Abstract
Central to the pathogenesis of many bacterial pathogens is the ability to deliver effector proteins directly into the cells of their eukaryotic host. EspF is one of many effector proteins exclusive to the attaching and effacing pathogen family that includes enteropathogenic (EPEC) and enterohemorrhagic (EHEC) Escherichia coli. Work in recent years has revealed EspF to be one of the most multifunctional effector proteins known, with defined roles in several host cellular processes, including disruption of the epithelial barrier, antiphagocytosis, microvillus effacement, host membrane remodelling, modulation of the cytoskeleton, targeting and disruption of the nucleolus, intermediate filament disruption, cell invasion, mitochondrial dysfunction, apoptosis, and inhibition of several important epithelial transporters. Surprisingly, despite this high number of functions, EspF is a relatively small effector protein, and recent work has begun to decipher the molecular events that underlie its multifunctionality. This review focuses on the activities of EspF within the host cell and discusses recent findings and molecular insights relating to the virulence functions of this fascinating bacterial effector.
Many of the world's most important diseases are caused by bacterial pathogens that deliver a large number of virulence proteins, termed effectors, into the cells of their host. One group of bacterial pathogens, the attaching and effacing (A/E) family, targets the mammalian intestinal tract, where they cause histopathological lesions on the apical surface of host enterocytes (51). A/E pathogens, which include enteropathogenic Escherichia coli (EPEC), enterohemorrhagic E. coli (EHEC), and Citrobacter rodentium, remain on the surface of host cells and inject a raft of effector proteins through the bacterium's type three secretion system (T3SS). These effector proteins cause major disruption to host cell physiology, ultimately leading to diarrheal disease.
Common to all A/E pathogens is a chromosomal pathogenicity island called the locus of enterocyte effacement (LEE), which carries many virulence genes, including the T3SS and six known effector proteins, Tir, Map, EspF, EspG, EspH, and EspZ (10). Tir is the best studied LEE effector and is known to target the host plasma membrane, where it serves as a receptor for the bacterial outer membrane protein Intimin. Tir is an essential virulence determinant (15, 31), possibly relating to its role in bacterial attachment, while a critical role for EspZ (previously known as SepZ) has also been demonstrated (15). For the remaining A/E pathogen effectors, it is widely accepted that most play a smaller, additive role in virulence, although the deletion of some effector genes has no obvious defects (25). Despite this, the biological functions of many A/E pathogen effectors have been determined in some depth, particularly those of the LEE-encoded effector EspF, which is becoming a model bacterial effector of multifunctionality, an emerging feature of effector proteins (27). Currently, EspF has the greatest number of reported functions among the A/E pathogen effectors and is one of the most multifunctional effector proteins known. Here, we review the diverse functions of EspF and discuss its role in disease processes in light of recent findings.
DISCOVERY OF EspF AND ITS MODULAR STRUCTURE
EspF was first identified for enteropathogenic E. coli by McNamara and Donnenberg, on the basis that it contained three eukaryotic-like proline-rich repeats (PRR) (34). Each PRR comprises two putative overlapping Src homology 3 (SH3]) binding domains with the consensus PxxP motif (Fig. 1) that is reported to mediate binding to a large number of eukaryotic signaling proteins containing the well-characterized SH3 domain (33). The presence of the PRR and SH3 binding motifs initially suggested that EspF could be an effector protein, and subsequent secretion and translocation assays revealed this to be the case (34, 35). EspF has since been found in all A/E pathogens, although its size varies, being dictated by the number of PRR modules, with the rabbit-specific EPEC strain RDEC-1 possessing two, prototypical EPEC three, EHEC four, and C. rodentium five (Fig. 1A). Some A/E pathogens carry additional EspF homologues, such as Tccp/EspF(U), which displays partial similarity to EspF and possesses the PRR regions (4, 18). However, Tccp/EspF(U) effectors have been reviewed elsewhere and will not be discussed here in detail (17).
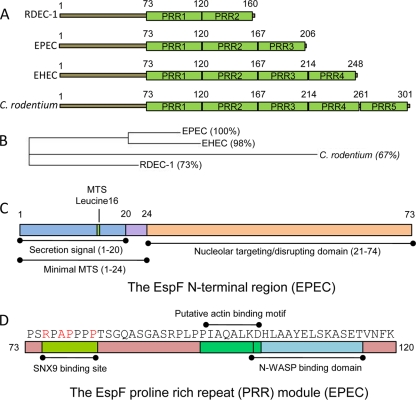
FIG. 1.
Modular architecture of EspF. (A) The different sizes of the four main EspF variants are determined by their number of proline-rich repeats (PRR), which are almost identical in size and sequence similarity. The EPEC and EHEC strains relate to E2348/69 and EDL933, respectively. (B) Phylogenetic tree of the N-terminal EspF region (residues 1 to 73) produced by using Clustal W software. Percent values indicate sequence identity relative to the EPEC variant. (C) Modular structure of the N-terminal region (residues 1 to 73) comprising the secretion signal, mitochondrial targeting signal (MTS), and nucleolar targeting domain. (D) Typical EspF PRR module taken from EPEC EspF, showing the host protein binding motifs; SNX9, sorting nexin 9.
Like most bacterial effectors, EspF exhibits a modular architecture composed of several distinct functional domains along its length (Fig. 1). These include a well-conserved N-terminal region containing the bacterial secretion signal (residues 1 to 20) that is sufficient for EspF secretion and translocation into host cells (7). Superimposed within the N-terminal region are two organelle-targeting domains (Fig. 1C) that direct EspF to the host mitochondria (residues 1 to 24) or the nucleolus (residues 21 to 74) (14, 40, 42). Phylogenetic analysis of the N-terminal region prior to the PRR regions (residues 1 to 74) (Fig. 1B) shows that EPEC and EHEC EspF exhibit high similarity, while the RDEC-1 and C. rodentium variants are more distantly related (Fig. 1B). This variation appears to have functional significance, as the disruption of the host nucleolus (an EspF-dependent event) was not observed with C. rodentium or RDEC-1 (14), suggesting that mutations within region 21 to 74 alter the nucleolus-disruptive behavior of EspF in these pathogens (14). The remaining C-terminal region of EspF (residue 74 and onward in all variants) (Fig. 1A) comprises the PRR modules, which are almost identical in size and sequence homology between the EspF variants (Fig. 1D). In addition to the SH3 binding domains described above, the PRR also contain a functional neuronal Wiskott-Aldrich syndrome (N-WASP) binding motif (1) toward the C-terminal end of each repeat (Fig. 1D).
