospirosis is a globally significant zoonosis caused by Leptospira spp. Iron is essential for growth of most bacterial species. Since iron availability is low in the host, pathogens have evolved complex iron acquisition mechanisms to survive and establish infection. In many bacteria, expression of iron uptake and storage proteins is regulated by Fur. L. interrogans encodes four predicted Fur homologs; we have constructed a mutation in one of these, la1857. We conducted microarray analysis to identify iron-responsive genes and to study the effects of la1857 mutation on gene expression. Under iron-limiting conditions, 43 genes were upregulated and 49 genes were downregulated in the wild type. Genes encoding proteins with predicted involvement in inorganic ion transport and metabolism (including TonB-dependent proteins and outer membrane transport proteins) were overrepresented in the upregulated list, while 54% of differentially expressed genes had no known function. There were 16 upregulated genes of unknown function which are absent from the saprophyte L. biflexa and which therefore may encode virulence-associated factors. Expression of iron-responsive genes was not significantly affected by mutagenesis of la1857, indicating that LA1857 is not a global regulator of iron homeostasis. Upregulation of heme biosynthetic genes and a putative catalase in the mutant suggested that LA1857 is more similar to PerR, a regulator of the oxidative stress response. Indeed, the la1857 mutant was more resistant to peroxide stress than the wild type. Our results provide insights into the role of iron in leptospiral metabolism and regulation of the oxidative stress response, including genes likely to be important for virulence.
Leptospirosis is a widespread zoonotic disease caused by pathogenic species of the spirochete Leptospira. Human infection occurs via contact with infected animals or with soil or water contaminated with urine of carrier animals (1, 36). In tropical countries, large outbreaks of human leptospirosis have occurred following severe flooding, while in developed countries, cases usually occur via occupational contact or recreational activities (27, 35). Clinical manifestations of leptospirosis in humans are extremely variable, ranging from a self-limiting, influenza-like syndrome through to rapidly fatal forms involving multiorgan failure, with death occurring in 5 to 25% of severe cases (8, 49). Transmission of Leptospira interrogans from rodents to humans is usually indirect, as L. interrogans can survive for considerable periods of time in contaminated soil or water. Currently, little is known about pathogenesis mechanisms or transcriptional regulation in Leptospira spp.
Iron is an essential cofactor in many biological processes and is therefore required for the growth of most organisms, including Leptospira spp. (23), although some organisms, such as Borrelia burgdorferi, have eliminated the need for iron (59). In the animal host, the concentration of free iron is insufficient for bacterial growth, as the majority of iron is bound to high-affinity protein carriers such as transferrin, lactoferrin, and ferritin (12). The low availability of iron is therefore one of the first barriers that bacterial pathogens must overcome in order to survive and establish infection in the host.
To cope with changes in iron availability, bacterial pathogens have evolved response mechanisms for regulation of iron homeostasis and complex strategies for iron acquisition from the host. In many bacteria, transcriptional regulation of various genes involved in iron acquisition is under the control of the iron-dependent negative regulator, Fur (ferric uptake regulator) (4). When iron is abundant, Fur forms a complex with ferrous iron and blocks transcription of target genes by binding to conserved promoter regions termed Fur boxes. Under iron-limiting conditions, the Fur-Fe2+ complexes dissociate and Fur can no longer bind to target genes, allowing expression to proceed. This system enables a rapid response to changes in iron levels. While most genes are negatively regulated by Fur, some genes are positively regulated at the transcriptional level, for example, pan1, norA, and nuoA in Neisseria meningitidis, which are involved in anaerobic and aerobic respiration (19). Various Escherichia coli genes involved in iron storage, iron metabolism, and defense against oxidative stress are also positively regulated, but at the posttranscriptional level via repression of a small RNA, RyhB (46). Virulence factors in various pathogens are iron regulated, for example, the Shiga-like toxin in Escherichia coli, diphtheria toxin, and the Pseudomonas exotoxin A (9, 13, 65). Various virulence-associated outer membrane proteins in Vibrio spp., Neisseria spp., and Yersinia spp. are also iron regulated (40).
Currently, very little is known about iron acquisition and regulatory mechanisms in pathogenic leptospires. Several outer membrane proteins have been shown to be iron regulated. For example, the TonB-dependent outer membrane receptor protein, LB191, is upregulated (6), while LipL36, LA0412 (pL24), and LA3469 (pL50) are downregulated under low-iron conditions (17). Processing of LipL32, the highly conserved major outer membrane protein of pathogenic Leptospira spp., has also been shown to be affected by iron availability, possibly due to iron-regulated proteases (17). Using random transposon mutagenesis with the saprophytic Leptospira biflexa, Louvel et al. (45) identified five hemin-requiring mutants; three of these had insertions in a gene encoding a protein with homology to FecA, the TonB-dependent ferric citrate receptor in E. coli, while the remaining two mutants had insertions in a FeoB-like protein. FeoB is conserved in many bacteria and plays a role in transport of ferrous iron (4). L. interrogans possesses a complete set of genes for de novo synthesis of heme (26) as well as a functional heme oxygenase (50) and is therefore capable of heme synthesis as well as using heme/hemoglobin as a sole iron source by scavenging iron from hemoglobin. Expression of genes involved in heme biosynthesis is likely to be regulated by a two-component system encoded between hemE and hemL (43). Heme (in the form of hemoglobin) is likely to be the main source of iron for pathogenic Leptospira spp. in vivo, and heme oxygenase is required for virulence and in vivo survival (52). L. interrogans can also bind to hemin via the hemin-binding proteins LB191 (also named HbpA) and LipL41 (6). The availability of genome sequences for L. interrogans has enabled identification of putative genes involved in iron acquisition and regulation (44, 54, 55, 60). The genomes of L. interrogans serovars (svs) Lai and Copenhageni encode four predicted Fur homologs; we have generated a transposon mutation (la1857) in one of these (51). Other bacterial species possess more than one Fur homolog. For example, Bacillus subtilis has three (Fur, PerR, and Zur) (11, 25, 68) which have significant sequence and structural similarities, but each responds to different environmental signals and regulates different target genes (24). All proteins in the Fur family are metalloregulatory proteins and include sensors of iron, zinc, nickel, and manganese, while other Fur family proteins, such as PerR, also bind to iron, but this converts PerR into an oxidation sensor rather than an iron sensor (37). Sensitivity of PerR to metal-catalyzed oxidation allows the protein to respond rapidly to low levels of hydrogen peroxide.
In the present study, we characterized the global transcriptional response of L. interrogans to iron limitation. Under low-iron conditions, 49 genes were downregulated and 43 genes were upregulated. However, 54% of differentially expressed genes were of unknown function. Some of these may encode unique leptospiral virulence factors or proteins necessary for in vivo survival. We also studied the transcriptional profile of the la1857 mutant and found little overlap with the transcriptional response to low-iron conditions. Instead, LA1857 appears to play a role in the response to oxidative stress, consistent with our finding that the la1857 mutant has an 8-fold increase in resistance to hydrogen peroxide. These data suggest that LA1857 functions as a PerR homolog, a member of the Fur family of regulators.
MATERIALS AND METHODS
Culture conditions.
L. interrogans sv Manilae was grown in EMJH medium (33) at 30°C. For the la1857 mutant, M776, kanamycin at a final concentration of 50 μg/ml was added. To study the effects of iron limitation, triplicate 25-ml cultures of the parent and la1857 mutant were grown to mid-log phase (4 × 108 to 6 × 108 cells/ml), and then the bacteria were pelleted (4,000 × g, 15 min, room temperature), washed in 10 ml of EMJH base (Difco), and resuspended in 1 ml EMJH base. The culture was then transferred to 100 ml EMJH medium with or without preincubation with 40 μM 2,2′-dipyridyl (Sigma) and grown to mid-log phase before harvesting of bacteria for RNA isolation.
RNA purification.
Leptospires were grown to a density of 2.5 × 108 to 6.5 × 108 cells/ml before harvesting of bacteria for RNA purification. The cell count was determined as described previously (2). Cultures were harvested and RNA was isolated from leptospires as described previously (41) and then further treated with a Turbo DNA-free kit (Ambion), according to the manufacturer's instructions. To confirm lack of contamination with genomic DNA, 0.5 μg of RNA was used as template in a PCR using primers toward la0615, encoding a conserved hypothetical protein in serovars Lai and Manilae (Table 1).
