Abstract
The multiple antigen peptide (MAP) approach is an effective method to chemically synthesize and deliver multiple T-cell and B-cell epitopes as the constituents of a single immunogen. Here we report on the design, chemical synthesis, and immunogenicity of three Plasmodium falciparum MAP vaccines that incorporated antigenic epitopes from the sporozoite, liver, and blood stages of the life cycle. Antibody and cellular responses were determined in three inbred (C57BL/6, BALB/c, and A/J) strains, one congenic (HLA-A2 on the C57BL/6 background) strain, and one outbred strain (CD1) of mice. All three MAPs were immunogenic and induced both antibody and cellular responses, albeit in a somewhat genetically restricted manner. Antibodies against MAP-1, MAP-2, and MAP-3 had an antiparasite effect that was also dependent on the mouse major histocompatibility complex background. Anti-MAP-1 (CSP-based) antibodies blocked the invasion of HepG2 liver cells by P. falciparum sporozoites (highest, 95.16% in HLA-A2 C57BL/6; lowest, 11.21% in BALB/c). Furthermore, antibodies generated following immunizations with the MAP-2 (PfCSP, PfLSA-1, PfMSP-142, and PfMSP-3b) and MAP-3 (PfRAP-1, PfRAP-2, PfSERA, and PfMSP-142) vaccines were able to reduce the growth of blood stage parasites in erythrocyte cultures to various degrees. Thus, MAP-based vaccines remain a viable option to induce effective antibody and cellular responses. These results warrant further development and preclinical and clinical testing of the next generation of candidate MAP vaccines that are based on the conserved protective epitopes from Plasmodium antigens that are widely recognized by populations of divergent HLA types from around the world.
Vaccinations against several deadly infectious agents continue to save millions of lives annually and have improved the quality of life of tens of millions of individuals by significantly preventing or reducing the transmission of several pandemic and locally transmitted infectious diseases. Thus, there are reasons to believe that a successful malaria vaccine would not only significantly reduce malaria mortality and morbidity but also become an important tool in disease control efforts.
The quest to develop a malaria vaccine began more than 6 decades ago with successful vaccination against malaria in birds (16). Since then, several decades of research in experimental models have demonstrated that both whole-parasite- and subunit (recombinant and synthetically produced)-based vaccines can induce protective immunity when delivered under optimal conditions. However, after hundreds of millions of dollars in investments and several dozen clinical trials, recombinant-protein-based candidate malaria vaccines have failed to induce the level of protection that would warrant production as licensed vaccines. The most successful recombinant vaccine, RTS,S, has undergone trials and, at its best, induced 53% protection against clinical malaria in a placebo-controlled clinical trial that involved 5- to 17-month-old children in Kenya and Tanzania (3). The limited success of recombinant vaccines has led to a surge in interest to produce and test whole attenuated parasite-based vaccines against malaria, most of which are based on live attenuated Plasmodium falciparum sporozoites (24, 59). Nonetheless, the whole-parasite-based vaccination approach presents unique challenges in terms of safety, residual virulence, and the potential for reversion in virulence, mode of delivery, and difficulties associated with sufficient production for en masse vaccination. Thus, given the limited amount of clinical immunity conferred by the recombinant-protein-based vaccines and the perceived hurdles with the whole-parasite-based vaccines, it is imperative that malaria researchers continue to apply alternative options to develop and test candidate malaria vaccines.
The complex multistage life cycle of malaria parasites presents unique challenges for vaccine development. Immunity against malaria parasites is stage dependent and species dependent. Many malaria researchers believe that a single-antigen vaccine representing only one stage of the life cycle will not be sufficient and that a multiantigen, multistage vaccine that targets different stages of parasite development is necessary to induce effective immunity. Based on these assumptions, it is reasonable to argue that separate malaria vaccines may be needed to target the different parasite developmental stages and also multiple Plasmodium species that may be prevalent in a given area. In this context, a synthetic-peptide-based approach where multiple protective epitopes representing different stages of the life cycle, possibly from more than one Plasmodium species, are assembled in a vaccine construct appears to be an attractive approach for malaria vaccine development.
The first peptide-based malaria vaccine was based on the repeat sequences of P. falciparum that underwent clinical testing in 1987 (21). Since then, a number of synthetic peptide vaccines have been produced for both murine (P. berghei and P. yoelii) and human (P. falciparum and P. vivax) malarias and tested for immunogenicity (1, 31, 39, 61) or immunogenicity and efficacy (32, 52, 56). However, in spite of the early momentum, several theoretical considerations and technological hurdles have slowed the progress of this vaccine development approach. Some of the perceived major arguments against the peptide-based vaccine approach include their short linear nature that prohibited the inclusion of multiple protective B-cell and T-cell epitopes in the same construct and the presumed inability to create the Cys-Cys disulfide bonds in conformation-dependent epitopes. However, recent studies suggest that protein domains that mimic the native protein structure can be created in chemically synthesized peptide constructs that include conformationally correct Cys-Cys bonds to create immunologically active molecules (7, 48, 49). In addition, recently it has been suggested that large-scale and cost-effective synthesis of peptide-based vaccines is possible (8).
Currently, a reasonably large database of unique B-cell and T-cell epitopes from Plasmodium proteins, including those from human P. falciparum and P. vivax malarias, has become available. By conducting a comprehensive meta-analysis of available data for Plasmodium immune epitopes, Vaughan et al. have identified more than 5,000 unique B-cell and T-cell epitopes for malaria parasites (60). Several of the P. falciparum and P. vivax epitopes were identified in extensive field studies conducted over the last 2 decades (12, 23, 35, 57) and by computer-based predictions of immune epitopes by analysis of genomic and proteomic databases; some of these predictions were validated in HLA-peptide binding studies and in vitro immunological studies (4, 11-13, 29). In this communication, we report the design, synthesis, and immunogenicity studies of three multiple antigen peptide (MAP)-based vaccines for P. falciparum malaria. These MAP vaccines were based on immunodominant B-cell and T-cell epitopes from the major malaria vaccine candidates, circumsporozoite protein (CSP), liver stage antigen 1 (LSA-1), merozoite surface protein 1 (MSP-1) and MSP-3, serine repeat antigen (SERA), and rhoptry-associated protein 1 (RAP-1) and RAP-2, representing the sporozoite, liver, and asexual blood stages of P. falciparum parasites. The synthesis of MAP vaccines was based on a novel technology developed by our group (6, 27). We are presenting data demonstrating the immunogenicity of these P. falciparum MAP vaccines in three inbred mouse strains (C57BL/6, BALB/c, and A/J), one congenic mouse strain (C57BL/6 strain expressing the HLA-A2 molecule), and one outbred mouse strain (CD1). The HLA-A2 transgenic mice were included in these studies to facilitate the determination of the immunogenicity of a CSP-based peptide in MAP-1 that was recognized by supertypes HLA-A*0202 and HLA-A*6802 (12).
