Abstract
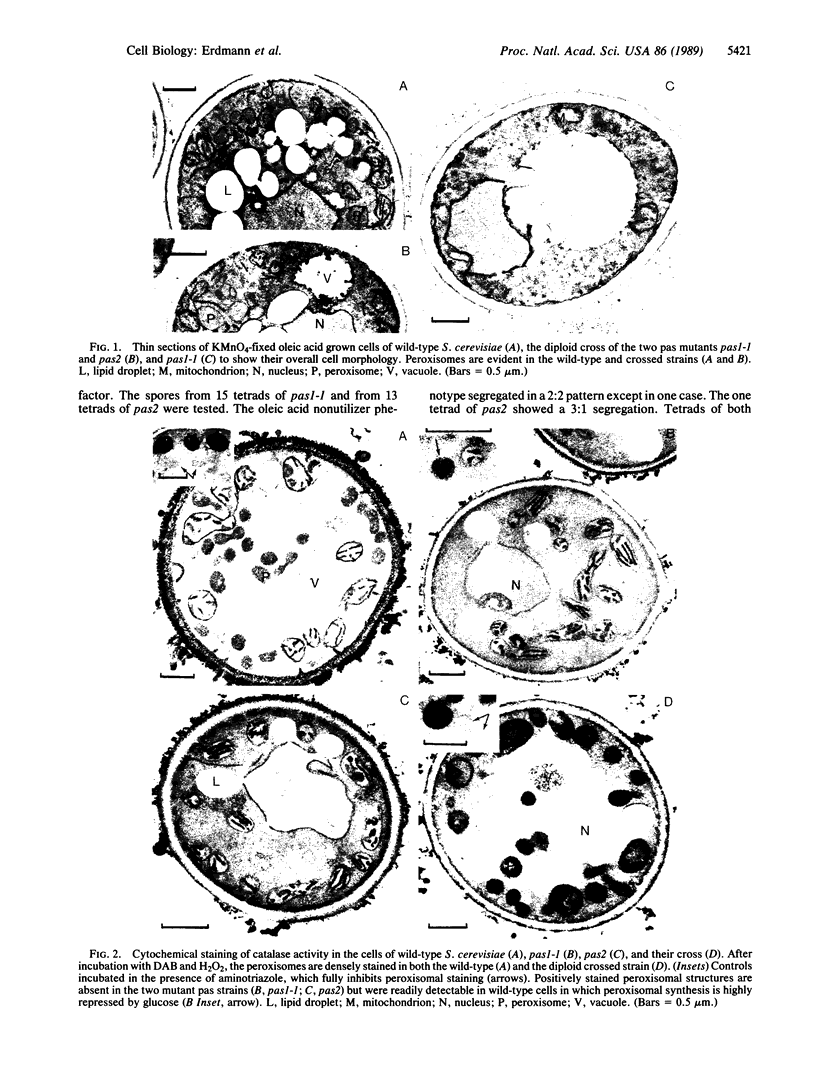
Two mutants of Saccharomyces cerevisiae affected in peroxisomal assembly (pas mutants) have been isolated and characterized. Each strain contains a single mutation that results in (i) the inability to grow on oleic acid, (ii) accumulation of peroxisomal matrix enzymes in the cytosol, and (iii) absence of detectable peroxisomes at the ultrastructural level. These lesions (pas1-1 and pas2) are shown to be nonallelic and recessive. Crossing of pas1-1 and pas2 strains resulted in diploid cells that had regained the ability to grow on oleic acid as sole carbon source and to form peroxisomes. These pas mutants may provide useful tools for future studies on the molecular mechanisms involved in peroxisomal assembly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankaitis V. A., Johnson L. M., Emr S. D. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P. How proteins get into microbodies (peroxisomes, glyoxysomes, glycosomes). Biochim Biophys Acta. 1986 May 5;866(4):179–203. doi: 10.1016/0167-4781(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brul S., Westerveld A., Strijland A., Wanders R. J., Schram A. W., Heymans H. S., Schutgens R. B., van den Bosch H., Tager J. M. Genetic heterogeneity in the cerebrohepatorenal (Zellweger) syndrome and other inherited disorders with a generalized impairment of peroxisomal functions. A study using complementation analysis. J Clin Invest. 1988 Jun;81(6):1710–1715. doi: 10.1172/JCI113510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brul S., Wiemer E. A., Westerveld A., Strijland A., Wanders R. J., Schram A. W., Heymans H. S., Schutgens R. B., Van den Bosch H., Tager J. M. Kinetics of the assembly of peroxisomes after fusion of complementary cell lines from patients with the cerebro-hepato-renal (Zellweger) syndrome and related disorders. Biochem Biophys Res Commun. 1988 May 16;152(3):1083–1089. doi: 10.1016/s0006-291x(88)80395-4. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Deshaies R. J., Kepes F., Böhni P. C. Genetic dissection of the early stages of protein secretion in yeast. Trends Genet. 1989 Mar;5(3):87–93. doi: 10.1016/0168-9525(89)90032-2. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol. 1987 Aug;105(2):633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., Wisniewski H. K., Ritch R. H., Norton W. T., Rapin I., Gartner L. M. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science. 1973 Oct 5;182(4107):62–64. doi: 10.1126/science.182.4107.62. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hruban Z., Vigil E. L., Slesers A., Hopkins E. Microbodies: constituent organelles of animal cells. Lab Invest. 1972 Aug;27(2):184–191. [PubMed] [Google Scholar]
- Kunau W. H., Bühne S., de la Garza M., Kionka C., Mateblowski M., Schultz-Borchard U., Thieringer R. Comparative enzymology of beta-oxidation. Biochem Soc Trans. 1988 Jun;16(3):418–420. doi: 10.1042/bst0160418. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Black V., Shio H., Fujiki Y., Hajra A. K., Datta N. S., Bangaru B. S., Dancis J. Zellweger syndrome: biochemical and morphological studies on two patients treated with clofibrate. Pediatr Res. 1985 Dec;19(12):1356–1364. doi: 10.1203/00006450-198512000-00030. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Moreno de la Garza M., Schultz-Borchard U., Crabb J. W., Kunau W. H. Peroxisomal beta-oxidation system of Candida tropicalis. Purification of a multifunctional protein possessing enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase and 3-hydroxyacyl-CoA epimerase activities. Eur J Biochem. 1985 Apr 15;148(2):285–291. doi: 10.1111/j.1432-1033.1985.tb08837.x. [DOI] [PubMed] [Google Scholar]