TABLE 1.
Sequences of primers used in this study
| Gene | Forward primer | Reverse primer |
|---|---|---|
| gyrB (la0005) | CCGACAAAAAAATTTCCACAA | CCCATGTAAACCCCCAGAA |
| la0615 | CAAAATTGTATGAAAAGCGGACG | GAGAATATCGTTAAGGTCGTGTTC |
| la0695 | GGTCCAACGGGAAGACAAGTT | GTGCCGTCGCTTCTACGGTA |
| la0706 | CAAAGATTACCCGGAGCTCAGA | CGCCGTAATTCCAGGAAGAG |
| la1857 | CTCACCGCCGATCAGGTTT | GCACGAGAAGCGTTTGGC |
| la2242 | CCTTCCGTTGGTTTGATTGT | CGCTTGAGAACCCCTATGAA |
| la2641 | TTTTGCTTCCGGTCTTGGAT | CGTTATCACCAGCGATTGAATC |
| la2824 | CACGGACGAGTTGGTTAGGG | CAATTGCCCCCACCATAATC |
| la2887 | TTTGCCTTACTCCGGTGGAA | GAATTGTGGGTTGTATAGAAACCGA |
| la3094 | TTCAAGAACAAGCAGCAAGAGAA | TCCGTAAATGTTCAAACTATGACCTG |
| la3468 | TACCGGAGTTGCTTCCTCTGA | TGACTTCGTGATCTCTTACGTTTCC |
| la3778 | GAATATTACGGATTCGACATACATCG | CCTTGGATGGTTACAACGGATT |
| lb011 | AGATTGGCTCGTTACTAGAGGCA | ACCAGTATGTTCCAAAGCGGG |
| lb183 | TGAAATCCATCTTCCGGGAG | GATGGTGATGATGACGGCTTG |
| lb186 | AATTATCGTACCGCGTTAGATTCC | GGCAAGAATGGATTGTTTTTCG |
| lb191 | CTAAAAAGGACAACGGGAATGG | CACAATCTGTGATTCTTCCGGA |
| lb014 | TCGTGCGATGTATCTTGCGA | CCGGAAAATCCTCACCTACAGA |
Microarray construction, hybridization, and analysis.
The L. interrogans microarray was printed as described previously (41). Labeled cDNA probes were synthesized from 2 μg of total RNA using a 3DNA Array 900MPX expression array detection kit (Genisphere) and then hybridized with the microarray slides, as described previously (41). Microarray hybridizations were scanned using a GMS 418 array scanner (Genetic MicroSystems). The Cy3 and Cy5 images were aligned and then overlaid with a grid using ImaGene, version 5.1 (Biodiscovery), to allow accurate gene identification and quantification of fluorescence intensity. Spots were examined manually, and poor spots with very low signals or inconsistent morphologies were flagged for elimination from the analysis.
Three independent RNA samples (biological replicates) from parent and mutant strains of L. interrogans serovar Manilae grown with or without 2,2′-dipyridyl were compared. The orientation of Cy3 to Cy5 labeling was the same for replicates 1 and 3, while replicate 2 was a dye swap. Raw data from the comparisons were analyzed using the web-based program BioArray Software Environment (BASE) (62). Raw data from replicate arrays (3 for each comparison) were combined and used for further analyses, as described previously (41). Genes were considered to be differentially expressed if they were at least 2-fold up- or downregulated with a P value of <0.05.
Validation of microarray data by real-time RT-PCR.
Real-time reverse transcription-PCR (RT-PCR) was performed as described previously (41). The primers used are shown in Table 1. Known concentrations of L. interrogans serovar Manilae genomic DNA were used to construct a gene-specific standard curve so that the concentration of template in each reaction could be determined. The gene encoding flagellum subunit B, flaB, was used as the normalizer for all reactions. Melting curve analysis confirmed that all PCRs amplified a single product.
Statistics for category comparisons.
Fisher's exact test was performed for pairwise comparisons of the frequencies of various groupings of leptospiral genes affected by iron limitation (Fig. 1).
FIG. 1.
Percentages of genes which were up- or downregulated under iron limitation and across the L. interrogans sv Lai genome in each COG category. The COG functional categories are as follows: information storage and processing (11% of coding sequences in L. interrogans serovar Lai genome) (includes J, translation; K, transcription; L, replication, recombination, and repair); cellular processes and signaling (20% of coding sequences in the serovar Lai genome) (includes D, cell cycle control, cell division, and chromosome partitioning; V, defense mechanisms; T, signal transduction mechanisms; M, cell wall, membrane, or envelope biogenesis; N, cell motility; U, intracellular trafficking, secretion, and vesicular transport; O, posttranslational modification, protein turnover, and chaperones); metabolism (19% of coding sequences in the serovar Lai genome) (includes C, energy production and conversion; G, carbohydrate transport and metabolism; E, amino acid transport and metabolism; F, nucleotide transport and metabolism; H, coenzyme transport and metabolism; I, lipid transport and metabolism; P, inorganic ion transport and metabolism; Q, secondary metabolite biosynthesis, transport, and catabolism); poorly characterized (50% of coding sequences in the serovar Lai genome) (includes R, general function prediction only; S, function unknown; and −, not in COGs). The asterisk indicates that genes predicted to encode inorganic ion transport and metabolic proteins were overrepresented among genes upregulated by low-iron conditions (Fisher's exact test, P < 10−5).
Phylogenetic analysis.
Sequence alignment and phylogenetic analysis were performed using the software Geneious Basic, version 4.7.5, to compare similarity between each of the putative L. interrogans Fur homologs with other Fur, Zur, and PerR proteins.
Oxidative stress assays.
Three biological replicates of mutant and wild-type cultures were tested in triplicate for sensitivity to hydrogen peroxide and cumene hydroperoxide. Hydrogen peroxide and cumene hydroperoxide were serially diluted using EMJH medium in a 96-well plate from 6.87 mM to 6 μM and 5 mM to 3 μM, respectively, in a 100-μl final volume. The mutant or wild-type cultures (5 × 106 cells in 100 μl EMJH medium) were added to the wells, and the plates were incubated overnight at 30°C. The minimum bactericidal concentration (MBC) was then determined by dark-field microscopy.
Microarray data accession number.
The data discussed in this publication have been deposited in the NCBI Gene Expression Omnibus database and are accessible through GEO series accession number GSE20422 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE20422).
RESULTS AND DISCUSSION
Iron limitation is one of the signals encountered by pathogens upon entry into the host. In order to characterize the global leptospiral response to iron limitation, L. interrogans sv Manilae cultures were grown in EMJH medium preincubated with 40 μM 2,2′-dipyridyl. The transcription patterns of these cultures were compared with those of cultures grown in normal EMJH medium. We used 40 μM 2,2′-dipyridyl, as this was the highest concentration at which growth was not severely inhibited. Genes which were up- or downregulated by at least 2-fold with 95% confidence were considered to be differentially expressed. The microarray data were validated by real-time RT-PCR analysis on 13 genes. A correlation coefficient (R2) of 0.7287 was observed between the fold change values obtained by microarray and real-time RT-PCR analyses (data not shown).
Effects of iron limitation on transcription.
We identified 49 and 43 genes which were downregulated and upregulated in response to low iron, respectively (Table 2 ). While the microarray data were generated using L. interrogans sv Manilae, for gene nomenclature we have used locus tags from L. interrogans sv Lai (LA numbers), except where the gene is unique to sv Copenhageni (LIC numbers) and is present in our draft sequence of the sv Manilae genome. Therefore, while some genes have been predicted to be part of a transcriptional unit in sv Lai and/or sv Copenhageni, it is unknown whether sv Manilae has a similar genome arrangement.
TABLE 2.
