Abstract
Escherichia coli is the most common Gram-negative organism causing neonatal meningitis. Previous studies demonstrated that E. coli K1 invasion of brain microvascular endothelial cells (BMEC) is required for penetration into the central nervous system, but the microbe-host interactions that are involved in this process remain incompletely understood. Here we report the involvement of vascular endothelial growth factor receptor 1 (VEGFR1) expressed on human brain microvascular endothelial cells (HBMEC) in E. coli K1 invasion of HBMEC. Our results showed that treatment of confluent HBMEC with pan-VEGFR inhibitors significantly inhibited E. coli K1 invasion of HBMEC. Immunofluorescence results indicated the colocalization of VEGFR1 with E. coli K1 during bacterial invasion of HBMEC. The E. coli-induced actin cytoskeleton rearrangements in HBMEC were blocked by VEGFR inhibitors but not by VEGFR2-specific inhibitors. The small interfering RNA (siRNA) knockdown of VEGFR1 in HBMEC significantly attenuated E. coli invasion and the concomitant actin filament rearrangement. Furthermore, we found an increased association of VEGFR1 with the p85 subunit of phosphatidylinositol 3-kinase (PI3K) in HBMEC infected with E. coli K1 and that E. coli K1-triggered Akt activation in HBMEC was blocked by VEGFR1 siRNA and VEGFR inhibitors. Taken together, our results demonstrate that VEGFR1 contributes to E. coli K1 invasion of HBMEC via recruitment of the PI3K/Akt signaling pathway.
Escherichia coli is the most common Gram-negative organism causing meningitis during the neonatal period (27). Neonatal E. coli meningitis is associated with significant mortality and morbidity (27). E. coli strains with the K1 capsular polysaccharide are the predominant isolates (about 80%) from neonatal E. coli meningitis (19, 30). Most cases of E. coli K1 meningitis result from hematogenous spread (8). Entry of circulating E. coli K1 into the central nervous system requires penetration across the blood-brain barrier (BBB), which is composed mainly of brain microvascular endothelial cells (BMEC) (15). The molecular mechanisms underlying E. coli K1 binding to and internalization into BMEC remain incompletely understood.
Many invasive pathogens exploit host cell signaling pathways to facilitate their entry into host cells. E. coli K1 also initiates host cell signaling cascades, leading to its subsequent uptake into mammalian cells (17). Encapsulated E. coli K1 interacts with host cells in a multistep process. At first, E. coli K1 binds to the surface of human brain microvascular endothelial cells (HBMEC) through specific bacterial structures, such as type 1 fimbriae. Then the rearrangements of the actin cytoskeleton occur, leading to the internalization of E. coli into HBMEC, a process called “invasion.” These events imply a complex interaction between bacterial virulence-related factors and the involved signaling molecules in HBMEC (17). Several E. coli K1 determinants contributing to HBMEC invasion, such as cnf1, fimH, ompA, ibeA, ibeB, and ibeC, have been identified (17). Previous studies have shown that activation of cellular signaling molecules, such as focal adhesion kinase (FAK), paxillin, phosphatidylinositol 3-kinase (PI3K), Src kinase, and Rho GTPases, is involved in E. coli K1 invasion of HBMEC (17, 20), but how those signaling molecules contribute to E. coli K1 invasion of HBMEC remains incompletely understood.
Vascular endothelial growth factor receptor (VEGFR) is a transmembrane tyrosine kinase receptor that is expressed mainly on vascular endothelial cells. VEGFR can participate in various biological functions, including cell survival, migration, and differentiation as well as vascular sprouting, stabilization, and permeability (34). Members of the VEGFR family that are expressed mainly on endothelial cells include VEGFR1 and VEGFR2, also known as Fms-like tyrosine kinase 1 (Flt-1) and fetal liver kinase 1 (Flk-1)/kinase insert domain receptor (KDR), respectively (25). In this study, we showed that VEGFR1, a member of the VEGFR family, contributes to E. coli K1 invasion of HBMEC via recruitment of the PI3K/Akt signaling pathway.
MATERIALS AND METHODS
Bacterial strains and media.
E. coli RS218 (O18:K1:H7) is a clinical isolate from the cerebrospinal fluid of a newborn infant with meningitis (14), and E44 is a spontaneous rifampin-resistant mutant of RS218. E44 was grown at 37°C in brain heart infusion broth (BD Biosciences, Sparks, MD) with rifampin (100 μg/ml).
Cell culture.
HBMEC (36) were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 10% Nu-serum (BD Biosciences), 2 mM glutamine, 1 mM sodium pyruvate, 1× nonessential amino acid, and 1× minimal essential medium (MEM) vitamin. Cells were incubated at 37°C in a 5% CO2, 95% air humidified atmosphere.
Bacterial invasion and adhesion assays with HBMEC.
E. coli K1 invasion of HBMEC was performed as described previously (20). Briefly, bacteria (107 CFU/well) were added to confluent monolayers of HBMEC in 24-well plates at a multiplicity of infection of 100. The monolayers were incubated at 37°C for 1.5 h to allow invasion to occur. The number of intracellular bacteria was determined after the extracellular bacteria were killed by incubation of the monolayers with experimental medium (RPMI 1640, 5% fetal bovine serum, and 1 mM sodium pyruvate) containing gentamicin (100 μg/ml) for 1 h at 37°C. The monolayers were washed and lysed with 0.5% Triton X-100. The released intracellular bacteria were enumerated by plating on LB agar plates. For inhibitor studies, HBMEC were incubated with SU5416, KRN633, 3-(3-thienyl)-6-(4-methoxyphenyl)pyrazolo[1,5a]pyrimidine (TMPPP), or SU1498 (Calbiochem, La Jolla, CA) for 30 min before addition of bacteria. The inhibitors were reconstituted in dimethyl sulfoxide (DMSO) and diluted in experimental medium according to the manufacturer's instructions. HBMEC treated with the same volume of DMSO without inhibitors were used as a vehicle control. DMSO alone did not have any effect on bacterial adhesion to and invasion of HBMEC (data not shown). A bacterial adhesion assay to determine the number of total cell-associated bacteria was done as described above except that the gentamicin step was omitted.