SUBCELLULAR TARGETS OF EspF
Microscopy analysis of infected host cells has consistently shown that almost all EspF is confined to host mitochondria (14, 29, 40, 42) (see Fig. 3). This finding suggested that mitochondria were either central to EspF's diverse functions or that other unidentified or transient target sites remained undiscovered. Alto et al. (1) revealed that when expressed in host cells, enhanced green fluorescent protein (EGFP)-EspF was highly dynamic and accumulated transiently in patches at the plasma membrane (1). This subcellular behavior was evident only because the N-terminal EGFP fusion abolishes EspF mitochondrial targeting (40), thereby uncovering EspF's extramitochondrial target sites. Recently, confocal sectioning of infected host cells revealed that in addition to being found in the mitochondria, EspF was found inside the host nucleolus (see Fig. 3C and D), a subnuclear structure responsible for ribosome biosynthesis. As targeting of the mitochondria and nucleolus are not required for the majority of EspF's functions (10, 14, 24, 47, 58) (see Fig. 3A), it is likely that a significant cytoplasmic pool of EspF exists, and Western blot analyses of fractionated host cells support this premise (29) (see Fig. 3E).
EspF'S EUKARYOTIC BINDING PARTNERS
EspF has been found to bind several host proteins (10), and while some putative interactions have been identified through yeast two-hybrid studies, many proven EspF binding partners have been determined and studied in greater detail. These include EspF interactions with actin, profilin, Arp2, N-WASP, sorting nexin 9, Abcf2, and the intermediate filament protein cytokeratin 18 (1, 43, 45, 57). In addition, affinity purification of glutathione S-transferase (GST)-tagged EspF expressed in yeast followed by mass spectrometry (MS) analysis has also revealed a number of interesting putative interactions, including a plasma membrane ATPase and E3 ubiquitin ligase, among others (see reference 22). Whether these EspF interactions in yeast translate to those in mammalian cells is unclear, particularly as the proven mammalian partners described above were not reported for yeast (22). Given the wide range of host cellular processes subverted by EspF (Fig. 2), it is likely that other eukaryotic binding partners remain to be discovered.
FIG. 2.
The multiple functions of EspF. (A) Table listing all known functions of EspF in A/E pathogens as described in the text. The far-left column indicates whether evidence for the EspF function is direct (host cell expression or biochemical studies) or related to infection with EspF-deficient bacterial mutants. (B) Diagram of intestinal cells illustrating the varied functions and subcellular behavior of EspF. Parentheses indicate the host protein interaction predicted or proven to play a role in the indicated function.
TARGETING THE MITOCHONDRIA: APOPTOSIS AND SELF-REGULATION
In silico analysis of the EspF protein indicates that it possesses a typical mitochondrial targeting sequence (MTS) within the first 101 amino acids (42). The N terminus of EspF has a predicted amphipathic α-helix, bearing a hydrophobic surface on one side and a positively charged hydrophilic face on the other side, features consistent with mitochondrial targeting sequences (41, 42). The N-terminal MTS of EPEC EspF has been verified experimentally with the first 24 amino acids sufficient for mitochondrial import (40), while residues 1 to 70 are important for full mitochondrial targeting (40, 42). There is also an essential role for leucine at position 16 in mitochondrial targeting (40, 42), and mutation of this residue to glutamic acid (L16E) has been a pivotal step in determining which of EspF's functions are dependent on mitochondrial targeting (see below) (Fig. 3 A). In addition, EspF exhibits a consensus Tom20 recognition motif in its N-terminal region (residues 13 to 17) (39), implying that its import into mitochondria is facilitated via the mitochondrial outer membrane protein Tom20 (40). Like many proteins imported into mitochondria, EspF is cleaved within the mitochondrial fraction of EPEC-infected cells, resulting in two distinct forms with approximate sizes of ∼28 kDa and ∼17 kDa (42) (Fig. 3). Processing of EspF is entirely dependent on an intact mitochondrial membrane potential, as the smaller cleaved form is not present in cells treated with the mitochondrial inhibitor valinomycin (14) (Fig. 3F).
FIG. 3.
Subcellular locations of EspF. (A) Table listing the predicted and proven subcellular locations for EspF's multiple functions, based on the assumption that EspF only targets the three locations given, although other sites are likely. Mito., mitochondrial; Nuc., nucleolar. (B) Colocalization of EspF with the mitochondrial marker DsRed in EPEC-infected HeLa cells. (C) EspF present in the mitochondria and nucleolus in late-stage-infected HeLa cells. (D) Nucleolar accumulation of the EspF variant (L16E) that cannot target the mitochondria. (E) Western blot (WB) showing EspF in both the mitochondrial (Mito.) and cytoplasmic (Cyto.) fractions of Caco-2 cells at all stages of infection. (F) Processing of EspF within the mitochondrial fraction of infected intestinal cells is dependent on an intact mitochondrial membrane potential (a) and is prevented with the mitochondrial inhibitor valinomycin (b).
Role in apoptosis.