L. interrogans sv Manilae genes differentially expressed under low-iron conditions, grouped by COGa category
| Regulation and locus tagb | Mean fold change | P value | COG | Gene | Description of gene product | Predicted locationc |
|---|---|---|---|---|---|---|
| Downregulated | ||||||
| LA0055 | −3.8 | 0.01 | —d | Hypothetical protein | CYT | |
| LA0135 | −5.1 | <0.01 | — | Hypothetical protein | CYT | |
| LA0137 | −2.7 | 0.01 | — | Hypothetical lipoprotein | NON-CYT | |
| LA0573 | −3.2 | 0.03 | — | Hypothetical protein | CYT | |
| LA0603 | −5.2 | <0.01 | — | Methyltransferase | CYT | |
| LA0983 | −5.9 | <0.01 | — | Conserved hypothetical protein | CYT | |
| LA1005 | −10.1 | <0.01 | — | Aminopeptidase | UNK | |
| LA1389 | −6.2 | <0.01 | — | Hypothetical protein | IM | |
| LA1472 | −2.7 | 0.02 | — | Hypothetical protein | CYT | |
| LA1475 | −3.4 | 0.01 | — | Hypothetical protein | CYT | |
| LA1478 | −2.1 | 0.03 | — | Conserved hypothetical protein | NON-CYT | |
| LA1535 | −7.4 | <0.01 | — | Hypothetical lipoprotein | NON-CYT | |
| LA1536 | −4.1 | <0.01 | — | Hypothetical protein | NON-CYT | |
| LA1539 | −4.3 | <0.01 | — | Conserved hypothetical protein | NON-CYT | |
| LA1593 | −7.1 | 0.01 | — | Conserved hypothetical protein | CYT | |
| LA1597 | −3.5 | <0.01 | — | Conserved hypothetical protein | CYT | |
| LA1601 | −4.0 | <0.01 | — | Sugar isomerase | CYT | |
| LA1759 | −7.7 | <0.01 | — | Conserved hypothetical lipoprotein | NON-CYT | |
| LA1796 | −9.8 | 0.01 | — | Hypothetical protein | CYT | |
| LA2628 | −2.1 | <0.01 | — | Conserved hypothetical protein | NON-CYT | |
| LA2753 | −3.0 | <0.01 | — | Hypothetical protein | CYT | |
| LA2824 | −5.0 | 0.02 | — | Hypothetical protein | IM | |
| LIC12649 | −4.8 | 0.04 | — | Hypothetical protein | NON-CYT | |
| LA0630 | −4.2 | 0.01 | C | Oxidoreductase | CYT | |
| LA0611 | −4.8 | 0.01 | D | ftsA | Cell division protein, actin-like ATPase | CYT |
| LA2459 | −2.4 | 0.01 | D | Conserved hypothetical protein | CYT | |
| LA1481 | −2.5 | 0.01 | E | proC | Pyrroline-5-carboxylate reductase | CYT |
| LA0634 | −8.5 | 0.01 | EP | Permease component of an ABC transporter complex | IM | |
| LA1457 | −3.0 | 0.02 | GM | Membrane protein of an ABC transporter complex | IM | |
| LA0632 | −2.7 | 0.01 | GT | ptsN | Protein-N(pi)-phosphohistidine-sugar phosphotransferase, subunit A | CYT |
| LA1451 | −2.0 | 0.01 | I | pssA | CDP-diacylglycerol-serine O-phosphatidyltransferase | IM |
| LA1596 | −6.1 | 0.01 | J | Methionyl-tRNA formyltransferase | CYT | |
| LA2691 | −3.8 | <0.01 | L | N6-adenine-specific DNA methylase | CYT | |
| LA1504 | −2.3 | 0.02 | M | Metallopeptidase | NON-CYT | |
| LA1582 | −2.1 | <0.01 | M | ADP-heptose synthase | CYT | |
| LA1595 | −2.0 | 0.05 | M | Pyridoxal phosphate-dependent aminotransferase | CYT | |
| LA1611 | −2.0 | <0.01 | MJ | Nucleoside diphosphate-sugar pyrophosphorylase | CYT | |
| LA2690 | −3.1 | <0.01 | P | bfr | Bacterioferritin (cytochrome b1) | CYT |
| LA0607 | −3.3 | 0.02 | Q | Substrate binding protein of an ABC transporter complex | PER | |
| LA1555 | −2.1 | 0.05 | Q | Multicopper oxidase | NON-CYT | |
| LA1081 | −2.0 | 0.02 | R | Permease of the major facilitator superfamily | IM | |
| LA1476 | −2.3 | 0.04 | R | Dehalogenase-like hydrolase | CYT | |
| LA1517 | −2.1 | <0.01 | R | Host factor I-related protein | CYT | |
| LA1592 | −3.7 | 0.03 | R | Methyltransferase | CYT | |
| LA0557 | −2.0 | 0.02 | S | Conserved hypothetical protein | IM | |
| LA0598 | −2.0 | <0.01 | S | Conserved hypothetical protein | CYT | |
| LA1526 | −2.4 | 0.02 | T | Signal transduction protein containing cyclic AMP-binding and CBS domains | CYT | |
| LA1681 | −2.0 | 0.03 | T | phoH | Phosphate starvation-inducible protein | CYT |
| LA1600 | −4.1 | 0.02 | V | ATP-binding protein of an ABC transporter complex | IM | |
| Upregulated | ||||||
| LA0569* | 3.9 | <0.01 | — | Conserved hypothetical protein | NON-CYT | |
| LA0570* | 5.1 | <0.01 | — | Conserved hypothetical protein | NON-CYT | |
| LA0571* | 3.2 | 0.01 | — | Conserved hypothetical protein | IM | |
| LA0589* | 6.2 | 0.01 | — | Conserved hypothetical protein | NON-CYT | |
| LA1375* | 2.3 | 0.01 | — | Conserved hypothetical protein | NON-CYT | |
| LA1767* | 2.5 | 0.01 | — | Hypothetical protein | CYT | |
| LA1847* | 2.7 | 0.02 | — | Hypothetical protein | CYT | |
| LA2060 | 2.1 | <0.01 | — | Conserved hypothetical protein | CYT | |
| LA2240* | 2.7 | <0.01 | — | Hypothetical lipoprotein | NON-CYT | |
| LA2241 | 4.6 | <0.01 | — | Hypothetical lipoprotein | NON-CYT | |
| LA2342* | 2.1 | 0.01 | — | Hypothetical protein | NON-CYT | |
| LA2882* | 2.7 | <0.01 | — | Hypothetical protein | CYT | |
| LA3867* | 2.1 | <0.01 | — | Conserved hypothetical lipoprotein | NON-CYT | |
| LA3974* | 2.2 | 0.01 | — | Conserved hypothetical protein | CYT | |
| LA4030* | 2.0 | 0.02 | — | Hypothetical protein | CYT | |
| LB194 | 10.1 | <0.01 | — | Hypothetical lipoprotein | NON-CYT | |
| LB225* | 2.0 | <0.01 | — | Conserved hypothetical lipoprotein | NON-CYT | |
| LA3470 | 5.6 | <0.01 | C | Thiol oxidoreductase | NON-CYT | |
| LA2061 | 2.2 | <0.01 | E | metX | Homoserine O-acetyltransferase | CYT |
| LA2062 | 2.3 | <0.01 | E | metY | O-Acetylhomoserine aminocarboxypropyltransferase | CYT |
| LB187 | 2.5 | <0.01 | G | Permease | IM | |
| LB191 | 5.8 | <0.01 | H | TonB-dependent outer membrane receptor | OM | |
| LA0568 | 5.0 | <0.01 | I | Outer membrane transport protein | OM | |
| LA0308 | 2.6 | 0.01 | J | hemK | Methylase of polypeptide chain release factors | CYT |
| LA0572 | 3.1 | 0.02 | P | TonB-dependent receptor | OM | |
| LA0726 | 2.7 | 0.01 | P | Sulfatase | IM | |
| LA1155 | 6.4 | <0.01 | P | Substrate binding protein of an ABC transporter complex | PER | |
| LA2242 | 2.7 | <0.01 | P | TonB-dependent protein | OM | |
| LA3468 | 2.2 | <0.01 | P | Outer membrane receptor for Fe3+-dicitrate/TonB-dependent receptor | OM | |
| LA3469 | 4.6 | <0.01 | P | Iron-regulated lipoprotein | OM | |
| LA4216 | 2.1 | <0.01 | P | cysI | Sulfite reductase (NADPH), alpha subunit | CYT |
| LB186 | 2.5 | <0.01 | P | Heme oxygenase | CYT | |
| LA0605 | 8.9 | 0.01 | R | SET family protein | CYT | |
| LA3107 | 2.1 | <0.01 | R | Hydrolase/acyltransferase | UNK | |
| LA3354 | 2.7 | <0.01 | R | Zn-dependent alcohol dehydrogenase | CYT | |
| LA3471* | 3.6 | <0.01 | R | Conserved hypothetical protein | NON-CYT | |
| LA3020* | 2.4 | 0.03 | S | Conserved hypothetical protein | CYT | |
| LA3410 | 2.2 | <0.01 | S | Conserved hypothetical protein | NON-CYT | |
| LA3104 | 4.4 | <0.01 | T | Signal transduction protein | CYT | |
| LA3234 | 2.1 | 0.01 | T | Adenylate/guanylate cyclase | UNK | |
| LA3909 | 2.0 | 0.04 | T | Signal transduction protein | CYT | |
| LB139 | 2.1 | <0.01 | TK | Regulator of sigma subunit | IM | |
| LIC10929 | 3.4 | 0.03 | V | Restriction endonuclease, type I | CYT |
COG categories are defined in the legend to Fig. 1.