Real-time reverse transcription (RT)-PCR.
The total RNA isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) was reverse transcribed using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, Madison, WI). Real-time PCR was performed on an ABI 7500 real-time PCR system (Applied BioSystems) with a SYBR premix Ex Taq kit (Takara Biotechnology, Dalian, China), according to the manufacturer's instructions. The primer sequences for human vascular endothelial growth factor receptor 1 (VEGFR1) were TTTAAAAGGCACCCAGCACAT (forward) and TTACTCACCATTTCAGGCAAAGAC (reverse); primer sequences for human VEGFR2 were GGCCCAATAATCAGAGTGGCA (forward) and TGTCATTTCCGATCACTTTTGGA (reverse). Primers for human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were described previously (21). The amplification conditions were as follows: 95°C for 30 s and 40 cycles of 95°C for 5 s and 60°C for 34 s. The comparative cycle threshold (CT) method was used to calculate the relative gene expression level, with GAPDH as the internal control. Real-time PCR products were analyzed on agarose gel electrophoresis and were verified by DNA sequencing.
RNA interference.
The small interfering RNA (siRNA) sequence targeting human VEGFR1 corresponding to the coding region, GCCGGAAGTTGTATGGTTAAA (nucleotides 1092 to 1112), was built by GenScript's siRNA design center (GenScript, Piscataway, NJ). A nonsilencing siRNA sequence (TTCTCCGAACGTGTCACGT) (21) was used as a control. The siRNA sequences were inserted into pRNA-U6.1/Neo vector (GenScript, Piscataway, NJ). Recombinant siRNA plasmids were transfected into HBMEC by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. At 24 h after transfection, the cells were passaged at a 1:10 dilution into fresh growth medium, and selection was performed with 300 μg/ml G418 (Invitrogen). The expression of VEGFR1 in the stable transfectants was examined by Western blot analysis.
Western blotting.
Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and prepared with radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) containing protease inhibitor cocktail (Roche, Indianapolis, IN). The samples were separated by SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) by using a semidry transfer cell (Bio-Rad, Hercules, CA). The PVDF membrane was blocked with 5% nonfat milk and incubated with the primary antibody at 4°C overnight. Then the blots were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotech, Santa Cruz, CA) for 1 h at room temperature. Immunoreactive bands were visualized by Super Signal West Pico chemiluminescent substrate (Pierce, Rockford, IL) by using an LAS-3000 mini (Fuji Film, Tokyo, Japan). The antibodies against VEGFR1, VEGFR2, Akt, phosphorylated Ser473 of Akt (p-Akt), and GAPDH were from Santa Cruz Biotech. Anti-p85 and anti-p110 antibodies were obtained from Millipore. For quantitative analysis, the mean density of each band was measured by Multi Gauge V3.1 software, and the band density of the activated form of the protein was divided by the density of the corresponding total protein band to obtain the normalized band density. Data are plotted as percentages of the control.
Immunoprecipitation.
Cells were washed with ice-cold PBS and lysed with lysis buffer (50 mM Tris, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% Triton X-100, 1 mM sodium orthovanadate, 25 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride) containing a protease inhibitor cocktail. The cell lysates were centrifuged, and the supernatant was collected. The protein content was determined by the Bradford method. A total of 1 mg of protein was incubated with appropriate antibody overnight at 4°C and incubated for 2 h with protein A/G-agarose (Santa Cruz Biotech). The proteins from immune complexes were eluted in SDS sample buffer for Western blot analysis.
Immunofluorescence.
HBMEC grown on coverslips were incubated with E. coli K1 for 15 min at 37°C. The cells were washed with PBS and fixed with 4% paraformaldehyde. Fixed cells were permeabilized with 0.2% Triton X-100 and then blocked with 5% bovine serum albumin (BSA) in PBS. Tetramethyl rhodamine isocyanate (TRITC)-labeled phalloidin (Sigma-Aldrich, St. Louis, MO) was used to stain the actin filaments. The cells were washed again, and the coverslips were mounted and analyzed using an immunofluorescence microscope (Olympus BX51, Tokyo, Japan). When indicated, HBMEC were incubated with SU5416 (20 μM), KRN633 (25 μM), TMPPP (20 μM), or SU1498 (20 μM) before bacterial infection. For analysis using a confocal laser scanning microscope (Olympus FluoView FV1000, Tokyo, Japan), the samples were prepared in the same way except that the cells were stained with VEGFR1 or p85 antibody and incubated with secondary antibody conjugated to Alexa 594 or Alexa 488 (Molecular Probes, Eugene, OR), respectively. When indicated, the bacteria were stained with fluorescein isothiocyanate (FITC)-conjugated E. coli-specific antibody (Abcam, HKSTP, Hong Kong).
Statistical analysis.
Statistical significance between two groups was analyzed by Student's t test. One-way analysis of variance (ANOVA) was used to compare multiple groups. A P value of <0.05 was considered significant.
RESULTS
VEGFR1 plays a role in E. coli K1 invasion of HBMEC.