MATERIALS AND METHODS
MAP synthesis and purification.
The tetrapeptide MAPs used in this study were synthesized by using a patented technology developed at CBER, FDA, that allows the assembly of up to four epitopes as one molecule (6). The individual malarial epitopes included in the MAP constructs were based on one or more of the following criteria: (i) identification as an immunodominant T- or B-cell epitope in field studies, (ii) evidence for neutralizing antibodies that block the invasion of liver cells by sporozoites or merozoite entry into erythrocytes, and (iii) reported function as a parasite ligand facilitating the merozoite invasion of erythrocytes.
The MAP technology utilizes a peptidyl core of three radially branched lysine residues onto which the malarial antigen sequences of interest were built using standard solid-phase chemistry. The lysine core is immunologically inert and generally accounts for less than 5% of the total molecular weight. The core template and the individual peptides were synthesized at a 0.1- to 0.25-mm synthesis scale on an Applied Biosystems model 433A peptide synthesizer with 9-fluorenylmethoxycarbonyl chemistry mediated by 2-[1-H-benzotriazole-1-yl]-1.13.3-tetramethyluronium hexafluorophosphate/1-hydroxybenzotriazole activation. Amino acids were purchased from Applied Biosystems and Anaspec (San Jose, CA). One millimole of each amino acid was used for single coupling. The following side chain-protecting groups were used: Lys(Boc), Ser(tBu), Glu(OtBu), His(trt), Asp(OtBu), Asn(Trt), Arg(Pbf), Gln(Trt), Trp(Boc), and Cys(Trt). Following final deprotection of the N-terminal amino acid by piperidine, peptides were cleaved for approximately 3 to 3.5 h with a cocktail containing trifluoroacetic acid (TFA), water, triisopropylsilane, and phenol (95:1.25:1.5:2.25) and 250 mg dithiothreitol per 10 ml of cocktail. Following cleavage, peptides were precipitated and washed with methyl tert-butyl ether. Peptide pellets were resuspended in 0.1% TFA or 5 to 10% acetic acid and either subjected immediately to reverse phase high-performance liquid chromatography (RP-HPLC) purification or lyophilized for long-term storage following semiprep purification of the crude mixture.
The peptides were purified by RP-HPLC using a Waters DeltaPak C18 column (19 mm by 300 mm, 15-μm particle size, 300-Å pore size) and a linear gradient of 0.1% TFA/water and 0.1% TFA/acetonitrile. Following purification, the acetonitrile was removed by low-stream nitrogen, followed by a rotary evaporator, and the peptides were then lyophilized for long-term storage.
Mice and immunization.
Female 6- to 12-week-old wild-type C57BL/6 mice, C57BL/6 mice expressing the human HLA-A2 transgene (HLA-A2), and BALB/c, A/J, and outbred CD1 mice were used in this study. The MAP vaccines were reconstituted in appropriate solvents. Groups of six mice each were immunized by three subcutaneous injections delivered at 4-week intervals. The vaccine formulation consisted of 20 μg of tetraepitope MAP in 100 μl phosphate-buffered saline (PBS) emulsified in 100 μl Montanide ISA 51 as an adjuvant. The control groups of mice received a similar volume of adjuvant in PBS only. Blood samples were drawn 2 days prior to each immunization and at 2 weeks after the last immunization. Serum samples from six mice per group were pooled and used in all serological assays. All of the experimental animals were housed, fed, and used in accordance with guidelines set forth in the National Institutes of Health manual Guide for the Care and Use of Laboratory Animals. The Center for Biologics Evaluation and Research Animal Care and Use Committee approved the animal study protocol.
Immunological assays. (i) ELISA.
Peptide-specific total IgG levels were assayed in the sera of MAP-immunized and control immunized mice by enzyme linked immunosorbent assay (ELISA). Briefly, flat-bottom Immulon II ELISA plates (Dynatech Laboratories) were coated overnight at 4°C with 50 μl of a 1-μg/ml solution of either MAP or individual peptides or recombinant PfCSP (rPfCSP) expressed in Escherichia coli in PBS, pH 7.4. The wells were blocked by incubation with 5% bovine serum albumin (BSA) in PBST (PBS containing 0.05% Tween 20) at 37°C for 1 h. Fifty-microliter volumes of 2-fold serial dilutions of the test sera or a control serum in 1% BSA-PBST were added to the wells and incubated for 60 min at 37°C. The wells were washed thoroughly after incubation and then incubated with an alkaline phosphatase conjugate for 1 h. This was followed by development of the plate using phosphatase substrate tablets (Sigma Aldrich Co.) and measurement of the optical density at 405 nm using an ELISA reader.
(ii) IIF.
Indirect immunofluorescence (IIF) was performed against the sporozoite and intraerythrocytic stages of P. falciparum parasites coating 12-well slides using the sera obtained at 2 weeks after the third immunization. Parasites were incubated with serial dilutions of sera and incubated in a moist chamber at 37°C. After 1 h of incubation, unbound material was removed by washing and Alexa Flour 488 goat anti-mouse IgG (Invitrogen Corporation) was added to the cells. Following washings, slides were mounted using VECTASHIELD mounting medium for fluorescence (Vector Laboratories Inc.) and evaluated using a fluorescence microscope. Slides processed with preimmune mouse sera were used as controls.
In vitro infection of HC-04 human hepatocytes and IIF.