Locus tags from L. interrogans sv Lai (LA number) have been used, except where the gene is unique to sv Copenhageni (LIC number) and is present in sv Manilae. *, the gene is absent from L. biflexa.
Predicted locations of proteins: noncytoplasmic proteins (NON-CYT); proteins located in the outer membrane (OM), periplasm (PER), inner membrane (IM), or cytoplasm (CYT); or unknown location (UNK).
—, not in COGs.
To determine if any groups of genes were overrepresented, the differentially expressed genes were sorted into functional categories on the basis of clusters of orthologous groups (COG) (66) (Fig. 1). Consistent with the high proportion of uncharacterized genes (∼50% of coding sequences in the L. interrogans genomes), the majority of differentially expressed genes encode proteins of hypothetical or uncharacterized function (56% and 53% of down- and upregulated genes, respectively, in COG categories R, S and no COG designation) (Fig. 1).
Growth under low-iron conditions did not affect expression of genes in COG categories F (nucleotide transport and metabolism), N (cell motility), O (posttranslational modification, protein turnover, and chaperones), or U (intracellular trafficking, secretion, and vesicular transport), suggesting little change to these cellular processes (Fig. 1). There were no upregulated genes in COG categories D (cell cycle control, cell division, and chromosome partitioning), M (cell membrane biogenesis), and Q (secondary metabolites biosynthesis, transport, and catabolism); but 16.7% of downregulated genes were in these categories (versus 7.1% across the genome; P = 0.012), possibly to divert cellular resources toward expression of essential proteins which are involved in iron acquisition and active transport. Genes assigned COG category P (inorganic ion transport and metabolism) were overrepresented in genes which were upregulated compared with the genome-wide frequency (18.6% and 2.4%, respectively; P < 10−5), a finding consistent with COG category P, including proteins predicted to be involved in transport of iron.
L. interrogans genes involved in iron transport and utilization.
Expression of the gene encoding LA0572, predicted to be involved in transport of ferrienterobactin (55), and expression of the gene encoding LA2242, a desferrioxamine receptor homolog, were upregulated (Table 2), similar to what was found in L. biflexa (44). Expression of la2242 is also induced by transition from low to physiological osmolarity conditions (48) and exposure to serum (57).
FecA is a surface protein which mediates transport of ferric citrate across the outer membrane (72). The gene encoding LA3468, a FecA homolog, was induced 2.2-fold in L. interrogans under low-iron conditions. In E. coli, the ferric iron transport genes (fecABCDE) are also upregulated under low-iron conditions (5). However, the ABC transport proteins involved in ferric citrate uptake by FecA in L. interrogans are unknown, since no other genes of the fec operon have been identified and the genes encoding the two-component signal transduction genes, fecIR (67), are also absent.
LB191 (or HbpA) from L. interrogans sv Lai is a hemin binding protein which is expressed in cultures grown under low-iron conditions (6). Our study confirmed upregulation of lb191 transcription under iron limitation. Genes encoding a heme oxygenase (LB186) and a putative heme permease (LB187) were also upregulated. The genes appear to be arranged in an operon and together with lb191 may be part of a heme acquisition and utilization locus. Since heme is likely to be the main source of iron in the host, upregulation of these genes was not surprising, especially given that heme oxygenase is required by L. interrogans for survival in the hamster model of infection (52). Genes encoding LB186 and LB187 were also upregulated in response to an increase in osmolarity (48) and exposure to serum (57), consistent with the importance of these genes for survival in the host.
Genes encoding predicted ferrous iron transport proteins, FeoA and FeoB (LA2578 and LA2579, respectively), were not differentially expressed under low-iron conditions, in contrast to the downregulation observed in L. biflexa (44). This difference may be due to differences in metabolism or iron uptake mechanisms between pathogenic and saprophytic leptospires. Iron transporter proteins require an energy transduction complex, consisting of TonB, ExbB, and ExbD, for uptake of iron (3). There are two predicted TonB or TonB-like proteins encoded in the L. interrogans genome, five ExbD-related biopolymer transport proteins, and four ExbB- or TolQ-like transport proteins grouped in four distinct loci. TolQ is structurally similar to ExbB and can substitute for ExbB activity (10, 21). None of these was differentially expressed, although la3246 (encoding a predicted ExbD-related biopolymer transport protein) was slightly upregulated (1.53-fold) under low-iron conditions. The constitutive expression of these genes suggests that the proteins are involved in transport of substances other than iron or their expression may respond to different iron sources. The gene bfr, encoding a putative bacterioferritin (LA2690), was 3.1-fold downregulated, consistent with the reduced need for iron storage proteins and other nonessential iron-containing proteins in order to release iron for crucial cellular processes. There was no change in transcription of genes encoding the other predicted bacterioferritins: Dps (LA3598), a DNA-binding ferritin-like protein, or LA4021, a predicted bacterioferritin-associated ferredoxin. In E. coli, Fur indirectly represses expression of bacterioferritin under iron-limiting conditions via derepression of RyhB, a small noncoding RNA which acts by hybridizing to the transcript and inhibiting translation (46). RyhB also negatively regulates a number of genes encoding iron-binding and metabolic proteins and thus, along with Fur, plays a major role in iron regulation in E. coli (47). Downregulation of bacterioferritin (LA2690) and other genes in L. interrogans may be regulated by a similar mechanism. However, there have been no small RNA species characterized in Leptospira spp. to date.
In some bacteria, Fur autoregulates its own transcription in response to iron levels (20, 30). This occurs via RyhB in E. coli (69). While transcription of the genes encoding Fur family proteins was not altered in L. interrogans under low-iron conditions (see Table S1 in the supplemental material), it is possible that their expression may also be regulated posttranscriptionally by small RNA species. Expression of the la1857 ortholog in L. biflexa (LEPBIa2461) was likewise unaffected by the iron concentration, but the other three fur-like genes were downregulated at least 10-fold under iron limitation conditions (44). The reasons for this discrepancy are unknown but further suggest different regulatory mechanisms in pathogenic and saprophytic Leptospira.
Effect of iron limitation on membrane proteins and putative virulence factors.
Various leptospiral outer membrane proteins are downregulated by iron, including LipL36 (LA0492), pL50 (LA3469), and pL24 (LA0412), a putative electron transfer flavoprotein (17). LA3469 (LruB) plays a role in equine recurrent uveitis and has an IrpA domain which is associated with iron metabolism regulation (70). Our results showed a 4.6-fold upregulation of la3469 with iron limitation. Also, in contrast with the findings of the study of Cullen et al. (17), we detected no change in transcription of la0412 or lipL36. The apparent discrepancy may be due to the differences in 2,2′-dipyridyl concentrations and the period of growth under iron-limiting conditions. However, we have observed previously that transcription levels do not necessarily reflect protein abundance (41, 42). LipL36, for example, is downregulated in vivo and during growth at 37°C (17, 28, 53), but our studies showed that lipL36 transcription was not temperature responsive, suggesting posttranscriptional regulation (41).