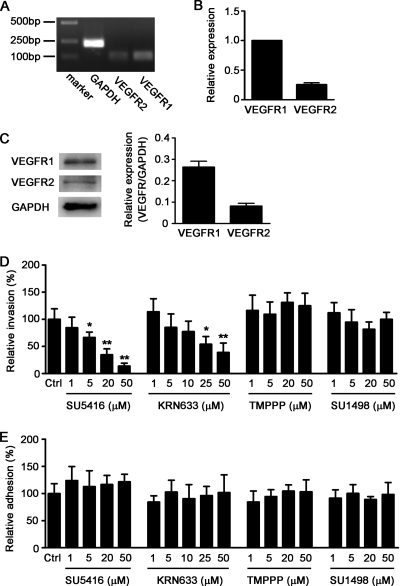
We examined the mRNA expression of VEGFR1 and VEGFR2 in HBMEC by real-time RT-PCR. The results showed that the transcripts of VEGFR1 and VEGFR2 are present in HBMEC (Fig. 1A), and the expression level of VEGFR1 is significantly higher (3.9-fold) than that of VEGFR2 (Fig. 1B). Western blot analysis illustrated that the protein level of VEGFR1 is 3.2-fold higher than that of VEGFR2 in HBMEC (Fig. 1C). In an attempt to analyze whether VEGFR is involved in E. coli K1 invasion of HBMEC, bacterial invasion and adhesion assays were performed in the presence of VEGFR inhibitors. SU5416 (11, 31) and KRN633 (23), which have been identified as pan-VEGFR tyrosine kinase inhibitors, were used to inhibit the activity of VEGFR in HBMEC. As shown in Fig. 1D, either SU5416 or KRN633 treatment significantly inhibited the ability of E. coli K1 strain E44 to invade HBMEC in a dose-dependent manner, while there was no effect on E. coli adhesion to HBMEC (Fig. 1E). In contrast, the specific VEGFR2 kinase inhibitors TMPPP (12) and SU1498 (37) did not exhibit any effect on E. coli K1 adhesion to and invasion of HBMEC (Fig. 1D and E). These results suggested that VEGFR1, but not VEGFR2, is likely to be involved in E. coli K1 invasion of HBMEC.
FIG. 1.
E. coli K1 invasion of HBMEC was abolished by VEGFR inhibitors, but not by VEGFR2-specific inhibitors. (A) Total RNA of HBMEC was isolated, and the expression of VEGFR1 and VEGFR2 was examined by RT-PCR. (B) Real-time RT-PCR was done to compare the relative expression levels of VEGFR1 and VEGFR2 in HBMEC. (C) HBMEC was lysed with RIPA buffer, and the expression of VEGFR1 and VEGFR2 was analyzed by Western blotting, with GAPDH as an internal control (n = 3). (D and E) Confluent HBMEC monolayers in a 24-well plate were treated with the indicated concentrations of SU5416, KRN633, TMPPP, or SU1498 for 30 min before addition of the bacteria. Then, bacterial invasion (D) and adhesion (E) assays were performed as described in Materials and Methods. Results are presented as relative percent invasion or adhesion of the parent E. coli K1 strain E44, which is defined as 100%. Values are means ± standard deviations (SD) of three independent experiments done in triplicate. “Ctrl” represents the vehicle control. *, P < 0.05; **, P < 0.01.
Knockdown of VEGFR1 in HBMEC blocked E. coli K1 invasion.
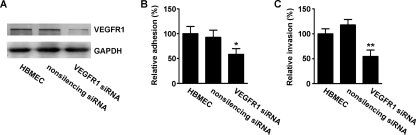
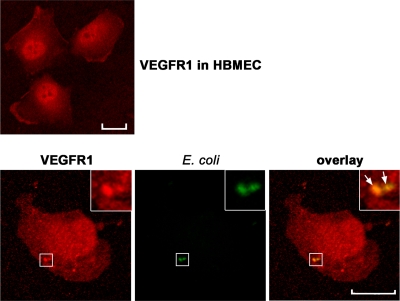
To further investigate the role of VEGFR1 in E. coli K1 invasion of HBMEC, siRNA was used to knock down VEGFR1 expression. Specific siRNA targeting VEGFR1 was designed, and the double-stranded siRNA was inserted into the pRNA-U6.1/Neo siRNA vector, with nonsilencing siRNA as a control. The siRNA constructs were stably transfected into HBMEC, and the silencing effect was examined by Western blot analysis. The results showed that the VEGFR1 siRNA construct was efficient in suppressing the expression of VEGFR1 in HBMEC, whereas the nonsilencing siRNA construct had no effect on VEGFR1 expression (Fig. 2A). The siRNA-transfected HBMEC were subjected to bacterial adhesion and invasion assays. VEGFR1 specific siRNA significantly reduced E. coli K1 adhesion to HBMEC compared to the nonsilencing siRNA control (Fig. 2B), and also the internalization of E. coli K1 was significantly decreased in HBMEC transfected with VEGFR1 siRNA compared with the control (Fig. 2C). These results suggested that knockdown of VEGFR1 in HBMEC prevents E. coli K1 adhesion to and invasion of HBMEC. To analyze whether E. coli K1 is associated with VEGFR1 in HBMEC during the infection process, the cellular localizations of E. coli and VEGFR1 were examined in infected HBMEC by immunofluorescence using confocal laser scanning microscopy. As shown in Fig. 3, a clear colocalization (yellow, indicated by arrows) of E. coli with VEGFR1 at the cell edge was observed with HBMEC infected with E. coli K1 strain E44. In contrast, the colocalization of E. coli with VEGFR1 was hardly discernible in HBMEC transfected with VEGFR1 siRNA (data not shown). These data indicated that VEGFR1, which is present in HBMEC, is involved in E. coli K1 internalization into HBMEC.
FIG. 2.
Knockdown of VEGFR1 blocked E. coli K1 adhesion to and invasion of HBMEC. (A) HBMEC were stably transfected with VEGFR1 siRNA plasmid or nonsilencing siRNA plasmid, and the expression of VEGFR1 in the cells was detected by Western blotting. To check for equal protein loadings, the blot was probed with a GAPDH-specific antibody. The stably transfected HBMEC were subjected to bacterial adhesion (B) and invasion (C) assays. Values are means ± SD of three independent experiments done in triplicate. *, P < 0.05; **, P < 0.01.
FIG. 3.