The sera from the mice immunized with MAP-2 were also tested for the ability to react with P. falciparum liver stage parasites. The HC-04 human hepatocyte cell line is known to support the development of P. falciparum liver forms (44). HC-04 cells (40,000/well) were seeded into Lab-Tek eight-chambered plastic slides precoated with ECL (elastin-collagen-laminin) 1 day before infection. Purified P. falciparum sporozoites of the 3D7 strain (50,000 in 50 μl Dulbecco's modified Eagle's medium [DMEM]-nutrient mixture F-12 containing 10% fetal bovine serum [FBS] and 1× penicillin-streptomycin) were added to each well containing HC-04 cells. Slides were incubated for 3 h at 37°C in 5% CO2, after which the P. falciparum sporozoite suspension was aspirated from each well and each well was washed three times with complete DMEM-nutrient mixture F-12. P. falciparum sporozoite-infected HC-04 cells were maintained at 37°C in 5% CO2 for 3 days with a daily change of medium. At day 3 postinfection with sporozoites, slides were treated with chilled methanol for 15 min at room temperature. The fixed parasites were washed for 5 min each at room temperature on a rocker platform with three changes of 1× PBS. The polyclonal antibody raised against MAP-2 was used as the primary antibody. A 100-μl aliquot of anti-MAP-2 antibody at a 1:50 dilution in PBS was added to each well of the Lab-Tek slide containing fixed liver stage parasites developing in HC-04 cells and incubated at 37°C for 1 h. After three washings, 5 min each at room temperature, a 100-μl aliquot of goat anti-mouse Alexa Fluor 488 (Invitrogen Corporation) at a 1:200 dilution in PBS containing a 1:1,000 dilution of 4′,6-diamidino-2-phenylindole (DAPI) from a 2-mg/ml stock and 0.001% Evans blue, was added to each well of the Lab-Tek slide. The slides were incubated for 1 h at 37°C, and then the wells were washed three times with PBS and the slide was mounted with a coverslip using VECTASHIELD mounting medium. The slide was observed under a fluorescence microscope using ×400 magnification using filters for green (for Alexa 488), red (Evans blue), and blue (DAPI). Images were captured from the same field viewed with these three filters and superimposed on one another to get the final multicolored images.
(iii) Enzyme-linked immunospot assay (ELISPOT) assay for detection of gamma interferon (IFN-γ)- and interleukin-4 (IL-4)-secreting cells.
Freshly isolated spleen cells taken from the mice on day 14 after immunization were used to detect IFN-γ- and IL-4-secreting cells. Plates were coated with either anti-IFN-γ or anti-IL-4 (BD Biosciences) capture antibody in PBS and kept at 4°C. The following morning, plates were blocked with 10% FBS-RPMI 1640 medium for 2 h. Freshly isolated spleen cells in complete medium (2 × 105/100 μl) were added to each well along with 100 μl of antigen (MAP or individual peptides) and cultured for 36 h at 37°C. The plates were washed with PBST, and biotinylated detection antibody was added for 2 h of incubation at room temperature. This was followed by washing and addition of streptavidin-horseradish peroxidase enzyme conjugate for 1 h. After repeated washing, the spots were revealed with DAB reagent (KPL Inc.) and enumerated using an AID EliSpot Reader System (Cell Technology Inc.). IFN-γ and IL-4 ex vivo responses were scored as the mean ± the standard deviation in triplicate wells. The results were expressed as the number of IFN-γ or IL-4 spot-forming cells (SFC) per 106 splenocytes.
Biological assays. (i) In vitro inhibition of sporozoite invasion (ISI) assay.
The sera from the mice immunized with MAP-1 were tested for the ability to inhibit sporozoite invasion in HepG2 (human hepatoma) cells. The assay was performed as described by Shi et al. (46). Briefly, HepG2 cells were collected, washed, and resuspended in complete minimal essential medium and subsequently plated at a density of 50,000 cells/0.3 ml in ECL-coated Lab-Tek glass slides and incubated for 2 days at 37°C in a CO2 incubator. The following day, the medium was removed and different dilutions of anti-MAP serum or the anti-CSP repeat monoclonal antibody (MAb) NFS1 (positive control) were added per well (in triplicate). This was immediately followed by the addition of 20,000 sporozoites in 50 μl of medium to each well. P. falciparum (strain NF54) sporozoites were obtained by dissection of the salivary glands of Anopheles stephensi mosquitoes as described by Ozaki et al. (38). The sporozoites were allowed to invade liver cells for 3 h, and then the cells were washed with PBS (pH 7.4). Subsequently, the cells were fixed with cold methanol. Sporozoites were visualized by immunostaining by using NFS1 as the primary antibody and anti-mouse IgG-peroxidase conjugate. Diaminobenzidine was used as the substrate. The slides were mounted with Paramount, and intracellular sporozoites were identified and counted. Percent inhibition of invasion was calculated as follows: % inhibition = 100 × [1 − (mean number of P. falciparum sporozoites invading HepG2 cells in test sample)/(mean number of P. falciparum sporozoites invading HepG2 cells in control samples)].
(ii) Merozoite growth inhibition assay (GIA).
The sera from the mice immunized with MAP-2 and MAP-3 were tested for the ability to inhibit merozoite growth by GIA as previously described (20). Briefly, P. falciparum (clone 3D7, FVO, and Camp strains) cultures were prepared in 48-well plates and kept in suspension culture angled on a rotator platform in heat-sealed plastic bags. Synchronized cultures adjusted to 0.2% late ring stages were mixed with sera (1:10 dilution) raised against the MAP vaccine or control serum to a final hematocrit of 4%. The cultures were harvested after 48 h, stained with Hoechst dye 33342, and analyzed by flow cytometry. The fluorescence signal was determined for a minimum of 40,000 erythrocytes gated on forward scatter. The percent inhibition was calculated from the mean parasitemia of triplicate test and control wells as 100 × [(control − test)/control].
RESULTS
Design and characterization of MAPs.