LA0695 (also known as Lsa24 [7], LenA [64], or LfhA [71]) is expressed during mammalian infection and can bind to the complement regulatory protein factor H (71), laminin, collagen IV, and fibronectin (7), suggesting roles in resistance to complement-mediated killing and adhesion to host cells. LA0695 is one of five L. interrogans proteins (LenA-LenF) that are structurally similar to mammalian endostatins (64). Previous studies showed that the transcript of la0695 was slightly upregulated by an increase in osmolarity (1.55-fold) (48), whereas there was no change in response to temperature (41). However, for unknown reasons, there was poor hybridization and therefore a low signal intensity of la0695 spots on the microarray slides, resulting in inconclusive data for la0695 transcripts. Given that LA0695 is likely to play a role in virulence, we selected this gene for real-time RT-PCR analysis and found that la0695 was upregulated 2.8-fold. Genes encoding two other endostatin-like proteins, lenB (LA3103) and lenD (LA1433), were slightly upregulated (1.6- and 1.8-fold, respectively) by iron limitation, whereas lenC, lenE, and lenF (la0563, la4324, and la4073, respectively) were not differentially expressed (see Table S1 in the supplemental material). lenD is also upregulated under conditions of increased temperature and osmolarity (41, 48). LenB has factor H-binding ability, while both LenB and LenD can bind to laminin (64). The Len proteins appear to provide functional redundancy, whereby expression of different proteins performing similar roles may be regulated by different mechanisms and/or environmental signals, as suggested by results of this and previous microarray studies (41, 48). Functional redundancy and differential regulation suggest that the individual Len proteins may play other as yet undefined roles in different stages of pathogenesis.
Using iTRAQ and 2DGE analyses, Eshghi et al. (22) identified five proteins in L. interrogans sv Copenhageni which were upregulated under in vivo-like conditions (iron limitation combined with the presence of serum): the essential virulence factor Loa22 (61), a putative glycosyl hydrolase (LIC13050/LA0505), a putative coagulase (LIC13166/LA3961), a putative catalase (KatE or LIC12032/LA1859), and a TolC-like outer membrane protein (LIC12575/LA1100). However, the corresponding genes were not differentially expressed in our study, which examined iron-limiting conditions alone.
We identified 20 genes which encode proteins with no similarity to proteins found in other bacteria with increased transcription under low-iron conditions. Of these, 16 were absent from the genome of the saprophyte L. biflexa (Table 2) (58) and may encode unique leptospiral virulence factors.
Genes involved in iron acquisition are transcriptionally regulated by iron availability through the action of Fur in many species of bacteria. Louvel et al. (45) were not able to identify putative Fur boxes in the promoter regions of genes predicted to be involved in iron uptake. Likewise, we also were unable to identify a clear consensus Fur box for genes upregulated by low iron.
Genes with altered expression in the la1857 mutant.
The genome of L. interrogans encodes four predicted Fur homologs, LA1857, LA2887, LA3094, and LB183; we have constructed a transposon insertion mutation in la1857 (51) with the insertion 62 bp into the 438-bp gene. Global transcription of the M776 mutant strain was compared with that of the wild-type serovar Manilae parent, both of which were grown under iron-replete conditions. If LA1857 does indeed function as a ferric uptake regulatory protein, we expected that its inactivation would have the same effect as growth under iron limitation. However, our results showed that this was not the case. Genes differentially expressed in the LA1857 mutant did not correspond to the iron-regulated genes in the wild-type strain, with only two downregulated and one upregulated gene being common to both data sets. Therefore, LA1857 does not play a significant role in regulation of iron homeostasis. Repeated attempts to complement the mutation were unsuccessful, but given the >1-kb intergenic region between la1857 and the next gene, there are unlikely to be downstream polarity effects.
We identified 11 and 20 genes which were down- and upregulated, respectively, in the mutant strain (Table 3). The microarray data were validated by real-time RT-PCR analysis of 13 genes, yielding a correlation coefficient (R2) of 0.7638 (data not shown). In the la1857 mutant, expression of LA1857 was negligible (Table 3) and likely represents nonspecific background fluorescence.
TABLE 3.
L. interrogans sv Manilae genes differentially expressed in the la1857 mutant versus wild type, grouped by COGa category
| Regulation and locus tagb | Mean fold change | P value | COG | Gene | Description of product | Predicted locationc | Transcriptional response in parent strain under iron limitationd |
|---|---|---|---|---|---|---|---|
| Downregulated | |||||||
| LA0951 | −7.04 | 0.02 | —e | Conserved hypothetical protein | IM | ||
| LA2020 | −2.05 | <0.01 | — | Hypothetical protein | NON-CYT | ||
| LA2882 | −4.61 | 0.04 | — | Hypothetical protein | CYT | ↑ | |
| LA0632 | −4.18 | <0.01 | GT | ptsN | Protein-N(pi)-phosphohistidine-sugar phosphotransferase, subunit A | CYT | ↓ |
| LA1603 | −2.45 | 0.02 | M | Glycosyltransferase | CYT | ||
| LA3247 | −3.33 | <0.01 | M | TonB-related protein | NON-CYT | ||
| LA1857 | −18.4 | <0.01 | P | fur | Ferric uptake regulation protein, Fur | CYT | |
| LA3242 | −2.42 | 0.03 | P | TonB-dependent receptor | OM | ||
| LA0598 | −2.27 | <0.01 | S | Conserved hypothetical protein | CYT | ↓ | |
| LA3244 | −2.86 | <0.01 | U | tolQ | Transport protein, TolQ-like | IM | |
| LA3245 | −2.47 | <0.01 | U | ExbD-related biopolymer transport protein | NON-CYT | ||
| Upregulated | |||||||
| LA0093 | 4.8 | <0.01 | — | Conserved hypothetical protein | CYT | ||
| LA0430 | 2.0 | <0.01 | — | Hypothetical protein | NON-CYT | ||
| LA0575 | 3.2 | 0.04 | — | Conserved hypothetical protein | CYT | ||
| LA1783 | 2.8 | <0.01 | — | Hypothetical protein | NON-CYT | ||
| LA1833 | 8.9 | 0.01 | — | Phage-related protein | CYT | ||
| LA2588 | 5.0 | 0.02 | — | Hypothetical protein | CYT | ||
| LIC12653 | 3.6 | 0.01 | — | Conserved hypothetical protein | UNK | ||
| LIC12658 | 3.1 | 0.03 | — | Hypothetical protein | CYT | ||
| LA0630 | 5.0 | <0.01 | C | Oxidoreductase | CYT | ||
| LA1209 | 2.7 | 0.04 | E | pepD | Dipeptidase | NON-CYT | |
| LB011 | 2.4 | <0.01 | H | hemCD | Bifunctional porphobilinogen deaminase/uroporphyrinogen synthase | CYT | |
| LB012 | 2.0 | <0.01 | H | hemB | Delta-aminolevulinic acid dehydratase | CYT | |
| LB013 | 2.5 | <0.01 | H | hemL | Glutamate-1-semialdehyde aminotransferase | CYT | |
| LA0084 | 3.5 | 0.04 | I | plsC | 1-Acylglycerol-3-phosphate O-acyltransferase | CYT | |
| LA0932 | 4.8 | 0.03 | I | pldB-2 | Lysophospholipase | CYT | |
| LA0943 | 8.5 | 0.02 | J | infB | Translation initiation factor 2 (IF-2; GTPase) | CYT | |
| LA1595 | 3.4 | 0.02 | M | Pyridoxal phosphate-dependent aminotransferase | CYT | ↓ | |
| LA0957 | 3.7 | 0.01 | MU | Outer membrane efflux protein related to TolC | OM | ||
| LA0666 | 5.7 | <0.01 | P | mauG-2 | Cytochrome c peroxidase | NON-CYT | |
| LA1859 | 2.3 | 0.01 | P | katE | Catalase | CYT |
COG categories are defined in the legend to Fig. 1.
Locus tags from L. interrogans sv Lai (LA number) have been used, except where the gene is unique to sv Copenhageni (LIC number) and is present in sv Manilae.
Predicted locations are defined in Table 2.
Transcriptional change effect from Table 2.
—, not in COGs.