Colocalization of E. coli K1 with VEGFR1 in HBMEC. HBMEC cultured on coverslips were left untreated (top) or incubated with E44 for 15 min (bottom), and then the cells were fixed, permeabilized, subsequently stained with rabbit anti-VEGFR1 antibody, and incubated with Alexa Fluor 594-conjugated anti-rabbit IgG. The bacteria were stained with FITC-conjugated E. coli-specific antibody. Samples were analyzed using confocal laser scanning microscopy. The arrows indicate colocalization (yellow) of VEGFR1 and E. coli. Scale bar = 20 μm.
VEGFR1 is required for E. coli K1-induced actin cytoskeleton rearrangements.
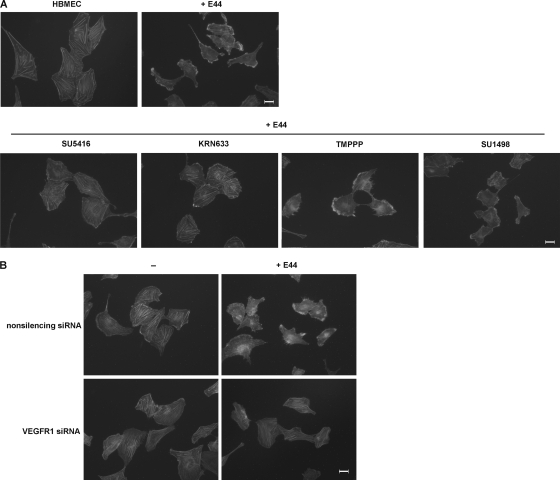
Previous studies showed that E. coli K1 internalization into HBMEC required the rearrangement of the actin cytoskeleton in host cells (17). To further evaluate the role of VEGFR1 in E. coli K1 invasion of HBMEC, HBMEC were treated with VEGFR inhibitors prior to bacterial infection, and the actin filaments were stained with TRITC-labeled phalloidin. As shown in Fig. 4A, normal HBMEC exhibited actin stress fibers extending across the cells, while the HBMEC infected with E. coli E44 exhibited prominent cytoskeleton rearrangements, with the actin filaments being accumulated at multiple sites along the cell periphery. We found that VEGFR inhibitors SU5416 and KRN633 markedly blocked E. coli-induced rearrangement of the actin cytoskeleton, whereas VEGFR2-specific inhibitors TMPPP and SU1498 had no such effect. Moreover, our results showed that E44 failed to induce actin filament changes in HBMEC transfected with VEGFR1 siRNA (Fig. 4B). These results demonstrated that VEGFR1 is involved in E. coli K1-induced actin cytoskeleton rearrangements in HBMEC.
FIG. 4.
E. coli K1-induced actin cytoskeleton rearrangements in HBMEC require VEGFR1. (A) HBMEC cultured on coverslips were treated with SU5416, KRN633, TMPPP, or SU1498 for 30 min, followed by incubation with E44 for 30 min. The actin filaments in HBMEC were stained with TRITC-phalloidin and visualized by immunofluorescence microscopy. (B) HBMEC stably transfected with VEGFR1 siRNA or nonsilencing siRNA were incubated with E44 for 30 min or left untreated, and the actin filaments were analyzed by immunofluorescence. Scale bar = 20 μm.
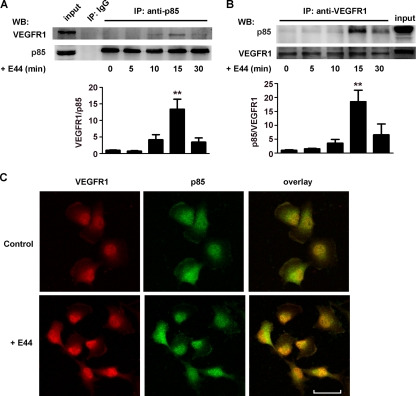
VEGFR1 interacts with PI3K in HBMEC infected with E. coli K1.
PI3K is involved in the regulation of actin cytoskeleton dynamics in eukaryotic cells (18). Previous studies revealed that PI3K in host cells plays an important role in the internalization of pathogens into host cells, including E. coli K1 invasion of HBMEC (1, 29, 32). Since it has been reported that the p85 subunits of PI3K and VEGFR1 are associated when overexpressed in Saccharomyces cerevisiae (yeast) cells (7), we next determined whether this interaction occurs in HBMEC infected with E. coli K1. E44-treated HBMEC were subjected to an immunoprecipitation assay with anti-p85 or anti-VEGFR1 antibody and to Western blotting with anti-VEGFR1 or anti-p85 antibody, as described for Fig. 5A and B. The results showed that VEGFR1 was associated with p85 in HBMEC in response to E44 treatment in a time-dependent manner, with a peak association at 15 min. Moreover, the expression of VEGFR1 and p85 in E44-infected HBMEC and noninfected HBMEC (control) was studied by confocal microscopy. We found that the colocalization of VEGFR1 and p85 was increased in HBMEC infected with E. coli E44 (Fig. 5C). These results suggested that the interaction of VEGFR1 with the p85 subunit of PI3K is enhanced in response to E. coli K1 in HBMEC.
FIG. 5.
E. coli K1 stimulation increased the association between VEGFR1 and p85 in HBMEC. Cell lysates of HBMEC treated with E44 at the indicated times were prepared, and 1 mg total proteins was immunoprecipitated (IP) with anti-p85 antibody (A) or anti-VEGFR1 antibody (B); the precipitated proteins were then analyzed by Western blotting (WB) using antibodies against VEGFR1 and p85 (n = 3). **, P < 0.01. (C) HBMEC cultured on coverslips were left untreated (Control) or incubated with E44 for 15 min; the cells were then stained with rabbit anti-VEGFR1 antibody and mouse anti-p85 antibody and incubated with Alexa Fluor 594-conjugated anti-rabbit IgG and Alexa Fluor 488-conjugated anti-mouse IgG. The coverslips were analyzed by confocal laser scanning microscopy. Yellow color indicates colocalization between VEGFR1 and p85 (scale bar, 40 μm).