MAP-1 included multiple epitopes from PfCSP, a major protein on the malaria sporozoite surface. CS proteins from different Plasmodium spp. have a few structural features in common, including a signal peptide, a variable central domain (composed of tandem repeats of amino acid sequences and two highly conserved regions), and a C-terminal hydrophobic sequence (30). In P. falciparum, the central region is composed of major repeat units of NANP and a minor repeat unit of NVDP (NANP × 37 and NVDP × 2 for the 3D7 strain of parasite). CSP repeats from the human and murine Plasmodium species are recognized as both B-cell and T-cell epitopes (18, 62). Antibodies against the repeat region can block sporozoites from entering cultured hepatocytes (2, 62, 63). These antibodies can also passively protect mice from sporozoite challenge in murine malarias (14, 40). Although the earliest immunological and vaccination studies were predominantly focused on the NANP repeat units, several additional B-cell and T-cell epitopes have been identified on PfCSP (5, 18, 19).
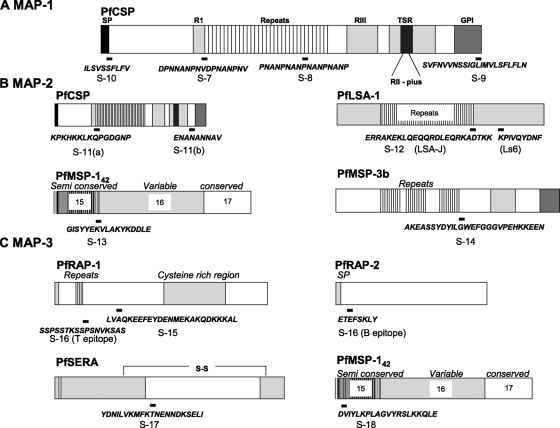
The peptide S-7 (amino acid sequence DPNNANPNVDPNANPNV) was chosen from the 5′ part of the minor repeat region that was identified as a CD4+ T-cell epitope from T-cell clones derived from a P. falciparum sporozoite-immunized volunteer (33). The peptide S-8 (amino acid sequence PNANPNANPNANPNANP) was chosen from the region of tandem repeats of NANP that contain an immunodominant B-cell epitope (33, 34). Major constraints to the development of subunit-based malaria vaccines are the HLA-restricted immune responses and antigenic polymorphism prevalent in the major malaria vaccine candidates (13, 23, 51, 54). Likewise, some of the immunodominant T-cell epitopes recognized by individuals in areas where malaria is endemic are either HLA dependent and/or located in the highly polymorphic regions of the molecule (12, 26, 42, 43, 55). To provide a wider immune responsiveness for the MAP-1 vaccine, epitopes S-9 and S-10 were included. Peptide S-9, located in the C-terminal hydrophobic polymorphic domain (amino acid sequence SVFNVVNSSIGLIMVLSFLFLN), has been identified as both a CD4+ T-cell and a CD8+ T-cell epitope, while peptide S-10, from the conserved N-terminal signal peptide region (amino acid sequence ILSVSSFLFV), was identified based on its binding with supertypes HLA-A*0202 and HLA-A*6802 and demonstrated cytotoxic T-lymphocyte (CTL) activity in peripheral blood mononuclear cells from an irradiated-sporozoite-immunized volunteer (12). Figure 1A shows the locations and amino acid sequences of MAP-1 peptides on PfCSP.
FIG. 1.
Amino acid sequences of the MAP peptides and their physical locations on P. falciparum antigens. (A) MAP-1 vaccine. (B) MAP-2 vaccine. (C) MAP-3 vaccine.
MAP-2 includes two pre-erythrocytic (PfCSP and LSA-1) and two blood stage (MSP-1 and MSP-3) antigens. We designed peptide S-11 as a fusion of sequences from two previously identified B-cell epitopes from PfCSP that are outside the repeat region, sequences KPKHKKLKQPGDGNP (S-11a) and ENANANNAV (S-11b). Epitope S-11a was recognized by sera from individuals living in Gabon, where malaria is endemic (9). Epitope S-11b was recognized more frequently by sera from Brazil and Papua New Guinea than by those from Kenya (47). Epitope S-12 is a combination of two strong T-cell epitopes from LSA-1. The LSA-J peptide is located at the junction of the last repeat unit and the next seven residues from the nonrepetitive region (amino acid sequence ERRAKEKLQEQQRDLEQRKADTKK), while Ls6 is from the C-terminal region of LSA-1 (amino acid sequence KPIVQYDNF). Both of these peptides have proven T-cell-stimulatory activity (15, 28). Peptide S-13 (amino acid sequence GISYYEKVLAKYKDDLE) was chosen from block 15-16, the dimorphic region of P. falciparum MSP-142. In a study in western Kenya, 55% of the individuals tested had proliferative responses to peptide S-13 (57). Finally, the fourth peptide, S-14 (amino acid sequence AKEASSYDYILGWEFGGGVPEHKKEEN), for MAP-2 was selected from MSP-3b. This 27-amino-acid peptide from MSP-3b was identified as the target for antibody-dependent cellular inhibition (ADCI) in the sera from African adults who had clinical immunity to malaria. Affinity-purified antibodies against MSP-3b peptides are effective in killing P. falciparum parasites in vitro through an ADCI mechanism that required the presence of monocytes (35). Figure 1B shows the locations and amino acid sequences of the MAP-2 peptides.
MAP-3 was based on asexual blood stage antigens of P. falciparum that are known to play important roles in the invasion of erythrocytes by merozoites. The first epitope (peptide S-15 [amino acid sequence LVAQKEEFEYDENMEKAKQDKKKAL]) is from RAP-1 and was recognized as a potent B-cell epitope. An anti-RAP-1 MAb that recognizes a sequence within this epitope has been shown to inhibit parasite growth in vitro (25). Peptide S-16 (amino acid sequence SSPSSTKSSPSNVKSASETEFSKLY) contains a T-cell epitope (SSPSSTKSSPSNVKSAS) from RAP-1 (45) and a B-cell epitope (ETEFSKLY) from RAP-2 (52). The third epitope of MAP-3 is based on a red-cell-binding domain from the serine repeat antigen (amino acid sequence YDNILVKMFKTNENNDKSELI) (41). The fourth epitope is again a red-cell-binding domain from the 33-kDa processing fragment of MSP-1 (amino acid sequence DVIYLKPLAGVYRSLKKQLE) and has been shown to be involved in a receptor ligand-type interaction between merozoites and red blood cells (58). Figure 1C shows the locations and amino acid sequences of MAP-3 peptides.