The Fur family of metalloregulatory proteins, Fur, Zur (zinc uptake regulator), and PerR (a peroxide stress regulator), are structurally similar and share high sequence similarity (29). Multiple Fur homologs have been described in other bacterial species; Campylobacter jejuni has two (Fur and PerR) and B. subtilis has three (Fur, Zur, and PerR) (11, 25, 68). In B. subtilis, while the paralogs are structurally similar, each performs distinct functions with very little or no regulon overlap (24). Likewise, the four predicted Fur homologs in L. interrogans may play distinct regulatory roles. B. burgdorferi does not require iron for growth, yet it possesses a Fur homolog, BosR, with 54.3% similarity to the B. subtilis PerR (59), which regulates dps, hemA, katA, and mrgA, all of which are involved in responses to oxidative stress and metal limitation (11, 14). In C. jejuni, PerR regulates the katA and ahpC genes (68). Therefore, on the basis of our microarray data (see below), LA1857 appears to be functionally more similar to PerR and is likely to function as a derepressor of genes encoding proteins involved in the oxidative stress response.
Catalase (KatA) and alkyl hydroperoxide reductase or peroxiredoxin (AhpC) are important bacterial enzymes in defense against oxidative stress. The katE gene, encoding a putative catalase, was upregulated 2.4-fold in M776. However, expression of ahpC (LA2809) was not altered. A gene encoding a putative cytochrome c peroxidase (mauG-2 or la0666) was also upregulated in the mutant. Cytochrome c peroxidase plays a role in protection against peroxide killing in Neisseria gonorrhoeae (63). Resistance to oxidative stress is likely to be important in the early stages of infection, allowing pathogenic leptospires to persist in phagocytes and disseminate (18). Genes in the heme biosynthesis pathway (hemCD, hemB, and hemL) were also upregulated more than 2-fold in the mutant strain. The first gene in the putative heme synthesis operon, hemA, was slightly upregulated at 1.8-fold (P < 0.001) (see Table S1 in the supplemental material).
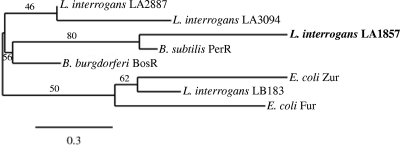
Phylogenetic analysis showed that LA1857 and LA2887 are indeed more similar to the B. subtilis PerR protein and BosR from B. burgdorferi, while LB183 and LA3094 are more similar to the E. coli Zur and Fur proteins, respectively (Fig. 2). B. burgdorferi BosR regulates the oxidative stress response and interfaces with the RpoS cascade and subsequent expression of known virulence factors; inactivation of BosR resulted in loss of infectivity in mice (31, 32, 56). However, the M776 mutant strain was not attenuated for virulence in the hamster model of infection (51), a finding that is consistent with derepression of oxidative stress genes potentially regulated by LA1857 and that indicates different roles for LA1857 and BosR.
FIG. 2.
Phylogenetic analysis of L. interrogans Fur homologs and other Fur family proteins generated by neighborhood joining with 100 bootstrap replicates and rooted at the midpoint. Branch support values are shown as percentages.
In B. subtilis, the Fe(II)-bound form of PerR is highly sensitive to metal-catalyzed oxidation by bound ferrous ions, resulting in Fe release and loss of DNA binding activity, leading to derepression of oxidative stress genes (38). Accordingly, we also observed that expression of katE was further increased by 4.3-fold in the mutant versus wild type when both were grown under low-iron conditions (see Table S1 in the supplemental material). In many bacteria, expression of KatA and AhpC is regulated by OxyR in response to oxidative stress (15). OxyR is an activator of peroxide stress genes and is not a metalloprotein. Instead, hydrogen peroxide catalyzes disulfide bond formation in OxyR, allowing it to bind to and induce expression of peroxide stress genes (73). Since genes encoding OxyR and SoxRS are absent from L. interrogans genomes (54, 60), we hypothesize that LA1857 is a PerR-like regulator and a functional analog of OxyR, although they clearly have different modes of action. C. jejuni also lacks oxyR, and the peroxide stress response is regulated by PerR (68). However, unlike C. jejuni, katE expression in L. interrogans was not iron responsive (see Table S1 in the supplemental material), and given that there was little overlap in the low-iron and mutant microarray data sets, regulation of responses to iron limitation and peroxide stress appear to be distinct. Since LA1857 is a Fur-like protein, divalent metal ions would be required to activate DNA binding activity. A number of residues (H37, D85, H91, H93, and D104) have been shown to be important for divalent metal binding in the B. subtilis PerR (38). An alignment of LA1857 and the B. subtilis PerR showed that the metal binding residues are also present in LA1857 (Fig. 3).
FIG. 3.
Alignment of amino acid sequences of LA1857 and B. subtilis PerR showing identical residues (*), conserved residues (:), and semiconserved residues (.). Residues shown to be important for divalent metal binding (Fe2+ or Mn2+) in B. subtilis PerR are also present in LA1857 (boxed).
While inactivation of la1857 significantly increased the expression of katE under iron-replete conditions, expression of the la1858 gene immediately upstream of katE was not altered in the mutant. The la1858 and katE genes in serovar Manilae overlap by 1 nucleotide, suggesting transcriptional coupling. However, analysis of the sequence upstream of katE revealed a putative PerR binding box with 13/15 matches to the consensus sequence (24), consistent with our hypothesis that LA1857 is a PerR homolog and regulator of katE.
Inactivation of PerR in C. jejuni results in hyperresistance to peroxide stress (68). Since mutation of la1857 resulted in derepression of katE and mauG-2 (Table 3), to determine whether this also results in increased resistance to oxidative stress, sensitivity to hydrogen peroxide between the mutant and wild-type strains was compared. The mutant was 8-fold more resistant to hydrogen peroxide; the MBC for the la1857 mutant was 430 μM, while the wild type was killed at 54 μM (Fig. 4). This finding is consistent with the higher levels of katE expression seen in the mutant, and therefore, LA1857 does indeed play a role in the oxidative stress response. However, we did not observe any difference in resistance to cumene hydroperoxide (Fig. 4). In Streptococcus pyogenes, PerR also regulates the peroxide stress response, but unlike C. jejuni and B. subtilis, the S. pyogenes PerR does not regulate ahpC (34). Glutathione peroxidase (GpoA) and AhpC are not required in the induced response to peroxide stress in S. pyogenes but do contribute to resistance against cumene hydroperoxide. Likewise, in L. interrogans PerR did not regulate ahpC (LA2809), which was not differentially expressed in the mutant versus the wild type. In addition, genes encoding other AhpC homologs (LA0734, LA0862, LA2312, LA2494, LA2809, LA3442, and LB117) and two GpoA homologs (LA1007 and LA4299) were also not differentially expressed in the LA1857 mutant, which may explain the lack of difference in resistance to cumene hydroperoxide between the LA1857 mutant and the wild type. The expression behavior of the ahpC and gpoA homologs in wild-type L. interrogans grown under peroxide stress conditions awaits further study.
FIG. 4.
Minimum bactericidal concentrations of hydrogen peroxide and cumene hydroperoxide for the LA1857 mutant (Mut) and wild type (WT). Three biological replicates were tested in triplicate, yielding identical results. The LA1857 mutant was 8-fold more resistant to killing by hydrogen peroxide, whereas there was no difference in resistance to cumene hydroperoxide.
It would be interesting to examine the roles of the other Fur homologs in L. interrogans. However, directed mutagenesis is extremely inefficient in pathogenic leptospires, with only two targeted mutations in a pathogenic species of Leptospira being reported to date (16, 39). Ongoing screening of our transposon library has not yet yielded mutations of the other Fur homologs. It is thus not possible to inactivate each of the homologs individually and/or in combination and study the subsequent effect on transcription patterns. This is the first report of peroxide stress regulation by a Fur-like protein in L. interrogans. However, since there are four predicted Fur homologs in L. interrogans svs Lai and Copenhageni, we suggest that each plays a distinct role in iron, metal, or stress responses.
Supplementary Material
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Katarzyna Rainczuk and Vicki Vallance.
This work was supported by the National Health and Medical Research Council and the Australian Research Council, Canberra, Australia (to B.A.), by Public Health Service grant AI-34431 from the National Institute of Allergy and Infectious Diseases (to D.A.H.), and by VA Medical Research Funds (to D.A.H.). G.L.M. is the recipient of a NHMRC Peter Doherty Fellowship.