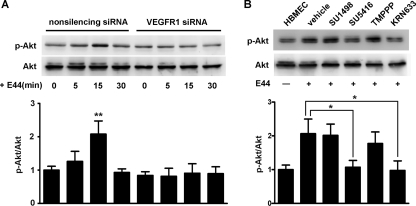
VEGFR1 is required for E. coli K1-induced Akt activation in HBMEC.
Previous studies demonstrated that Akt, a serine/threonine kinase, is a downstream effector of PI3K in E. coli K1-induced signaling in HBMEC (29). Also, Akt is required for PI3K-induced remodeling of actin filaments (18). Here, the levels of Akt activation, as measured by a phosphorylation-specific antibody, p-Akt, were determined to assess the activation of PI3K in HBMEC after bacterial infection. E. coli-induced Akt phosphorylation was abolished by transfection with VEGFR1 siRNA in HBMEC (Fig. 6A). Moreover, treatment with VEGFR inhibitors SU5416 and KRN633 prevented the increased Akt phosphorylation in HBMEC challenged with E44. In contrast, treatment with VEGFR2-specific inhibitors TMPPP and SU1498 had no effect on the phosphorylation level of Akt (Fig. 6B). These data indicated that VEGFR1 is required for E. coli K1-induced PI3K/Akt activation in HBMEC.
FIG. 6.
VEGFR1 is required for E. coli K1-induced Akt phosphorylation. (A) HBMEC stably transfected with VEGFR1 siRNA or nonsilencing siRNA were incubated with E44 for indicated periods of time. The cell lysates were prepared, separated by SDS-PAGE, and immunoblotted with either p-Akt or Akt antibody as described in Materials and Methods (n = 3). **, P < 0.01. (B) Confluent HBMEC were treated with SU5416, KRN633, TMPPP, or SU1498 for 30 min and then incubated with E44 for 15 min. The level of Akt phosphorylation in HBMEC was examined by Western blotting (n = 3). *, P < 0.05.
DISCUSSION
E. coli meningitis develops following a complex and multifactorial pathogen-host interaction. Isolated human brain microvascular endothelial cells (HBMEC) have been used as an in vitro model to explore the mechanisms of microbial traversal across the BBB (15, 17), a key step in the pathogenesis of meningitis. E. coli K1 invades HBMEC through a zipper-like mechanism (16), but the underlying mechanisms remain incompletely understood. In the present study, we found for the first time that vascular endothelial growth factor receptor 1 (VEGFR1), expressed on HBMEC, contributes to E. coli K1 invasion of HBMEC via recruitment of PI3K/Akt signaling.
The function of VEGFR family proteins was largely restricted to angiogenesis and regulation of vasculogenesis (25). Although it has been reported that activation of VEGFR2 is associated with the Tat protein of human immunodeficiency virus (2, 13), whether VEGFR is involved in bacterial infection of host cells remains unknown. In this study, the expression of VEGFR1 and VEGFR2 was demonstrated with HBMEC. We found that pan-VEGFR tyrosine kinase inhibitors SU5416 and KRN633 prevented E. coli K1 invasion of HBMEC without affecting E. coli adhesion to HBMEC. In contrast, VEGFR2-specific kinase inhibitors TMPPP and SU1498 did not affect E. coli K1 adhesion to and invasion of HBMEC. It has been reported that SU5416 and KRN633 could inhibit several tyrosine and serine/threonine kinases in addition to VEGFR (22, 23). To exclude the potential nonspecific effects of the pharmacological inhibitor, HBMEC with stable transfection of VEGFR1 siRNA was established. We found that knockdown of VEGFR1 in HBMEC effectively blocked E. coli K1 adhesion to and invasion of HBMEC, as well as the E. coli K1-induced rearrangements of actin filament in HBMEC. These findings indicated that VEGFR1 is necessary for E. coli K1 internalization into HBMEC. It should be noted that there is a discrepancy regarding the effect of VEGFR1 knockdown and pan-VEGFR inhibitors (SU5416 and KRN633) on bacterial adhesion to HBMEC. We consider that this difference is due to the different expression levels of VEGFR1 in the HBMEC affecting bacterial adhesion assay. Our immunofluorescence results revealed that VEGFR1 is colocalized with E. coli K1 in infected HBMEC, suggesting the binding of E. coli K1 with VEGFR1 on HBMEC. Therefore, when the expression of VEGFR1 was downregulated by VEGFR1-specific siRNA, E. coli K1 could not effectively bind to VEGFR1 on HBMEC, leading to the decreased bacterial adhesion to HBMEC. On the contrary, SU5416 and KRN633 are small molecular chemical compounds targeting VEGFR kinase activation (11, 23, 31), with no effect on VEGFR1 expression in HBMEC (see Fig. S1 in the supplemental material). Hence, E. coli adhesion to HBMEC is not affected by treatment with pan-VEGFR inhibitors.
It has been identified that PI3K/Akt signaling results in activation of cofilin, which promotes reorganization of the actin cytoskeleton for E. coli K1 entry into HBMEC (5, 29). PI3K is a heterodimer consisting of an 85-kDa regulatory subunit (p85) and a 110-kDa catalytic subunit (p110). The p85 regulatory subunit is crucial in mediating the activation of PI3K by receptor tyrosine kinases (RTKs) (9). The SH2 domains of p85 bind to phosphotyrosine residues in the sequence context pYXXM on RTKs (38, 35). This binding both relieves the basal inhibition of p110 by p85 and recruits the p85-p110 heterodimers to its substrate (phosphatidylinositol-4,5-bisphosphate) at the plasma membrane (43, 44). Activation of PI3K/Akt by VEGFR-1 has been reported previously (3, 40), but the involved mechanism remains unclear. Studies have shown that VEGFR1 interacts with p85 in yeast cells (7), and p85 is associated with VEGFR1 in the human multiple myeloma cell line (26) and bovine retinal pericytes (39). In our study, the physical association between VEGFR1 and p85 in HBMEC was identified by coimmunoprecipitation, and this VEGFR1-p85 complex is significantly increased upon E. coli K1 infection. We found that the expression and phosphorylation of VEGFR1 in HBMEC were weakly affected by E. coli K1 (data not shown), and this may be due to the low level of VEGFR1 phosphorylation in response to stimulation (33, 41). Furthermore, VEGFR1 knockdown in HBMEC and treatment of HBMEC with pan-VEGFR inhibitors prevented the PI3K/Akt activation induced by E. coli K1. These results suggested that E. coli K1 induces significant molecular interaction between VEGFR1 and p85 without obvious effect on VEGFR1 phosphorylation, which results in the activation of PI3K/Akt signaling in HBMEC. Taken together, our findings indicated that E. coli K1 adheres to HBMEC through VEGFR1, leading to increased interaction of VEGFR1 with p85 and activation of PI3K/Akt accompanied by actin cytoskeleton rearrangements, ultimately contributing to E. coli K1 invasion into HBMEC.