Characterization of MAPs was performed by matrix-assisted laser desorption ionization-time of flight mass spectrometry and amino acid analysis (data not shown). In addition to MAPs, individual peptides were also synthesized and characterized for use in immunological assays.
Immunogenicity of MAPs. (i) Antibody responses measured by ELISA.
The pooled sera from mice that were collected 2 weeks after the third immunization were used to measure the total IgG responses by ELISA. The responses against MAP-1 vaccine were evaluated in HLA-A2, C57BL/6, BALB/c, A/J, and CD1 strains of mice. The responses against MAP-2 and MAP-3 vaccines were evaluated in C57BL/6, BALB/c, A/J, and CD1 strains of mice.
(a) Responses to MAP-1.
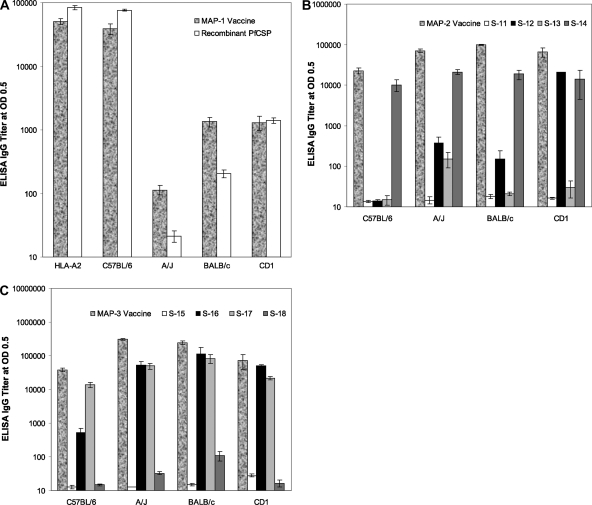
MAP-1 is a combination of four epitopes from PfCSP. ELISA anti-MAP-1 IgG titers were measured using either MAP-1 or individual MAP-1 peptides or rPfCSP as a coating antigen. The highest mean anti-MAP-1 IgG titer was observed in congenic HLA-A2 (50,659 ± 5,431), followed by C57BL/6 mice (39,030 ± 7,454) when MAP-1 was used as a coating antigen. In comparison, MAP-1 was significantly less immunogenic in BALB/c (1,137 ± 222) and A/J (114 ± 19) mice. The response in CD1 mice was similar to that seen in BALB/c mice, with an IgG titer of 1,306 ± 320 (Fig. 2A).
FIG. 2.
ELISA IgG responses in HLA-A2, C57BL/6, A/J, BALB/c, and CD1 strains of mice immunized by three subcutaneous injections of MAP vaccines in Montanide ISA 51 or injected with Montanide ISA 51 alone. ELISA IgG titers were determined in pooled sera (n = 6) from each immunization group. (A) Sera from MAP-1-immunized mice tested against MAP-1 or rPfCSP as a coating antigen. (B) Sera from MAP-2-immunized mice tested against MAP-2 or individual peptides. (C) Sera from MAP-3-immunized mice tested against MAP-3 or individual peptides. ELISA IgG titers are calculated values determined as interpolated titers at an optical density (OD) of 0.5. The bars represent mean ELISA IgG titers, and the error bars reflect the standard deviations of triplicate samples.
When rPfCSP was used as a coating antigen, the responses were again highest in HLA-A2 mice (84,302 ± 6,191), followed by C57BL/6 mice (76,304 ± 2,182) and BALB/c mice (204 ± 28), and negligible in A/J mice (Fig. 2A). The response in CD1 mice was 60-fold lower than in HLA-A2 mice (84,302 ± 6,191 versus 1,405 ± 133). The IgG titers in mice receiving the Montanide ISA 51 control were almost negligible, ranging from 14 to 40. No ELISA IgG reactivities were observed in the sera from MAP-1 immunized mice when individual peptides were used as a coating antigens (data not shown), suggesting that these synthetic peptides, while serving as potent B-cell immunizing epitopes, are not optimal ELISA coating antigens.
(b) Responses to MAP-2.
MAP-2 includes B- and T-cell epitopes from two pre-erythrocytic-stage (CSP and LSA-1) and two erythrocytic-stage (MSP-1 and MSP-3) antigens. This vaccine induced high-level antibody responses in all four strains of immunized mice when MAP-2 was used as a coating antigen. The highest mean IgG titers were in BALB/c mice (98,621 ± 2,990), followed by A/J mice (70,640 ± 6,307) and CD1 mice (65,969 ± 17,399), while lower titers were observed in C57BL/6 mice (22,688 ± 3,869). These results show that the combination epitopes contained within MAP-2 were able to induce relatively high-level, wide-ranging immune responses in diverse major histocompatibility complex (MHC) types (Fig. 2B).
Although MAP-2 contained a strong B-cell epitope from PfCSP (peptide S-11), sera from all four strains of mice failed to react when rPfCSP was used as a coating antigen (data not shown). We further tested the reactivity of sera from mice immunized with MAP-2 with individual peptides. The sera from the four strains of mice also failed to recognize the S-11 peptide. S-12 is a T-cell epitope from LSA-1. When S-12 was used as a coating antigen, there was a moderate but consistent reactivity with sera from A/J (372 ± 155), BALB/c (401 ± 90), and CD1 mice (447 ± 133). However, sera from C57BL/6 mice failed to recognize the S-12 peptide. These results strongly indicate that peptide sequence S-12 also contains a previously unrecognized, novel B-cell epitope. Peptide S-13, a known T-cell epitope, also showed a low level of recognition in A/J mice (154 ± 63) which was ∼12-fold higher than the sera from Montanide ISA 51 control mice (13 ± 1). Peptide S-14 had both a T- and a B-cell epitope from MSP-3. Among the four epitopes from MAP-2, S-14 was maximally recognized and sera from all four strains of mice reacted with the S-14 peptide. The ELISA IgG titers were 21,222 ± 2,525 (A/J mice), 18,759 ± 4,933 (BALB/c mice), 10,177 ± 3,270 (C57BL/6 mice), and 13,887 ± 9,445 (CD1 mice) (Fig. 2B). There was no detectable reactivity with the sera of mice immunized with Montanide ISA 51 only (data not shown).