Until recently, only one of EspF's functions, the induction of apoptosis, was dependent on mitochondrial targeting (40). EspF initiates the induction of the intrinsic apoptotic pathway by disrupting mitochondrial membrane potential (MMP), which releases cytochrome c into the cytoplasm, leading to caspase 9 cleavage (42). The mechanism has been dissected in more detail (42) with EspF shown to bind and cause depletion of the host protein Abcf2 within the mitochondria (43). RNA interference (RNAi) knockdowns also revealed an important role for Abcf2 in impeding apoptotic induction and provided a rationale for why EspF targets this particular protein (43). This interesting study highlighted the usefulness of using bacterial effectors in eukaryotic cell biology, as the work proposed that the relatively unknown protein Abcf2 was an antiapoptotic factor (43).
Whether apoptosis is EspF's primary role for targeting mitochondria is open to debate. Although EPEC induces apoptosis in nonpolarized cell types, such as HeLa cells in vitro (9, 40, 42), the apoptotic index is quite low, particularly compared to that of other pathogens (8, 13). In addition, target cell types such as polarized intestinal cells are relatively resistant to EPEC-mediated apoptosis (58), and late-stage apoptotic events are rarely observed for EPEC-infected cells (23). Collectively, these data suggest that apoptosis may not be the desired outcome of EspF mitochondrial targeting, at least with EPEC. This idea is further supported by a recent study which revealed that EPEC and C. rodentium employ the effector NleH to counteract apoptosis and promote host cell survival (23). However, in direct contrast to this finding, Nagai et al. (40) demonstrated that late-stage apoptosis (assessed by DNA fragmentation) is evident in the colons of mice infected with C. rodentium, mediated by EspF mitochondrial targeting. Two separate studies also showed high levels of apoptosis in the intestines of C. rodentium-infected mice (55, 56) that was also dependent on EspF (55). In the latter study, a pathophysiological role for EspF-mediated apoptosis was proposed, as it stimulated a specific immune response involving interleukin-17-producing T helper cells [T(H)17] within the mouse intestine (55). The authors concluded that apoptosis induced by Citrobacter preferentially invokes T(H)17 immune responses (55), although it is unclear what benefit such immune responses would confer during infection. Thus, although a role for apoptosis has not been demonstrated in vivo with EPEC, evidence is accumulating that apoptosis is a specific EspF-mediated event associated with C. rodentium infection. It is unclear why there are differences in the results of in vivo infection studies, but it is possible that different experimental conditions may affect the balance between pro- and antiapoptotic signaling and thus explain the different reported outcomes.
Case for spatio-temporal self-regulation.
Recent work has provided an alternative possibility (14) for why EspF targets the mitochondria and relates to the exploitation of mitochondria to control EspF's cellular functions. As most EspF is rapidly imported into active mitochondria during early infection, the effector is restricted in targeting other cellular sites (Fig. 3), an example of regulation by sequestration. However, as MMP progressively decreases with infection, induced by EspF (and other EPEC effectors) (26), mitochondrial import diminishes and newly delivered EspF is free to target other infection sites. One of these sites was recently revealed to be the nucleolus and chemical inhibition of MMP or using the EspF L16E variant that does not target the mitochondria, resulted in rapid nucleolar uptake of EspF (Fig. 3D). This kinetic regulatory role of the mitochondria enables EspF to perform its transient cytoplasmic roles (Fig. 3) en route to active mitochondria but also ensures that these EspF activities are tightly regulated and not overtly deleterious to the host cell. Taken together, the primary function of mitochondrial targeting and dysfunction by EspF may be a regulatory one with EspF-induced apoptosis, at least in EPEC-infected cells, possibly an undesirable in vitro observed outcome.
NUCLEOLUS: A NEW TARGET FOR BACTERIAL EFFECTORS
The nucleolus is a non-membrane-bound organelle within the nucleus of eukaryotic cells that is responsible primarily for ribosome biogenesis (2). Until recently, no bacterial pathogens were known to target the nucleolus despite almost all known viruses targeting proteins to this subnuclear structure. EspF was identified as the first bacterial protein to target the nucleolus (14), particularly during late-stage infection and also when expressed in host cells, driven by an N-terminal targeting domain (residues 21 to 74) (14). Within the nucleolus, EspF caused the mobilization of a subset of nucleolar factors, particularly the most abundant nucleolar protein nucleolin, which aberrantly entered the cytoplasm, depleting its nucleolar levels (14). This appears to be an important event, as nucleolin's role in ribosomal processing is dependent on its nucleolar location. Not surprisingly, ribosomal processing was also shown to be impaired by EspF, when ectopically expressed in host cells (14). Although the role of nucleolar targeting in disease is unclear, the disruption of ribosome biogenesis and protein translation would likely prevent de novo defense responses during long-term bacterial infection. However, this potential infection strategy may not be universal; unlike EPEC and EHEC, mobilization of nucleolin was not observed during infection with C. rodentium or RDEC-1 (14).
MEMBRANE REMODELLING AND CYTOSKELETAL MODULATION
EPEC EspF has six putative PxxP SH3 binding motifs within its three PRR domains, and two studies have revealed that it specifically binds to the SH3 domain of the host protein sorting nexin 9 (SNX9). EspF interacts with SNX9 in the cytosol during infection to induce the formation of membrane tubules (1, 29). Alto et al. (1) used phage display to determine that the preferred EspF binding site of SNX9 is RxAPxxP, a repeat sequence found at the N-terminal end of each PRR module. In the host cell, SNX9 is normally involved in host membrane-cytoskeleton processes, and the binding of EspF to SNX9 appears to regulate host membrane alterations (1). However, the role of membrane remodelling during bacterial infection has not been determined and is unlinked to the disruption of tight junctions (TJ) (1), NHE3 inhibition (see below), or antiphagocytosis (59). Recently, it has been suggested that EspF employs SNX9 for the invasion of intestinal epithelial cells (59) (as described below).