In HBMEC transfected with VEGFR1 siRNA, the adhesion and invasion were reduced to ∼50%, whereas the E. coli K1-induced actin cytoskeletal rearrangements and Akt phosphorylation in HBMEC were markedly blocked (Fig. 4 and 6). We consider that this discrepancy may be due to the complex mechanism underlying E. coli K1 invasion of host cells. Studies showed that the host cell cytoskeleton plays a key role during bacterial invasion into eukaryotic cells. As for E. coli K1 invasion of HBMEC, although the important role of the actin cytoskeleton during bacterial invasion has been documented, it was observed that microtubule assembly inhibitors nocodazole, colchicine, vincristine and vinblastine and the microtubule-stabilizing agent taxol could also inhibit E. coli K1 invasion of bovine brain microvascular endothelial cells (28). Therefore, it is likely that the invasion of E. coli into BMEC involves not only the actin cytoskeleton but also microtubules. Moreover, it has been demonstrated that E. coli K1 invasion of HBMEC occurs as the result of bacterial interactions with receptors on HBMEC (CD48, gp96, and a 67-kDa laminin receptor) and involves many signaling molecules, such as FAK, PI3K/Akt, Src kinase, Rho GTPases, paxillin, cytosolic phospholipase A2α, and 5-lipoxygenase (17). In our present study, although the E. coli-induced Akt phosphorylation and actin cytoskeletal rearrangements in HBMEC were effectively blocked by VEGFR1 siRNA, additional signaling pathways contributing to bacterial invasion may remain unaffected. As a result, VEGFR1 siRNA transfection could result in moderately reduced (∼50%) E. coli K1 adhesion to and invasion of HBMEC.
We examined the effect of E. coli K1 on phosphorylation of VEGFR1 and found that phosphorylation of VEGFR1 in HBMEC was weakly affected by E. coli K1 (see Fig. S2 in the supplemental material). It has been reported that even the specific ligands of VEGFR1 (such as VEGF-B and placental growth factor) are less able to induce a strong VEGFR1 phosphorylation in endothelial cells, although these ligands can still regulate specific cellular processes, such as endothelial P-selectin translocation (24, 42). Hence, phosphorylation of VEGFR1 may not be clearly discernible in connection with VEGFR1 kinase activity. The specific role of VEGFR1 in bacterial adhesion, invasion, and subsequent events was clearly demonstrated by our results from VEGFR1 siRNA studies. Although E. coli K1 has a weak effect on VEGFR1 phosphorylation, we found that E. coli K1 infection significantly promoted the physical association between VEGFR1 and p85 in HBMEC (Fig. 5). These findings suggest the possibility that E. coli K1 infection could induce a distinct conformation of the intracellular domain in VEGFR1 that may facilitate the coupling of VEGFR1 to intracellular effector PI3K.
VEGFR1 is a 180-kDa transmembrane glycoprotein and belongs to the VEGFR receptor tyrosine protein family that is involved in the development and growth of the vascular endothelial system (10, 25). To date, VEGFR1 remains the elusive member of the VEGFR family. Although the signaling potential of VEGFR1 remains controversial, it has seemed that VEGFR1 plays a role in inducing signaling responses, especially in the cell types that do not express or express low levels of VEGFR2 (4, 6). We found that PI3K/Akt signaling mediated by VEGFR1, but not VEGFR2, is involved in E. coli K1 invasion of HBMEC, and our results showed that the expression level of VEGFR1 is higher than that of VEGFR2 in HBMEC. Therefore, it is tempting to speculate that VEGFR1 is more predominant than VEGFR2 and involved in initiating signal transduction cascades in response to E. coli K1 in HBMEC.
In summary, our findings indicated the involvement of VEGFR1 in E. coli K1 invasion of HBMEC. Bacterial stimulation promotes the physical association between VEGFR1 and p85 subunit of PI3K. VEGFR1 is necessary for PI3K/Akt activation and actin cytoskeleton rearrangements induced by E. coli K1. Further studies are needed to determine the structure(s) of E. coli K1 that uses VEGFR1 for binding to and invasion of HBMEC.
Supplementary Material
Acknowledgments
We are grateful to Sheng-He Huang (Department of Pediatrics, University of Southern California, Los Angeles, CA) for technical assistance.