(c) Responses to MAP-3.
MAP-3 contains sequences only from blood stage antigens that include epitopes from PfRAP-1 and PfRAP-2 and two epitopes from red-cell-binding domains (SERA and MSP-1). This vaccine induced extremely high-level antibody responses in all of the mouse strains tested when MAP-3 was used as a coating antigen. The highest titers were found in A/J (309,801 ± 18,765) and BALB/c (245,734 ± 30,307) mice, followed by CD1 mice (73,433 ± 33,807); the lowest (but still substantial) responses were observed in C57BL/6 mice (37,995 ± 5,018) (Fig. 2C).
IgG responses to individual peptides were also evaluated. Although peptide S-15 is a potent B-cell epitope from RAP-1, sera from mice immunized with MAP-3 failed to react with the S-15 peptide. The sera from all four strains of mice reacted strongly with the S-16 peptide, which possesses sequences representing both T- and B-cell epitopes from RAP-1 and RAP-2. When S-16 was used as a coating antigen, the highest titers were observed in BALB/c mice (112,172 ± 65,755). The A/J and CD1 mice had comparable IgG titers of 51,400 ± 16,322 and 49,912 ± 5,668, respectively, while the responses were lower in C57BL/6 mice (529 ± 177). Peptide S-17, which contains sequences from the red-cell-binding antigen (SERA), was immunogenic in all of the mouse strains tested. With the S-17 peptide as a coating antigen, BALB/c mice responded maximally, with an IgG titer of 82,888 ± 23,910, followed by A/J mice (50,049 ± 10,114). The responses were comparable in the CD1 and C57BL/6 strains of mice (with IgG titers of 21,551 ± 2,792 in CD1 mice and 13,880 ± 2,567 in C57BL/6 mice). The S-18 peptide, a red blood cell-binding epitope from MSP-1, was nonimmunogenic in all groups of immunized mice (Fig. 2C).
(ii) Antibody responses measured by IIF.
We next wanted to know if antibodies against MAP vaccines react with native antigens expressed on the parasite during their respective stages of the life cycle. To accomplish this, we evaluated the reactivity of pooled sera from mice immunized with MAP vaccines with sporozoite stage, liver stage, or asexual blood stage parasites by IIF. Sera from MAP-1 (four epitopes from CSP)-immunized mice were tested against P. falciparum sporozoites. MAP-1 vaccine induced extremely high titers of antibodies against CSP present on the sporozoite surface. The sera from HLA-A2 mice reacted with the sporozoites with an endpoint titer of 409,600. This was followed by the reactivity of sera from BALB/c (102, 400) and C57BL/6 (25, 600) mice. The sera from CD1 and A/J mice also recognized the sporozoites, but their endpoint titers were lower (800 for CD1 mice and 100 for A/J mice) (Table 1).
TABLE 1.
IIF IgG titers in sera from HLA-A2, C57BL/6, A/J, BALB/c, and CD1 strains of mice immunized with MAP vaccines delivered in Montanide ISA 51
| Mouse strain | Endpoint IIF titera |
||
|---|---|---|---|
| MAP-1 | MAP-2 | MAP-3 | |
| HLA-A2 | 409,600 | NDb | ND |
| C57BL/6 | 25,600 | 400 | 400 |
| A/J | 100 | 1,600 | 800 |
| BALB/c | 102,400 | 400 | 1,600 |
| CD1 | 800 | 200 | 3,200 |
Sera from MAP-1-immunized mice were tested against P. falciparum sporozoites. Sera from MAP-2 and MAP-3 immunized mice were tested against P. falciparum asexual blood stage parasites.
ND, not done.
Since the MAP-2 vaccine construct contains a peptide based on the LSA-1 sequence, we determined the reactivity of sera from the mice immunized with MAP-2 with the liver stage malaria parasites. P. falciparum sporozoites were allowed to develop to the liver stage form in human hepatocyte cell line HC-04 for 3 days. When tested at a 1:50 dilution, sera from immunized BALB/c, C57BL/6, and CD1 mice recognized the liver stage parasite (Fig. 3B, C, and D); the intensity of fluorescence of these sera was equivalent to the reactivity seen with a polyclonal mouse antibody generated by immunizing with a DNA plasmid containing the LSA-1 gene (A). The recognition of liver stage P. falciparum parasites by MAP-2-immunized mouse sera indicates that the LSA-1 epitope (peptide S-12) in the MAP-2 vaccine was able to generate antibodies that may be effective against liver stage parasites.
FIG. 3.
IIF IgG reactivity against P. falciparum liver stage parasites in C57BL/6, A/J, BALB/c, and CD1 strains of mice that received three subcutaneous injections of MAP vaccines in Montanide ISA 51. The reactivity for liver stage parasites was tested against (A) polyclonal mouse anti-LSA-1 antibody (positive control), (B) MAP-2-immunized BALB/c mouse sera, (C) C57BL/6-immunized mouse sera, (D) CD1-immunized mouse sera, and (E) normal mouse serum. The arrow points to the developing P. falciparum parasites. The hepatocyte nucleus is stained blue with DAPI.
MAP-2 and MAP-3 contained epitopes from the asexual blood stages of the life cycle, and consequently, sera raised against the MAP-2 and MAP-3 vaccines were tested against the asexual blood stage P. falciparum parasites by IIF. MAP-2 vaccine induced various levels of antiparasite antibodies in the four strains of mice tested. The highest reciprocal endpoint titer was observed in A/J mice (1:1,600). The titer in BALB/c and C57BL/6 mice was 1:400, followed by 1:200 in CD1 mice (Table 1). The sera from MAP-3-immunized mice also recognized the blood stage schizonts. The titer was highest in CD1 mice (1:3,200), followed by BALB/c (1:1,600), A/J (1:800), and C57BL/6 (1:400) mice (Table 1). The antibody responses measured by ELISA and IIF showed that MAPs were generally highly immunogenic and they induced antibodies that specifically recognized the epitopes on the P. falciparum sporozoite, liver, or asexual blood stage of the life cycle.
(iii) Induction of T-cell responses.