- Moser H. W. New approaches in peroxisomal disorders. Dev Neurosci. 1987;9(1):1–18. doi: 10.1159/000111604. [DOI] [PubMed] [Google Scholar]
- Pollock R. A., Hartl F. U., Cheng M. Y., Ostermann J., Horwich A., Neupert W. The processing peptidase of yeast mitochondria: the two co-operating components MPP and PEP are structurally related. EMBO J. 1988 Nov;7(11):3493–3500. doi: 10.1002/j.1460-2075.1988.tb03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Lazarow P. B. Peroxisomal integral membrane proteins in control and Zellweger fibroblasts. J Biol Chem. 1988 Jul 25;263(21):10502–10509. [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Small G. M., Lazarow P. B. Peroxisomal membrane ghosts in Zellweger syndrome--aberrant organelle assembly. Science. 1988 Mar 25;239(4847):1536–1538. doi: 10.1126/science.3281254. [DOI] [PubMed] [Google Scholar]
- Santos M. J., Ojeda J. M., Garrido J., Leighton F. Peroxisomal organization in normal and cerebrohepatorenal (Zellweger) syndrome fibroblasts. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6556–6560. doi: 10.1073/pnas.82.19.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram A. W., Strijland A., Hashimoto T., Wanders R. J., Schutgens R. B., van den Bosch H., Tager J. M. Biosynthesis and maturation of peroxisomal beta-oxidation enzymes in fibroblasts in relation to the Zellweger syndrome and infantile Refsum disease. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6156–6158. doi: 10.1073/pnas.83.16.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutgens R. B., Heymans H. S., Wanders R. J., van den Bosch H., Tager J. M. Peroxisomal disorders: a newly recognised group of genetic diseases. Eur J Pediatr. 1986 Feb;144(5):430–440. doi: 10.1007/BF00441734. [DOI] [PubMed] [Google Scholar]
- Skoneczny M., Chełstowska A., Rytka J. Study of the coinduction by fatty acids of catalase A and acyl-CoA oxidase in standard and mutant Saccharomyces cerevisiae strains. Eur J Biochem. 1988 Jun 1;174(2):297–302. doi: 10.1111/j.1432-1033.1988.tb14097.x. [DOI] [PubMed] [Google Scholar]
- Snow R. An enrichment method for auxotrophic yeast mutants using the antibiotic 'nystatin'. Nature. 1966 Jul 9;211(5045):206–207. doi: 10.1038/211206a0. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Mateblowski M., Kunau W. H., Harder W. Proliferation of microbodies in Saccharomyces cerevisiae. Yeast. 1987 Jun;3(2):77–84. doi: 10.1002/yea.320030204. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Kos M., Roest B., Meijer A. J., Schrakamp G., Heymans H. S., Tegelaers W. H., van den Bosch H., Schutgens R. B., Tager J. M. Activity of peroxisomal enzymes and intracellular distribution of catalase in Zellweger syndrome. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1054–1061. doi: 10.1016/s0006-291x(84)80240-5. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Schutgens R. B., Tager J. M. Peroxisomal matrix enzymes in Zellweger syndrome: activity and subcellular localization in liver. J Inherit Metab Dis. 1985;8 (Suppl 2):151–152. doi: 10.1007/BF01811504. [DOI] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller R. A., Raetz C. R. Isolation of animal cell mutants deficient in plasmalogen biosynthesis and peroxisome assembly. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5170–5174. doi: 10.1073/pnas.83.14.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijken J. P., Veenhuis M., Vermeulen C. A., Harder W. Cytochemical localization of catalase activity in methanol-grown Hansenula polymorpha. Arch Microbiol. 1975 Nov 7;105(3):261–267. doi: 10.1007/BF00447145. [DOI] [PubMed] [Google Scholar]