In addition to the SNX9 binding sites, the putative N-WASP binding motif of each PRR has also been shown to be functional, mediating the direct interaction of EspF with the Cdc42/Rac-interactive binding (CRIB) domain of N-WASP (1). All EspF variants possess the N-WASP binding sites, including the distantly related homologue Tccp/EspF(U), suggesting some level of functional similarity between these effectors. In vitro biochemical studies have revealed that EspF binds and activates N-WASP, which subsequently induces actin polymerization (1), and work on Tccp/EspF(U) has revealed that N-WASP activation is promoted by the synergistic actions of the EspF repeat regions, with multiple repeat regions conferring higher N-WASP activation than a single repeat alone (3, 44). Interestingly, a recent study has shown a direct interaction between actin itself and EspF from rabbit EPEC strain E22 (45). In the latter study, EspF was shown to contribute to the extent and size of actin-based pedestals in EPEC-infected cells, presumably through its direct modulation of the cytoskeleton, although no role for N-WASP was demonstrated (45). EspF modulation of actin may be linked to several prominent host cellular changes, including microvillus elongation (49), microvillus effacement (12), TJ disruption (35), and antiphagocytosis (47). Therefore, the ability of EspF to bind actin and signal through N-WASP may have important disease-relevant implications (45). Finally, EspF also binds cytokeratin 18, a protein that forms part of the intermediate filament network in epithelial cells, and this interaction was suggested to facilitate collapse of this important cellular structure (57).
ANTIPHAGOCYTOSIS, INTESTINAL CELL INVASION, AND M-CELL TRANSLOCATION
EPEC was known for several years to inhibit macrophage phagocytosis, but the effector(s) responsible remained elusive (6). EspF was originally discounted to play a role in antiphagocytosis (6), but two more recent studies showed that EspF is the main effector responsible for this function (30, 47), with roles for other effectors in the process (30). Indeed, EspF has been shown to inhibit PI-3 kinase-dependent uptake of the bacteria into macrophages, with a requirement for the N-terminal half of EspF but no role for mitochondrial targeting (47). More recently, Martinez-Argudo et al. have shown that EPEC inhibits its own uptake into intestinal M cells, dependent on EspF that probably inhibits signaling pathways similar to those observed with macrophages (32).
Although EspF inhibits bacterial uptake in macrophage/M-cell lines, it appears to have the converse role in intestinal enterocytes, for which it actively promotes cell invasion (59). EspF-mediated cell invasion was dependent on the SNX9 binding motifs of EspF, suggesting that SNX9 is involved in the bacterial internalization process (59). However, the intestinal cells used in this study were only partially polarized, and as EPEC is considered a noninvasive pathogen on polarized epithelia, the role of EPEC invasiveness in disease is unclear. In any case, this EspF-mediated mechanism is apparently missing from Hep-2 cells, as a separate study found no role for EspF in EPEC invasion of these cell types (34).
EspF-MEDIATED CELLULAR CHANGES LINKED DIRECTLY TO DIARRHEA
Several mechanisms contribute to the onset of diarrhea, including a loss of intestinal microvilli, disruption of epithelial tight junctions, and inhibition of epithelial transporter activity, all of which are mechanisms induced by A/E pathogens (19). Although these cellular processes are quite different, EspF has been demonstrated to play important roles in each, as described here.
Tight junctions.
Tight junctions (TJ) between adjacent cells form an effective barrier to the paracellular movement of water and small molecules, and their disruption is associated with diarrhea. Disruption of epithelial tight junctions is a well-documented EPEC event, measured by a drop in electrical resistance of an epithelial monolayer (5, 46, 52) and has also been shown in vivo with C. rodentium (28). This function was the first ascribed to EspF (35) and was also used to identify EspF's molecular chaperone CesF (16). The authors of the former study speculated that EspF may not be acting alone in the disruption of TJs (35), and indeed, subsequent studies have shown that EspF cooperates with the EPEC effectors Map, Tir, EspG, and NleA to mediate rapid TJ disruption (11, 13, 53, 54). The molecular details of EspF-mediated TJ disruption are slowly becoming unraveled, and while mitochondrial targeting is not involved (58), the recruitment of tight junctional proteins to bacterium-induced pedestals by EspF has been implicated (45). The EspF domains involved in TJ disruption remain undefined, but studies suggest that they likely exist in the PRR of the effector, as there is no obvious role for mitochondrial or nucleolar targeting (14, 58). Importantly, EspF has been shown to contribute to TJ disruption in vivo, suggesting that this cellular function plays an important role during infection, at least in the mouse model (20).
It has been known for some time that the outer membrane protein Intimin is essential for EPEC to mediate TJ disruption (11). Recent evidence suggests that the interaction of Intimin with the Tir effector (13) is a prerequisite event that is necessary for the TJ-disrupting activities of the other effectors, providing EPEC with additional control over effector function (13). This shows that EspF requires additional EPEC-mediated signaling to disrupt the TJs, and this is further supported by a separate study in which expression of EspF alone within epithelial cells had no observable effect on tight junctions (1).
Epithelial transporters.
In addition to barrier dysfunction, disruption of water reabsorption is a common mechanism linked to diarrhea. The reabsorption of water is dependent on epithelial transporters, such as the sodium glucose cotransporter SGLT1, the water channel aquaporin, or the sodium hydrogen exchanger NHE3. Surprisingly, all three of these epithelial transporters are targeted and impaired by EspF during bacterial infection (12, 21, 24). SGLT-1 is the main intestinal water pump, and its importance is demonstrated in human genetic disorders in which a mutant form of SGLT-1 results in excessive diarrhea and infant death (36). SGLT-1 is rapidly inactivated prior to the loss of microvilli by the cooperative activities of EspF, Map, Tir, EspG, and EspH (12), with EspF having been shown to play a significant role (P. Dean, S. Quitard, N. Garmy, C. Gillespie, and B. Kenny, unpublished data). NHE3, in contrast, mediates the exchange of sodium-hydrogen ions in the intestine and has been linked to diarrhea in NHE3-knockout mice (50). Hodges et al. (24) revealed that NHE3 is targeted by EPEC in a time-dependent manner and that EspF was almost entirely responsible, given that an espF mutant was highly defective in this subversive event. Finally, EspF also acts upon a third class of epithelial surface protein, the intestinal water channel aquaporin (21). Mice infected with C. rodentium display a marked alteration in the cellular location of aquaporin within the mouse colon, which was linked to diarrheal symptoms (21), and EspF was shown to play a significant role in this process.