D.A.H. is a consultant for Merial, which markets vaccines for prevention of leptospirosis.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 30 August 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Adler, B., and A. de la Pena Moctezuma. 2010. Leptospira and leptospirosis. Vet. Microbiol. 140:287-296. [DOI] [PubMed] [Google Scholar]
- 2.Adler, B., and S. Faine. 1976. Susceptibility of mice treated with cyclophosphamide to lethal infection with Leptospira interrogans serovar pomona. Infect. Immun. 14:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmer, B. M., M. G. Thomas, R. A. Larsen, and K. Postle. 1995. Characterization of the exbBD operon of Escherichia coli and the role of ExbB and ExbD in TonB function and stability. J. Bacteriol. 177:4742-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 5.Angerer, A., and V. Braun. 1998. Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Arch. Microbiol. 169:483-490. [DOI] [PubMed] [Google Scholar]
- 6.Asuthkar, S., S. Velineni, J. Stadlmann, F. Altmann, and M. Sritharan. 2007. Expression and characterization of an iron-regulated hemin-binding protein, HbpA, from Leptospira interrogans serovar Lai. Infect. Immun. 75:4582-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbosa, A. S., P. A. Abreu, F. O. Neves, M. V. Atzingen, M. M. Watanabe, M. L. Vieira, Z. M. Morais, S. A. Vasconcellos, and A. L. Nascimento. 2006. A newly identified leptospiral adhesin mediates attachment to laminin. Infect. Immun. 74:6356-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, J. M. Vinetz, and Peru-United States Leptospirosis Consortium. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757-771. [DOI] [PubMed] [Google Scholar]
- 9.Bjorn, M. J., B. H. Iglewski, S. K. Ives, J. C. Sadoff, and M. L. Vasil. 1978. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect. Immun. 19:785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun, V. 1989. The structurally related exbB and tolQ genes are interchangeable in conferring tonB-dependent colicin, bacteriophage, and albomycin sensitivity. J. Bacteriol. 171:6387-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 12.Bullen, J. J. 1981. The significance of iron in infection. Rev. Infect. Dis. 3:1127-1138. [DOI] [PubMed] [Google Scholar]
- 13.Calderwood, S. B., and J. J. Mekalanos. 1987. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J. Bacteriol. 169:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, L., L. P. James, and J. D. Helmann. 1993. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J. Bacteriol. 175:5428-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christman, M. F., G. Storz, and B. N. Ames. 1989. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc. Natl. Acad. Sci. U. S. A. 86:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croda, J., C. P. Figueira, E. A. Wunder, Jr., C. S. Santos, M. G. Reis, A. I. Ko, and M. Picardeau. 2008. Targeted mutagenesis in pathogenic Leptospira species: disruption of the LigB gene does not affect virulence in animal models of leptospirosis. Infect. Immun. 76:5826-5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullen, P. A., S. J. Cordwell, D. M. Bulach, D. A. Haake, and B. Adler. 2002. Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect. Immun. 70:2311-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis, J. M., D. A. Haake, and L. Ramakrishnan. 2009. Leptospira interrogans stably infects zebrafish embryos, altering phagocyte behavior and homing to specific tissues. PLoS Negl. Trop. Dis. 3:e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delany, I., R. Rappuoli, and V. Scarlato. 2004. Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis. Mol. Microbiol. 52:1081-1090. [DOI] [PubMed] [Google Scholar]
- 20.Delany, I., G. Spohn, A. B. Pacheco, R. Ieva, C. Alaimo, R. Rappuoli, and V. Scarlato. 2002. Autoregulation of Helicobacter pylori Fur revealed by functional analysis of the iron-binding site. Mol. Microbiol. 46:1107-1122. [DOI] [PubMed] [Google Scholar]
- 21.Eick-Helmerich, K., and V. Braun. 1989. Import of biopolymers into Escherichia coli: nucleotide sequences of the exbB and exbD genes are homologous to those of the tolQ and tolR genes, respectively. J. Bacteriol. 171:5117-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eshghi, A., P. A. Cullen, L. Cowen, R. L. Zuerner, and C. E. Cameron. 2009. Global proteome analysis of Leptospira interrogans. J. Proteome Res. 8:4564-4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faine, S. 1959. Iron as a growth requirement for pathogenic Leptospira. J. Gen. Microbiol. 20:246-251. [DOI] [PubMed] [Google Scholar]
- 24.Fuangthong, M., and J. D. Helmann. 2003. Recognition of DNA by three ferric uptake regulator (Fur) homologs in Bacillus subtilis. J. Bacteriol. 185:6348-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaballa, A., and J. D. Helmann. 1998. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J. Bacteriol. 180:5815-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guegan, R., J. M. Camadro, I. Saint Girons, and M. Picardeau. 2003. Leptospira spp. possess a complete haem biosynthetic pathway and are able to use exogenous haem sources. Mol. Microbiol. 49:745-754. [DOI] [PubMed] [Google Scholar]
- 27.Haake, D. A., M. Dundoo, R. Cader, B. M. Kubak, R. A. Hartskeerl, J. J. Sejvar, and D. A. Ashford. 2002. Leptospirosis, water sports, and chemoprophylaxis. Clin. Infect. Dis. 34:e40-e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haake, D. A., C. Martinich, T. A. Summers, E. S. Shang, J. D. Pruetz, A. M. McCoy, M. K. Mazel, and C. A. Bolin. 1998. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late-log-phase growth and mammalian infection. Infect. Immun. 66:1579-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hantke, K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4:172-177. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez, J. A., A. M. Muro-Pastor, E. Flores, M. T. Bes, M. L. Peleato, and M. F. Fillat. 2006. Identification of a furA cis antisense RNA in the cyanobacterium Anabaena sp. PCC 7120. J. Mol. Biol. 355:325-334. [DOI] [PubMed] [Google Scholar]
- 31.Hyde, J. A., D. K. Shaw, R. Smith Iii, J. P. Trzeciakowski, and J. T. Skare. 2009. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol. Microbiol. 74:1344-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyde, J. A., D. K. Shaw, R. Smith III, J. P. Trzeciakowski, and J. T. Skare. 2010. Characterization of a conditional bosR mutant in Borrelia burgdorferi. Infect. Immun. 78:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, R. C., J. Walby, R. A. Henry, and N. E. Auran. 1973. Cultivation of parasitic leptospires: effect of pyruvate. Appl. Microbiol. 26:118-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King, K. Y., J. A. Horenstein, and M. G. Caparon. 2000. Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes. J. Bacteriol. 182:5290-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko, A. I., M. Galvao Reis, C. M. Ribeiro Dourado, W. D. Johnson, Jr., and L. W. Riley. 1999. Urban epidemic of severe leptospirosis in Brazil. Lancet 354:820-825. [DOI] [PubMed] [Google Scholar]
- 36.Ko, A. I., C. Goarant, and M. Picardeau. 2009. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat. Rev. Microbiol. 7:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, J. W., and J. D. Helmann. 2007. Functional specialization within the Fur family of metalloregulators. Biometals 20:485-499. [DOI] [PubMed] [Google Scholar]
- 38.Lee, J. W., and J. D. Helmann. 2006. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 440:363-367. [DOI] [PubMed] [Google Scholar]