This work was supported by the National Natural Science Foundation of China (30500277, 30840013, and 30970120), the Doctoral Seeding Fund of Liaoning Province (20051036), and the Innovation Team Program Foundation of Liaoning Province (2006T131).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 30 August 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Agarwal, V., and S. Hammerschmidt. 2009. Cdc42 and the phosphatidylinositol 3-kinase-Akt pathway are essential for Pspc-mediated internalization of pneumococci by respiratory epithelial cells. J. Biol. Chem. 284:19427-19436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albini, A., R. Soldi, D. Giunciuclio, E. Girauo, R. Benelli, L. Primo, D. Noonan, M. Salio, G. Camussi, W. Rock, and F. Bussolino. 1996. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat. Med. 2:1371-1375. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, S., S. Mehta, I. Haque, K. Sengupta, K. Dhar, S. Kambhampati, P. J. Van Veldhuizen, and S. K. Banerjee. 2008. VEGF-A165 induces human aortic smooth muscle cell migration by activating neuropilin-1-VEGFR1-PI3K axis. Biochemistry 47:3345-3351. [DOI] [PubMed] [Google Scholar]
- 4.Bellik, L., M. C. Vinci, S. Filippi, F. Ledda, and A. Parenti. 2005. Intracellular pathways triggered by the selective FLT-1-agonist placental growth factor in vascular smooth muscle cells exposed to hypoxia. Br. J. Pharmacol. 146:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Y.-H., S. H.-M. Chen, A. Jong, Z. Y. Zhou, L. Wei, K. Suzuki, and S.-H. Huang. 2002. Enhanced Escherichia coli invasion of human brain microvascular endothelial cells is associated with alterations in cytoskeleton induced by nicotine. Cell. Microbiol. 4:503-514. [DOI] [PubMed] [Google Scholar]
- 6.Clauss, M., H. Weich, G. Breier, U. Knies, W. Röckl, J. Waltenberger, and W. Risau. 1996. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. J. Biol. Chem. 271:17629-17634. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham, S. A., M. N. Waxham, P. M. Arrate, and T. A. Brock. 1995. Interaction of the Flt-1 tyrosine kinase receptor with the p85 subunit of phosphatidylinositol 3-kinase. J. Biol. Chem. 270:20254-20257. [DOI] [PubMed] [Google Scholar]
- 8.Dietzman, D. E., G. W. Fischer, and F. D. Schoenknecht. 1974. Neonatal Escherichia coli septicemia-bacterial counts in blood. J. Pediatr. 85:128-130. [DOI] [PubMed] [Google Scholar]
- 9.Engelman, J. A., L. Ji, and L. C. Cantley. 2006. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7:606-619. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, C., M. Mazzone, B. Jonckx, and P. Carmeliet. 2008. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat. Rev. Cancer 8:942-956. [DOI] [PubMed] [Google Scholar]
- 11.Fong, T. A. T., L. K. Shawver, L. Sun, C. Tang, H. App, T. J. Powell, Y. H. Kim, R. Schreck, X. Wang, W. Risau, A. Ullrich, K. P. Hirth, and G. McMahon. 1999. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 59:99-106. [PubMed] [Google Scholar]
- 12.Fraley, M. E., W. F. Hoffman, R. S. Rubino, R. W. Hungate, A. J. Tebben, R. Z. Rutledge, R. C. McFall, W. R. Huckle, R. L. Kendall, K. E. Coll, and K. A. Thomas. 2002. Synthesis and initial SAR studies of 3,6-disubstituted pyrazolo[1,5-a]pyrimidines: a new class of KDR kinase inhibitors. Bioorg. Med. Chem. Lett. 12:2767-2770. [DOI] [PubMed] [Google Scholar]
- 13.Ganju, R. K., N. Munshi, B. C. Nair, Z.-Y. Liu, P. Gill, and J. E. Groopman. 1998. Human immunodeficiency virus Tat modulates the Flk-1/KDR receptor, mitogen-activated protein kinases, and components of focal adhesion in Kaposi's sarcoma cells. J. Virol. 72:6131-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang, S.-H., Y.-H. Chen, Q. Fu, M. Stins, Y. Wang, C. Wass, and K. S. Kim. 1999. Identification and characterization of an Escherichia coli invasion gene locus, ibeB, required for penetration of brain microvascular endothelial cells. Infect. Immun. 67:2103-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, S.-H., M. F. Stins, and K. S. Kim. 2000. Bacterial penetration across the blood-brain barrier during the development of neonatal meningitis. Microb. Infect. 2:1237-1244. [DOI] [PubMed] [Google Scholar]
- 16.Kim, B. Y., J. Kang, and K. S. Kim. 2005. Invasion processes of pathogenic Escherichia coli. Int. J. Med. Microbiol. 295:463-470. [DOI] [PubMed] [Google Scholar]
- 17.Kim, K. S. 2008. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 6:625-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kölsch, V., P. G. Charest, and R. A. Firtel. 2008. The regulation of cell motility and chemotaxis by phospholipid signaling. J. Cell Sci. 121:551-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korhonen, T. K., M. V. Valtonen, J. Parkkinen, V. Vaisanen-Rhen, J. Finne, F. Orskov, I. Orskov, S. B. Svenson, and P. H. Makela. 1985. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect. Immun. 48:486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, W., W.-D. Zhao, J.-C. Yan, Z.-Y. Ren, W.-G. Fang, L. Zhu, D.-S. Shang, and Y.-H. Chen. 2010. Involvement of Src tyrosine kinase in Escherichia coli invasion of human brain microvascular endothelial cells. FEBS Lett. 584:27-32. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Y.-J., D.-W. Guo, L. Tian, D.-S. Shang, W.-D. Zhao, B. Li, W.-G. Fang, L. Zhu, and Y.-H. Chen. 2010. Peripheral T cells derived from Alzheimer's disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-alpha-dependent. Neurobiol. Aging 31:175-188. [DOI] [PubMed] [Google Scholar]