Microvillus effacement.
Effacement (or loss) of host microvilli is a defining feature of the A/E pathogens and often considered to be the major contributor to diarrhea. Although multiple effector proteins are involved (12), EspF has a well-defined role in initially causing peripheral microvilli to elongate (49) and also in inducing the effacement of peripheral microvilli around the bacteria (12). This EspF-effacing activity continues during infection, resulting in increasing zones of effacement around the attached bacteria (12). However, reports of this distinctive phenotype are only from in vitro studies, and it is still not clear whether such an activity occurs in vivo. Also, it is uncertain whether the elongation and effacement of peripheral microvilli are a manifestation of a common cellular function in different cell types, as the former has been described for ex vivo tissue (49), while the latter occurs on intestinal cell lines (12).
Overall, the targeting of cellular processes that are directly linked to diarrhea-related symptoms appears to represent a significant proportion of the functional repertoire of EspF. This suggests that EspF is likely to play an important role in disease caused by the A/E pathogens.
EspF AS A VIRULENCE FACTOR
A major obstacle in defining the importance of a particular effector in disease is often the high level of functional redundancy between effectors. EspF is no exception, as it displays some of the highest levels of functional overlap described for a bacterial effector (10). Thus, any defects in the virulence of an EspF-deficient mutant may be masked by the actions of other effectors with a similar function. With this caveat in mind, EspF has been shown to be of moderate importance in the virulence of A/E pathogens. Although two separate studies with mice infected with C. rodentium showed that EspF contributes to mouse lethality, intestinal histopathology, and colonization (15, 40), a different study suggested only partial involvement (38). Rabbit infection with EHEC also revealed that EspF is an important colonization factor and suppressor of the host inflammatory response (48). Whether EspF is important in human infection with EPEC or EHEC has not been determined, but if the animal model results are to be extrapolated to humans, combined with the growing number of EspF functions on human cell lines, it seems highly probable that EspF plays an important role during EPEC/EHEC infection. Notably, and as put forward in this review, many functions of EspF disrupt cellular processes that have been proven to play a role in diarrheal symptoms and strongly point to EspF being a prominent effector in the manifestation of diarrhea. Also notable is that EspF has its own dedicated chaperone CesF, unlike most other A/E pathogen effectors which share and compete for a common chaperone binding partner. Whether this has any bearing on the importance of EspF is unclear, but it suggests that A/E pathogens seem to give EspF special attention and ensure that it does not have to compete with other effectors during the translocation process. Finally, the importance of EspF may be drawn from findings that relate to effector levels delivered into host intestinal cells. Mills et al. (37) revealed that both Tir and EspZ, two critical virulence factors (15), are delivered at very high levels in Caco-2 intestinal cells (see supplemental data set in reference 37). Only EspF was delivered at comparable levels, with much lower intracellular concentrations for the remaining effectors that were tested (37). Thus, the level of EspF delivered into host cells is undoubtedly sufficient to affect the normal physiology of the host intestine during infection and compares with that of other essential effectors.
CONCLUDING REMARKS
The multifunctionality of EspF is not an exception but typifies many well-studied effector proteins from several important pathogens (see reference 27). Indeed, the number of reported activities for the remaining A/E pathogen effectors continues to grow, revealing an ever-increasing and awe-inspiring level of complexity within the infected host cell. What is striking about EspF is that most of its reported functions are unrelated to each other, suggesting that at the molecular level, EspF is a highly versatile protein with the ability to hijack diverse eukaryotic processes. Given the tremendous efforts that have shed light on EspF's multiple functions, we are now in a position to start dissecting these functions and determining their role in the disease process.
Acknowledgments
We thank Brendan Kenny and Roger Parton for critically reviewing the manuscript. We also thank Trevor Booth (Newcastle Bio-imaging) for confocal microscopy expertise and Sabine Quitard for technical assistance.
This work was supported by a University Fellowship to P.D., a DFG research fellowship to S.M., and an MRC-DTG studentship to A.H. A.J.R. is supported by the BBSRC (grant BB/G011389/1).
Biography
 Ashleigh Holmes was born in Sydney, Australia, and moved to Scotland at age 6. She completed her undergraduate masters (M.Sci.) degree in microbiology at the University of Glasgow in 2007 and is currently undertaking a Ph.D. at the same institution. Her MRC-funded research, supervised by Dr. Andrew Roe and Prof. Tim Mitchell, focuses on protein translocation systems of pathogenic bacteria.
Ashleigh Holmes was born in Sydney, Australia, and moved to Scotland at age 6. She completed her undergraduate masters (M.Sci.) degree in microbiology at the University of Glasgow in 2007 and is currently undertaking a Ph.D. at the same institution. Her MRC-funded research, supervised by Dr. Andrew Roe and Prof. Tim Mitchell, focuses on protein translocation systems of pathogenic bacteria.
 Sabrina Mühlen was born and raised in Braunschweig, Germany. She obtained her undergraduate degree from the University of Osnabrück, Germany, in molecular biology before completing her graduate studies in microbiology in Victoria, Canada, and Osnabrück. For her Ph.D., she decided to do something different and went to the German Cancer Research Centre in Heidelberg and received her Ph.D. in cancer biology in 2008. Since 2009, she has been at the Institute for Cell and Molecular Biology, Newcastle (United Kingdom), investigating the effects of EPEC effector proteins on host cellular processes.