- 39.Liao, S., A. Sun, D. M. Ojcius, S. Wu, J. Zhao, and J. Yan. 2009. Inactivation of the fliY gene encoding a flagellar motor switch protein attenuates mobility and virulence of Leptospira interrogans strain Lai. BMC Microbiol. 9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litwin, C. M., and S. B. Calderwood. 1993. Role of iron in regulation of virulence genes. Clin. Microbiol. Rev. 6:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo, M., D. M. Bulach, D. R. Powell, D. A. Haake, J. Matsunaga, M. L. Paustian, R. L. Zuerner, and B. Adler. 2006. Effects of temperature on gene expression patterns in Leptospira interrogans serovar Lai as assessed by whole-genome microarrays. Infect. Immun. 74:5848-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo, M., S. J. Cordwell, D. M. Bulach, and B. Adler. 2009. Comparative transcriptional and translational analysis of leptospiral outer membrane protein expression in response to temperature. PLoS Negl. Trop. Dis. 3:e560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louvel, H., J. M. Betton, and M. Picardeau. 2008. Heme rescues a two-component system Leptospira biflexa mutant. BMC Microbiol. 8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louvel, H., S. Bommezzadri, N. Zidane, C. Boursaux-Eude, S. Creno, A. Magnier, Z. Rouy, C. Medigue, I. Saint Girons, C. Bouchier, and M. Picardeau. 2006. Comparative and functional genomic analyses of iron transport and regulation in Leptospira spp. J. Bacteriol. 188:7893-7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louvel, H., I. Saint Girons, and M. Picardeau. 2005. Isolation and characterization of FecA- and FeoB-mediated iron acquisition systems of the spirochete Leptospira biflexa by random insertional mutagenesis. J. Bacteriol. 187:3249-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masse, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:4620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masse, E., C. K. Vanderpool, and S. Gottesman. 2005. Effect of RyhB small RNA on global iron use in Escherichia coli. J. Bacteriol. 187:6962-6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsunaga, J., M. Lo, D. M. Bulach, R. L. Zuerner, B. Adler, and D. A. Haake. 2007. Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect. Immun. 75:2864-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McBride, A. J., D. A. Athanazio, M. G. Reis, and A. I. Ko. 2005. Leptospirosis. Curr. Opin. Infect. Dis. 18:376-386. [DOI] [PubMed] [Google Scholar]
- 50.Murray, G. L., K. M. Ellis, M. Lo, and B. Adler. 2008. Leptospira interrogans requires a functional heme oxygenase to scavenge iron from hemoglobin. Microbes Infect. 10:791-797. [DOI] [PubMed] [Google Scholar]
- 51.Murray, G. L., V. Morel, G. M. Cerqueira, J. Croda, A. Srikram, R. Henry, A. I. Ko, O. A. Dellagostin, D. M. Bulach, R. W. Sermswan, B. Adler, and M. Picardeau. 2009. Genome-wide transposon mutagenesis in pathogenic Leptospira species. Infect. Immun. 77:810-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray, G. L., A. Srikram, R. Henry, A. Puapairoj, R. W. Sermswan, and B. Adler. 2009. Leptospira interrogans requires heme oxygenase for disease pathogenesis. Microbes Infect. 11:311-314. [DOI] [PubMed] [Google Scholar]
- 53.Nally, J. E., J. F. Timoney, and B. Stevenson. 2001. Temperature-regulated protein synthesis by Leptospira interrogans. Infect. Immun. 69:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nascimento, A. L., A. I. Ko, E. A. Martins, C. B. Monteiro-Vitorello, P. L. Ho, D. A. Haake, S. Verjovski-Almeida, R. A. Hartskeerl, M. V. Marques, M. C. Oliveira, C. F. Menck, L. C. Leite, H. Carrer, L. L. Coutinho, W. M. Degrave, O. A. Dellagostin, H. El-Dorry, E. S. Ferro, M. I. Ferro, L. R. Furlan, M. Gamberini, E. A. Giglioti, A. Goes-Neto, G. H. Goldman, M. H. Goldman, R. Harakava, S. M. Jeronimo, I. L. Junqueira-de-Azevedo, E. T. Kimura, E. E. Kuramae, E. G. Lemos, M. V. Lemos, C. L. Marino, L. R. Nunes, R. C. de Oliveira, G. G. Pereira, M. S. Reis, A. Schriefer, W. J. Siqueira, P. Sommer, S. M. Tsai, A. J. Simpson, J. A. Ferro, L. E. Camargo, J. P. Kitajima, J. C. Setubal, and M. A. Van Sluys. 2004. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J. Bacteriol. 186:2164-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nascimento, A. L., S. Verjovski-Almeida, M. A. Van Sluys, C. B. Monteiro-Vitorello, L. E. Camargo, L. A. Digiampietri, R. A. Harstkeerl, P. L. Ho, M. V. Marques, M. C. Oliveira, J. C. Setubal, D. A. Haake, and E. A. Martins. 2004. Genome features of Leptospira interrogans serovar Copenhageni. Braz. J. Med. Biol. Res. 37:459-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ouyang, Z., M. Kumar, T. Kariu, S. Haq, M. Goldberg, U. Pal, and M. V. Norgard. 2009. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol. Microbiol. 74:1331-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patarakul, K., M. Lo, and B. Adler. 2010. Global transcriptomic response of Leptospira interrogans serovar Copenhageni upon exposure to serum. BMC Microbiol. 10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Picardeau, M., D. M. Bulach, C. Bouchier, R. L. Zuerner, N. Zidane, P. J. Wilson, S. Creno, E. S. Kuczek, S. Bommezzadri, J. C. Davis, A. McGrath, M. J. Johnson, C. Boursaux-Eude, T. Seemann, Z. Rouy, R. L. Coppel, J. I. Rood, A. Lajus, J. K. Davies, C. Medigue, and B. Adler. 2008. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS One 3:e1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Posey, J. E., and F. C. Gherardini. 2000. Lack of a role for iron in the Lyme disease pathogen. Science 288:1651-1653. [DOI] [PubMed] [Google Scholar]
- 60.Ren, S. X., G. Fu, X. G. Jiang, R. Zeng, Y. G. Miao, H. Xu, Y. X. Zhang, H. Xiong, G. Lu, L. F. Lu, H. Q. Jiang, J. Jia, Y. F. Tu, J. X. Jiang, W. Y. Gu, Y. Q. Zhang, Z. Cai, H. H. Sheng, H. F. Yin, Y. Zhang, G. F. Zhu, M. Wan, H. L. Huang, Z. Qian, S. Y. Wang, W. Ma, Z. J. Yao, Y. Shen, B. Q. Qiang, Q. C. Xia, X. K. Guo, A. Danchin, I. Saint Girons, R. L. Somerville, Y. M. Wen, M. H. Shi, Z. Chen, J. G. Xu, and G. P. Zhao. 2003. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422:888-893. [DOI] [PubMed] [Google Scholar]
- 61.Ristow, P., P. Bourhy, F. W. McBride, C. P. Figueira, M. Huerre, P. Ave, I. S. Girons, A. I. Ko, and M. Picardeau. 2007. The OmpA-Like protein Loa22 is essential for leptospiral virulence. PLoS Pathog. 3:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saal, L. H., C. Troein, J. Vallon-Christersson, S. Gruvberger, A. Borg, and C. Peterson. 2002. BioArray Software Environment (BASE): a platform for comprehensive management and analysis of microarray data. Genome Biol. 3:software0003.0001-0003.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seib, K. L., H. J. Tseng, A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2004. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J. Infect. Dis. 190:136-147. [DOI] [PubMed] [Google Scholar]
- 64.Stevenson, B., H. A. Choy, M. Pinne, M. L. Rotondi, M. C. Miller, E. Demoll, P. Kraiczy, A. E. Cooley, T. P. Creamer, M. A. Suchard, C. A. Brissette, A. Verma, and D. A. Haake. 2007. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS One 2:e1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tai, S. P., A. E. Krafft, P. Nootheti, and R. K. Holmes. 1990. Coordinate regulation of siderophore and diphtheria toxin production by iron in Corynebacterium diphtheriae. Microb. Pathog. 9:267-273. [DOI] [PubMed] [Google Scholar]
- 66.Tatusov, R. L., E. V. Koonin, and D. J. Lipman. 1997. A genomic perspective on protein families. Science 278:631-637. [DOI] [PubMed] [Google Scholar]
- 67.Van Hove, B., H. Staudenmaier, and V. Braun. 1990. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J. Bacteriol. 172:6749-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Vliet, A. H., M. L. Baillon, C. W. Penn, and J. M. Ketley. 1999. Campylobacter jejuni contains two fur homologs: characterization of iron-responsive regulation of peroxide stress defense genes by the PerR repressor. J. Bacteriol. 181:6371-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vecerek, B., I. Moll, and U. Blasi. 2007. Control of Fur synthesis by the non-coding RNA RyhB and iron-responsive decoding. EMBO J. 26:965-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verma, A., S. Artiushin, J. Matsunaga, D. A. Haake, and J. F. Timoney. 2005. LruA and LruB, novel lipoproteins of pathogenic Leptospira interrogans associated with equine recurrent uveitis. Infect. Immun. 73:7259-7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verma, A., J. Hellwage, S. Artiushin, P. F. Zipfel, P. Kraiczy, J. F. Timoney, and B. Stevenson. 2006. LfhA, a novel factor H-binding protein of Leptospira interrogans. Infect. Immun. 74:2659-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagegg, W., and V. Braun. 1981. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA. J. Bacteriol. 145:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng, M., F. Aslund, and G. Storz. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718-1721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.