- 22.Mologni, L., E. Sala, S. Cazzaniga, R. Rostagno, T. Kuoni, M. Puttini, J. Bain, L. Cleris, S. Redaelli, B. Riva, F. Formelli, L. Scapozza, and C. Gambacorti-Passerini. 2006. Inhibition of RET tyrosine kinase by SU5416. J. Mol. Endocrinol. 37:199-212. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura, K., A. Yamamoto, M. Kamishohara, K. Takahashi, E. Taguchi, T. Miura, K. Kubo, M. Shibuya, and T. Isoe. 2004. KRN633: a selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase that suppresses tumor angiogenesis and growth. Mol. Cancer Ther. 3:1639-1649. [PubMed] [Google Scholar]
- 24.Neagoe, P., C. Lemieux, and M. Sirois. 2005. Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J. Biol. Chem. 280:9904-9912. [DOI] [PubMed] [Google Scholar]
- 25.Olsson, A.-K., A. Dimberg, J. Kreuger, and L. Claesson-Welsh. 2006. VEGF receptor signalling—in control of vascular function. Nat. Rev. Mol. Cell Biol. 7:359-371. [DOI] [PubMed] [Google Scholar]
- 26.Podar, K., Y.-T. Tai, B. K. Lin, R. P. Narsimhan, M. Sattler, T. Kijima, R. Salgia, D. Gupta, D. Chauhan, and K. C. Anderson. 2002. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with beta1 integrin- and phosphatidylinositol 3-kinase-dependent PKC alpha activation. J. Biol. Chem. 277:7875-7881. [DOI] [PubMed] [Google Scholar]
- 27.Polin, R. A., and M. C. Harris. 2001. Neonatal bacterial meningitis. Semin. Neonatol. 6:157-172. [DOI] [PubMed] [Google Scholar]
- 28.Prasadarao, N. V., C. A. Wass, M. F. Stins, H. Shimada, and K. S. Kim. 1999. Outer membrane protein A-promoted actin condensation of brain microvascular endothelial cells is required for Escherichia coli invasion. Infect. Immun. 67:5775-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy, M. A., N. V. Prasadarao, C. A. Wass, and K. S. Kim. 2000. Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J. Biol. Chem. 275:36769-36774. [DOI] [PubMed] [Google Scholar]
- 30.Robbins, J., G. J. McCracken, E. Gotschlich, F. Orskov, I. Orskov, and L. Hanson. 1974. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N. Engl. J. Med. 290:1216-1220. [DOI] [PubMed] [Google Scholar]
- 31.Roskoski, R., Jr. 2007. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem. Biophys. Res. Commun. 356:323-328. [DOI] [PubMed] [Google Scholar]
- 32.Sason, H., M. Milgrom, A. M. Weiss, N. Melamed-Book, T. Balla, S. Grinstein, S. Backert, I. Rosenshine, and B. Aroeti. 2009. Enteropathogenic Escherichia coli subverts phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate upon epithelial cell infection. Mol. Biol. Cell 20:544-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seetharam, L., N. Gotoh, Y. Maru, G. Neufeld, S. Yamaguchi, and M. Shibuya. 1995. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene 10:135-147. [PubMed] [Google Scholar]
- 34.Shibuya, M., and L. Claesson-Welsh. 2006. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 312:549-560. [DOI] [PubMed] [Google Scholar]
- 35.Songyang, Z., S. E. Shoelson, M. Chaudhuri, G. Gish, T. Pawson, W. G. Haser, F. King, T. Roberts, S. Ratnofsky, R. J. Lechleider, B. G. Neel, R. B. Birge, J. E. Fajardo, M. M. Chou, H. Hanafusa, B. Schaffhausen, and L. C. Cantley. 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72:767-778. [DOI] [PubMed] [Google Scholar]
- 36.Stins, M. F., F. Gilles, and K. S. Kim. 1997. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 76:81-90. [DOI] [PubMed] [Google Scholar]
- 37.Strawn, L. M., G. McMahon, H. App, R. Schreck, W. R. Kuchler, M. P. Longhi, T. H. Hui, C. Tang, A. Levitzki, A. Gazit, I. Chen, G. Keri, L. Orfi, W. Risau, I. Flamme, A. Ullrich, K. P. Hirth, and L. K. Shawver. 1996. Flk-1 as a target for tumor growth inhibition. Cancer Res. 56:3540-3545. [PubMed] [Google Scholar]
- 38.Suenaga, A., N. Takada, M. Hatakeyama, M. Ichikawa, X. Yu, K. Tomii, N. Okimoto, N. Futatsugi, T. Narumi, M. Shirouzu, S. Yokoyama, A. Konagaya, and M. Taiji. 2005. Novel mechanism of interaction of p85 subunit of phosphatidylinositol 3-kinase and ErbB3 receptor-derived phosphotyrosyl peptides. J. Biol. Chem. 280:1321-1326. [DOI] [PubMed] [Google Scholar]
- 39.Suzuma, K., K. Naruse, I. Suzuma, N. Takahara, K. Ueki, L. P. Aiello, and G. L. King. 2000. Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1, and phosphatidylinositol 3-kinase-Akt-dependent pathways in retinal vascular cells. J. Biol. Chem. 275:40725-40731. [DOI] [PubMed] [Google Scholar]
- 40.Vogel, C., A. Bauer, M. Wiesnet, K. T. Preissner, W. Schaper, H. H. Marti, and S. Fischer. 2007. Flt-1, but not Flk-1 mediates hyperpermeability through activation of the PI3-K/Akt pathway. J. Cell. Physiol. 212:236-243. [DOI] [PubMed] [Google Scholar]
- 41.Waltenberger, J., L. Claesson-Welsh, A. Siegbahn, M. Shibuya, and C. H. Heldin. 1994. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem. 269:26988-26995. [PubMed] [Google Scholar]
- 42.Warner, A. J., J. Lopez-Dee, E. L. Knight, J. R. Feramisco, and S. A. Prigent. 2000. The Shc-related adaptor protein, Sck, forms a complex with the vascular-endothelial-growth-factor receptor KDR in transfected cells. Biochem. J. 347:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu, J., C. Wjasow, and J. M. Backer. 1998. Regulation of the p85/p110alpha phosphatidylinositol 3′-kinase. Distinct roles for the N-terminal and C-terminal SH2 domains. J. Biol. Chem. 273:30199-30203. [DOI] [PubMed] [Google Scholar]
- 44.Yu, J., Y. Zhang, J. McIlroy, T. Rordorf-Nikolic, G. A. Orr, and J. M. Backer. 1998. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol. Cell. Biol. 18:1379-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.