Sabrina Mühlen was born and raised in Braunschweig, Germany. She obtained her undergraduate degree from the University of Osnabrück, Germany, in molecular biology before completing her graduate studies in microbiology in Victoria, Canada, and Osnabrück. For her Ph.D., she decided to do something different and went to the German Cancer Research Centre in Heidelberg and received her Ph.D. in cancer biology in 2008. Since 2009, she has been at the Institute for Cell and Molecular Biology, Newcastle (United Kingdom), investigating the effects of EPEC effector proteins on host cellular processes.
 Andrew Roe completed his undergraduate research at the University of Aberdeen, Scotland, and then conducted his Ph.D. research at the same institution under Prof. Ian Booth, studying the mechanisms of weak acid resistance in Escherichia coli. For several years, he worked as a postdoctoral researcher with Prof. David Gally at the University of Edinburgh, investigating the regulation of virulence genes in E. coli O157:H7. He is currently a lecturer at the University of Glasgow, where his group is focused on studying gene expression and the development of small molecules that interfere with pathogenesis.
Andrew Roe completed his undergraduate research at the University of Aberdeen, Scotland, and then conducted his Ph.D. research at the same institution under Prof. Ian Booth, studying the mechanisms of weak acid resistance in Escherichia coli. For several years, he worked as a postdoctoral researcher with Prof. David Gally at the University of Edinburgh, investigating the regulation of virulence genes in E. coli O157:H7. He is currently a lecturer at the University of Glasgow, where his group is focused on studying gene expression and the development of small molecules that interfere with pathogenesis.
 Paul Dean earned his undergraduate degree from the University of Bath (United Kingdom) and did his Ph.D. at the same institution, working under Profs. Stuart Reynolds and Keith Charnley on fungal and bacterial pathogens of insects. He moved to the University of Bristol (United Kingdom) to start his postdoctoral research with Prof. Brendan Kenny, working on the functions of EPEC effector proteins. He is currently a research fellow in the Institute of Cell and Molecular Biosciences at the University of Newcastle (United Kingdom), funded by a Faculty Fellowship. His major passion is elucidating the functions of bacterial virulence proteins, and he is continually inspired by the work of various laboratories in this field.
Paul Dean earned his undergraduate degree from the University of Bath (United Kingdom) and did his Ph.D. at the same institution, working under Profs. Stuart Reynolds and Keith Charnley on fungal and bacterial pathogens of insects. He moved to the University of Bristol (United Kingdom) to start his postdoctoral research with Prof. Brendan Kenny, working on the functions of EPEC effector proteins. He is currently a research fellow in the Institute of Cell and Molecular Biosciences at the University of Newcastle (United Kingdom), funded by a Faculty Fellowship. His major passion is elucidating the functions of bacterial virulence proteins, and he is continually inspired by the work of various laboratories in this field.
Editor: H. L. Andrews-Polymenis
Footnotes
Published ahead of print on 2 August 2010.
REFERENCES
- 1.Alto, N. M., A. W. Weflen, M. J. Rardin, D. Yarar, C. S. Lazar, R. Tonikian, A. Koller, S. S. Taylor, C. Boone, S. S. Sidhu, S. L. Schmid, G. A. Hecht, and J. E. Dixon. 2007. The type III effector EspF coordinates membrane trafficking by the spatiotemporal activation of two eukaryotic signaling pathways. J. Cell Biol. 178:1265-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boisvert, F. M., S. van Koningsbruggen, J. Navascues, and A. I. Lamond. 2007. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 8:574-585. [DOI] [PubMed] [Google Scholar]
- 3.Campellone, K. G., H. C. Cheng, D. Robbins, A. D. Siripala, E. J. McGhie, R. D. Hayward, M. D. Welch, M. K. Rosen, V. Koronakis, and J. M. Leong. 2008. Repetitive N-WASP-binding elements of the enterohemorrhagic Escherichia coli effector EspF(U) synergistically activate actin assembly. PLoS Pathog. 4:e1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campellone, K. G., D. Robbins, and J. M. Leong. 2004. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev. Cell 7:217-228. [DOI] [PubMed] [Google Scholar]
- 5.Canil, C., I. Rosenshine, S. Ruschkowski, M. S. Donnenberg, J. B. Kaper, and B. B. Finlay. 1993. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect. Immun. 61:2755-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celli, J., M. Olivier, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 20:1245-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charpentier, X., and E. Oswald. 2004. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J. Bacteriol. 186:5486-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crane, J. K., S. Majumdar, and D. F. Pickhardt III. 1999. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect. Immun. 67:2575-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane, J. K., B. P. McNamara, and M. S. Donnenberg. 2001. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell. Microbiol. 3:197-211. [DOI] [PubMed] [Google Scholar]
- 10.Dean, P., and B. Kenny. 2009. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr. Opin. Microbiol. 12:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean, P., and B. Kenny. 2004. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol. Microbiol. 54:665-675. [DOI] [PubMed] [Google Scholar]