We also investigated whether the T-cell epitopes contained in the MAP vaccines were able to generate cell-mediated immunity by assessing the ability of the spleen cells to generate IFN-γ and IL-4 responses following immunization with MAP-1 or MAP-2 vaccine. Spleen cells were harvested from MAP-1-immunized HLA-A2, C57BL/6, and BALB/c mice and MAP-2-immunized C57BL/6 and BALB/c mice on day 14 after the third immunization and cultured for 36 h in the presence of MAP constructs or individual peptides.
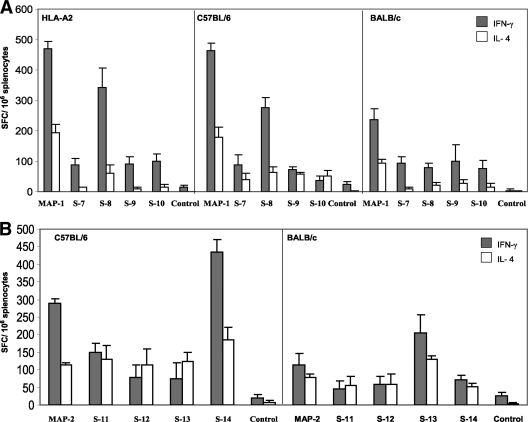
The spleen cells from the three strains of MAP-1-immunized mice were able to produce IFN-γ and IL-4 after in vitro stimulation with MAP-1 (Fig. 4A). The IFN-γ responses were comparable in the spleen cells from HLA-A2 (469 SFC/106 splenocytes) and C57BL/6 (463 SFC/106 splenocytes) mice, while 235 SFC/106 splenocytes were observed in BALB/c mice. While investigating the contributions of individual peptides, we found that peptide S-8, a NANP repeat and a known T- and B-cell epitope, was responsible for the induction of a large portion of the IFN-γ-secreting cells by MAP-1 in HLA-A2 (341 SFC/106 splenocytes) and C57BL/6 (275 SFC/106 splenocytes) mice. The IFN-γ responses induced following stimulation with the S-8 peptide in spleen cells from BALB/c mice were lower (80 spots/106 cells), suggesting that the cellular response induced by the S-8 peptide in the MAP-1 vaccine was under the regulation of MHC genes. The spleen cells also responded to the other three MAP-1 peptides, but the responses were significantly lower (Fig. 4A). The IL-4 production by spleen cells followed the IFN-γ production pattern, except at a lower level, when the cells were cultured in the presence of the MAP-1 construct. The responses were equivalent in HLA-A2 (195 SFC/106 splenocytes) and C57BL/6 (178 SFC/106 splenocytes) mice, followed by that of BALB/c (94 SFC/106 splenocytes) mice.
FIG. 4.
(A) ELISPOT IFN-γ and IL-4 responses in HLA-A2, C57BL/6, and BALB/c mice immunized by three subcutaneous injections of MAP-1 vaccine in Montanide ISA 51. Freshly isolated spleen cells were pooled from five mice per group and cultured in the presence of MAP-1 or individual peptides (10 μg/ml). Data are presented as mean numbers of antigen-specific IFN-γ/IL-4 SFC per million spleen cells, and error bars reflect the standard deviation of triplicate samples. (B) ELISPOT IFN-γ and IL-4 responses in C57BL/6 and BALB/c mice immunized by three subcutaneous injections of MAP-2 vaccine in Montanide ISA 51. Freshly isolated spleen cells from five mice per group were pooled and cultured in the presence of MAP-2 or individual peptides (10 μg/ml). Data are presented as mean numbers of antigen-specific IFN-γ/IL-4 SFC per million spleen cells, and error bars reflect the standard deviations of triplicate samples.
We then evaluated IFN-γ and IL-4 production in response to MAP-2 immunization in C57BL/6 and BALB/c mice. The spleen cells from both strains of mice induced IFN-γ and IL-4 production when cultured in the presence of either the MAP-2 construct or individual peptides (Fig. 4B). The responses of C57BL/6 mice were always stronger than those of BALB/c mice. The frequency of IFN-γ-secreting spleen cells in C57BL/6 mice (436 SFC/106 splenocytes) was highest in response to the S-14 peptide, which is an ADCI epitope from MSP-3. This epitope has been previously described as a T-cell epitope recognized by the majority of individuals in areas where malaria is endemic (36, 50). This was followed by response to the MAP-2 construct (290 SFC/106 splenocytes) and the S-11 peptide (150 SFC/106 splenocytes), which is a B-cell epitope from PfCSP. The responses to the S-12 (77 SFC/106 splenocytes) and S-13 (76 SFC/106 splenocytes) constructs, though lower, were also significant. In BALB/c mice, the highest IFN-γ responses were observed in response to the S-13 peptide (205 SFC/106 splenocytes), which is a known T-cell epitope from MSP-1. This was again followed by 112 SFC/106 splenocytes in response to the MAP-2 construct and 70 SFC/106 splenocytes in response to the S-14 peptide. The responses to the S-11 and S-12 peptides were weaker (Fig. 4B).
We also investigated IL-4 responses in MAP-2-immunized mice. The IL-4 responses also followed the pattern of IFN-γ responses, but at a lower level. In C57BL/6 mice, the responses to the S-14 peptide were the strongest (186 SFC/106 splenocytes). The IL-4 production was equivalent in response to MAP-2 or the individual peptide S-11, S-12, or S-13. In BALB/c mice, the IL-4 (130 SFC/106 splenocytes) responses to the S-13 peptide, which is a T-cell epitope from MSP-1, were the strongest. The responses to the MAP-2 construct and individual peptides S-11, S-12, and S-14 were comparable. Overall, while MAP vaccines and their component peptides were able to generally induce high levels of cellular responses in mice of disparate MHC types, in some instances, these responses were under the regulation of the host genetic background.
Biological activity of anti-MAP antibodies. (i) In vitro ISI in HepG2 cells.