- 12.Dean, P., M. Maresca, S. Schuller, A. D. Phillips, and B. Kenny. 2006. Potent diarrheagenic mechanism mediated by the cooperative action of three enteropathogenic Escherichia coli-injected effector proteins. Proc. Natl. Acad. Sci. U. S. A. 103:1876-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean, P., S. Muhlen, S. Quitard, and B. Kenny. 2010. The bacterial effectors EspG and EspG2 induce a destructive calpain activity that is kept in check by the co-delivered Tir effector. Cell. Microbiol. 12:1308-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean, P., J. A. Scott, A. A. Knox, S. Quitard, N. J. Watkins, and B. Kenny. 2010. The enteropathogenic E. coli effector EspF targets and disrupts the nucleolus by a process regulated by mitochondrial dysfunction. PLoS Pathog. 6:e1000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng, W., J. L. Puente, S. Gruenheid, Y. Li, B. A. Vallance, A. Vazquez, J. Barba, J. A. Ibarra, P. O'Donnell, P. Metalnikov, K. Ashman, S. Lee, D. Goode, T. Pawson, and B. B. Finlay. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. U. S. A. 101:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott, S. J., C. B. O'Connell, A. Koutsouris, C. Brinkley, M. S. Donnenberg, G. Hecht, and J. B. Kaper. 2002. A gene from the locus of enterocyte effacement that is required for enteropathogenic Escherichia coli to increase tight-junction permeability encodes a chaperone for EspF. Infect. Immun. 70:2271-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel, G., and A. D. Phillips. 2008. Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal. Cell. Microbiol. 10:549-556. [DOI] [PubMed] [Google Scholar]
- 18.Garmendia, J., A. D. Phillips, M. F. Carlier, Y. Chong, S. Schuller, O. Marches, S. Dahan, E. Oswald, R. K. Shaw, S. Knutton, and G. Frankel. 2004. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell. Microbiol. 6:1167-1183. [DOI] [PubMed] [Google Scholar]
- 19.Guttman, J. A., and B. B. Finlay. 2008. Subcellular alterations that lead to diarrhea during bacterial pathogenesis. Trends Microbiol. 16:535-542. [DOI] [PubMed] [Google Scholar]
- 20.Guttman, J. A., Y. Li, M. E. Wickham, W. Deng, A. W. Vogl, and B. B. Finlay. 2006. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell. Microbiol. 8:634-645. [DOI] [PubMed] [Google Scholar]
- 21.Guttman, J. A., F. N. Samji, Y. Li, W. Deng, A. Lin, and B. B. Finlay. 2007. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell. Microbiol. 9:131-141. [DOI] [PubMed] [Google Scholar]
- 22.Hardwidge, P. R., S. Donohoe, R. Aebersold, and B. B. Finlay. 2006. Proteomic analysis of the binding partners to enteropathogenic Escherichia coli virulence proteins expressed in Saccharomyces cerevisiae. Proteomics 6:2174-2179. [DOI] [PubMed] [Google Scholar]
- 23.Hemrajani, C., C. N. Berger, K. S. Robinson, O. Marches, A. Mousnier, and G. Frankel. 2010. NleH effectors interact with Bax inhibitor-1 to block apoptosis during enteropathogenic Escherichia coli infection. Proc. Natl. Acad. Sci. U. S. A. 107:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodges, K., N. M. Alto, K. Ramaswamy, P. K. Dudeja, and G. Hecht. 2008. The enteropathogenic Escherichia coli effector protein EspF decreases sodium hydrogen exchanger 3 activity. Cell. Microbiol. 10:1735-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly, M., E. Hart, R. Mundy, O. Marches, S. Wiles, L. Badea, S. Luck, M. Tauschek, G. Frankel, R. M. Robins-Browne, and E. L. Hartland. 2006. Essential role of the type III secretion system effector NleB in colonization of mice by Citrobacter rodentium. Infect. Immun. 74:2328-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenny, B., and M. Jepson. 2000. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell. Microbiol. 2:579-590. [DOI] [PubMed] [Google Scholar]
- 27.Kenny, B., and R. Valdivia. 2009. Host-microbe interactions: bacteria. Curr. Opin. Microbiol. 12:1-3. [DOI] [PubMed] [Google Scholar]
- 28.Ma, C., M. E. Wickham, J. A. Guttman, W. Deng, J. Walker, K. L. Madsen, K. Jacobson, W. A. Vogl, B. B. Finlay, and B. A. Vallance. 2006. Citrobacter rodentium infection causes both mitochondrial dysfunction and intestinal epithelial barrier disruption in vivo: role of mitochondrial associated protein (Map). Cell. Microbiol. 8:1669-1686. [DOI] [PubMed] [Google Scholar]
- 29.Marches, O., M. Batchelor, R. K. Shaw, A. Patel, N. Cummings, T. Nagai, C. Sasakawa, S. R. Carlsson, R. Lundmark, C. Cougoule, E. Caron, S. Knutton, I. Connerton, and G. Frankel. 2006. EspF of enteropathogenic Escherichia coli binds sorting nexin 9. J. Bacteriol. 188:3110-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marches, O., V. Covarelli, S. Dahan, C. Cougoule, P. Bhatta, G. Frankel, and E. Caron. 2008. EspJ of enteropathogenic and enterohaemorrhagic Escherichia coli inhibits opsono-phagocytosis. Cell. Microbiol. 10:1104-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marches, O., J. P. Nougayrede, S. Boullier, J. Mainil, G. Charlier, I. Raymond, P. Pohl, M. Boury, J. De Rycke, A. Milon, and E. Oswald. 2000. Role of tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect. Immun. 68:2171-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Argudo, I., C. Sands, and M. A. Jepson. 2007. Translocation of enteropathogenic Escherichia coli across an in vitro M cell model is regulated by its type III secretion system. Cell. Microbiol. 9:1538-1546. [DOI] [PubMed] [Google Scholar]
- 33.Mayer, B. J. 2001. SH3 domains: complexity in moderation. J. Cell Sci. 114:1253-1263. [DOI] [PubMed] [Google Scholar]
- 34.McNamara, B. P., and M. S. Donnenberg. 1998. A novel proline-rich protein, EspF, is secreted from enteropathogenic Escherichia coli via the type III export pathway. FEMS Microbiol. Lett. 166:71-78. [DOI] [PubMed] [Google Scholar]
- 35.McNamara, B. P., A. Koutsouris, C. B. O'Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. 2001. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Invest. 107:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meinild, A., D. A. Klaerke, D. D. Loo, E. M. Wright, and T. Zeuthen. 1998. The human Na+-glucose cotransporter is a molecular water pump. J. Physiol. 508:15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills, E., K. Baruch, X. Charpentier, S. Kobi, and I. Rosenshine. 2008. Real-time analysis of effector translocation by the type III secretion system of enteropathogenic Escherichia coli. Cell Host Microbe 3:104-113. [DOI] [PubMed] [Google Scholar]
- 38.Mundy, R., C. Jenkins, J. Yu, H. Smith, and G. Frankel. 2004. Distribution of espI among clinical enterohaemorrhagic and enteropathogenic Escherichia coli isolates. J. Med. Microbiol. 53:1145-1149. [DOI] [PubMed] [Google Scholar]
- 39.Muto, T., T. Obita, Y. Abe, T. Shodai, T. Endo, and D. Kohda. 2001. NMR identification of the Tom20 binding segment in mitochondrial presequences. J. Mol. Biol. 306:137-143. [DOI] [PubMed] [Google Scholar]
- 40.Nagai, T., A. Abe, and C. Sasakawa. 2005. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J. Biol. Chem. 280:2998-3011. [DOI] [PubMed] [Google Scholar]
- 41.Neupert, W. 1997. Protein import into mitochondria. Annu. Rev. Biochem. 66:863-917. [DOI] [PubMed] [Google Scholar]
- 42.Nougayrede, J. P., and M. S. Donnenberg. 2004. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell. Microbiol. 6:1097-1111. [DOI] [PubMed] [Google Scholar]
- 43.Nougayrede, J. P., G. H. Foster, and M. S. Donnenberg. 2007. Enteropathogenic Escherichia coli effector EspF interacts with host protein Abcf2. Cell. Microbiol. 9:680-693. [DOI] [PubMed] [Google Scholar]
- 44.Padrick, S. B., H. C. Cheng, A. M. Ismail, S. C. Panchal, L. K. Doolittle, S. Kim, B. M. Skehan, J. Umetani, C. A. Brautigam, J. M. Leong, and M. K. Rosen. 2008. Hierarchical regulation of WASP/WAVE proteins. Mol. Cell 32:426-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peralta-Ramirez, J., J. M. Hernandez, R. Manning-Cela, J. Luna-Munoz, C. Garcia-Tovar, J. P. Nougayrede, E. Oswald, and F. Navarro-Garcia. 2008. EspF Interacts with nucleation-promoting factors to recruit junctional proteins into pedestals for pedestal maturation and disruption of paracellular permeability. Infect. Immun. 76:3854-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philpott, D. J., D. M. McKay, P. M. Sherman, and M. H. Perdue. 1996. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am. J. Physiol. 270:G634-G645. [DOI] [PubMed] [Google Scholar]
- 47.Quitard, S., P. Dean, M. Maresca, and B. Kenny. 2006. The enteropathogenic Escherichia coli EspF effector molecule inhibits PI-3 kinase-mediated uptake independently of mitochondrial targeting. Cell. Microbiol. 8:972-981. [DOI] [PubMed] [Google Scholar]
- 48.Ritchie, J. M., and M. K. Waldor. 2005. The locus of enterocyte effacement-encoded effector proteins all promote enterohemorrhagic Escherichia coli pathogenicity in infant rabbits. Infect. Immun. 73:1466-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw, R. K., J. Cleary, M. S. Murphy, G. Frankel, and S. Knutton. 2005. Interaction of enteropathogenic Escherichia coli with human intestinal mucosa: role of effector proteins in brush border remodeling and formation of attaching and effacing lesions. Infect. Immun. 73:1243-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shull, G. E., M. L. Miller, and P. J. Schultheis. 2000. Lessons from genetically engineered animal models. VIII. Absorption and secretion of ions in the gastrointestinal tract. Am. J. Physiol. Gastrointest Liver Physiol. 278:G185-G190. [DOI] [PubMed] [Google Scholar]
- 51.Spears, K. J., A. J. Roe, and D. L. Gally. 2006. A comparison of enteropathogenic and enterohaemorrhagic Escherichia coli pathogenesis. FEMS Microbiol. Lett. 255:187-202. [DOI] [PubMed] [Google Scholar]
- 52.Spitz, J., R. Yuhan, A. Koutsouris, C. Blatt, J. Alverdy, and G. Hecht. 1995. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am. J. Physiol. 268:G374-G379. [DOI] [PubMed] [Google Scholar]
- 53.Thanabalasuriar, A., A. Koutsouris, A. Weflen, M. Mimee, G. Hecht, and S. Gruenheid. 2010. The bacterial virulence factor NleA is required for the disruption of intestinal tight junctions by enteropathogenic Escherichia coli. Cell. Microbiol. 12:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomson, F. L., V. K. Viswanathan, K. J. Kanack, R. P. Kanteti, K. V. Straub, M. Menet, J. B. Kaper, and G. Hecht. 2005. Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol. Microbiol. 56:447-464. [DOI] [PubMed] [Google Scholar]
- 55.Torchinsky, M. B., J. Garaude, A. P. Martin, and J. M. Blander. 2009. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature 458:78-82. [DOI] [PubMed] [Google Scholar]
- 56.Vallance, B. A., W. Deng, K. Jacobson, and B. B. Finlay. 2003. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect. Immun. 71:3443-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viswanathan, V. K., S. Lukic, A. Koutsouris, R. Miao, M. M. Muza, and G. Hecht. 2004. Cytokeratin 18 interacts with the enteropathogenic Escherichia coli secreted protein F (EspF) and is redistributed after infection. Cell. Microbiol. 6:987-997. [DOI] [PubMed] [Google Scholar]
- 58.Viswanathan, V. K., A. Weflen, A. Koutsouris, J. L. Roxas, and G. Hecht. 2008. Enteropathogenic E. coli-induced barrier function alteration is not a consequence of host cell apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 294:G1165-G1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weflen, A. W., N. M. Alto, V. K. Viswanathan, and G. Hecht. 2010. E. coli secreted protein F promotes EPEC invasion of intestinal epithelial cells via an SNX9-dependent mechanism. Cell. Microbiol. 12:919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]