Having established that immunizations with MAP-1 vaccine that incorporated CSP molecule sequences induced high levels of antibodies in mice of diverse MHC types, we wanted to determine if these antibodies have any effect on the growth of liver stage parasites in vitro. To this end, we measured the biological activity in sera by testing the ability to inhibit sporozoite invasion of the hepatoma cell line HepG2. The ISI assay revealed that antibodies against the MAP-1 vaccine have the capacity to inhibit the invasion of HepG2 cells by P. falciparum sporozoites in vitro in mouse strains of diverse MHC types (Table 2). Pooled sera from immunized HLA-A2 mice showed the highest level of inhibition (95.16%) of sporozoite invasion, followed by sera from C57BL/6 (88.72%) and CD1 (66.08%) mice. The ISI effect observed in sera from immunized HLA-A2 mice was almost equivalent to that of an anti-CSP MAb (NFS1) used as a positive control. The ability of the antibodies generated against the MAP-1 construct to inhibit sporozoite invasion in vitro shows that the antibodies are biologically active and effective in reducing the invasion of liver cells by sporozoites.
TABLE 2.
In vitro ISI of HepG2 cells by sera from mice immunized with MAP-1 vaccine
| Vaccine and mouse strain | No. of P. falciparum sporozoites/well (mean ± SD)a | % ISIb |
|---|---|---|
| MAP-1 | ||
| HLA-A2 | 20, 22, 24 (22 ± 2) | 95.16 |
| C57BL/6 | 59, 54, 41 (51.3 ± 9.3) | 88.72 |
| A/J | 362, 370, 360 (364 ± 5.3) | 20 |
| BALB/c | 406, 405, 401 (404 ± 2.6) | 11.21 |
| CD1 | 162, 176, 125 (154.3 ± 26.3) | 66.08 |
| Montanide ISA 51 control | ||
| HLA-A2 | 429, 414, 482 (441.7 ± 35.7) | 0 |
| C57BL/6 | 548, 621, 544 (571 ± 43.3) | 0 |
| A/J | 500, 543, 537 (526.7 ± 23.3) | 0 |
| BALB/c | 433, 478, 410 (440.3 ± 34.6) | 3.22 |
| CD1 | 489, 534,512 (511.7 ± 22.5) | 0 |
| NFS1 (positive control) | 28, 27, 26 (27 ± 1) | 94.06 |
| Medium control | 478, 452, 435 (455 ± 21.6) | 0 |
The mean and standard deviation of P. falciparum sporozoite counts were calculated from triplicate wells after staining with NFS1 (100 μg/ml), an anti-P. falciparum CSP MAb.
ISI was calculated as follows: 100 × [1 − (mean number of P. falciparum sporozoites invading HepG2 cells in test sample/mean number of P. falciparum sporozoites invading HepG2 cells in medium control culture)].
(ii) Merozoite GIA.
Since both MAP-2 and MAP-3 contained epitopes from the asexual blood stage of P. falciparum, antibodies generated against these MAPs were tested to determine if they could affect the growth of blood stage parasites in erythrocytes. GIA was performed with the P. falciparum 3D7, FVO, and Camp strains in suspension cultures. MAP-2 contained epitopes from two blood stage antigens, PfMSP-142 and PfMSP-3b (Fig. 1B). Immunizations with MAP-2 had induced high ELISA IgG antibody levels but moderate levels of IIF IgG antibodies in all four strains of mice tested. Various levels of P. falciparum strain-dependent GIA activity were seen in sera from MAP-2-immunized BALB/c mice (3D7, 4%; FVO, 16%; Camp, 19%) and CD1 mice (3D7, 7%; FVO, 25%; Camp, 35%); sera from C57BL/6 and A/J mice were not effective in the GIA. In MAP-3, all four epitopes were from antigens present in the blood stage of the life cycle (Fig. 1C). Sera from immunized C57BL/6 mice demonstrated the highest level of GIA activity against all three P. falciparum strains tested (3D7, 54%; FVO, 73%; Camp, 62%), while the lowest GIA activity was seen in CD1 mice (3D7, 11%; FVO, 24%; Camp, 29%) (Fig. 5). Thus, the generation of growth-inhibitory antibodies by MAP vaccines appeared to be dependent on the mouse MHC background. We also noted some variations in the level of GIA activity in immunized sera when they were tested against different P. falciparum strains, suggesting that sequence diversity in antigenic epitopes incorporated in the MAP constructs may influence the protective efficacy of MAP vaccine.
FIG. 5.
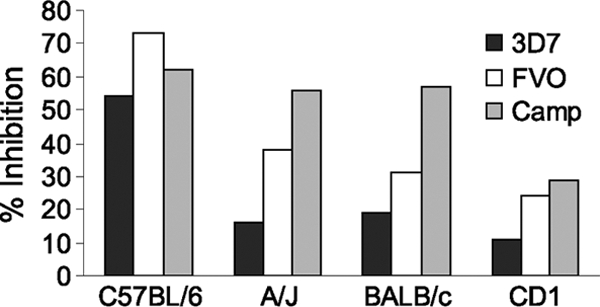
Merozoite growth/invasion-inhibitory activity of anti-MAP-3 sera against the P. falciparum 3D7, FVO, and Camp strains of asexual blood stage parasites. Synchronized ring stage parasites at 0.2% parasitemia were mixed with anti-MAP-3 sera (1:10 dilution) from the C57BL/6, A/J, BALB/c, and CD1 strains of mice in 48-well plates in suspension state. The cultures were harvested after 48 h, and parasitemia was evaluated by enumerating parasites stained with Hoechst dye 33342 and analyzing the fluorescence signal of approximately 40,000 erythrocytes by flow cytometry. Percent inhibition was calculated from the mean parasitemia of triplicate test and control wells as 100 × [(control − test/control)].
DISCUSSION
In this study, we have revisited the concept of a multiantigen, multiepitope vaccine by assembling several immunodominant B-cell and T-cell epitopes from the antigens from the sporozoite, liver, and asexual blood stages of P. falciparum as synthetic MAP vaccines. Previously, we have reported on the design, novel methodology for synthesis, and immunogenicity studies of three MAP vaccines in mice (27). Now we report on the next generation of three MAP vaccines that are based on the better-characterized effective T-cell and B-cell epitopes from P. falciparum that are available in the literature, and we demonstrate their antibody and cellular responses in different MHC haplotype mice.
MAP-1 was based on the antigenic sites from the P. falciparum CSP that contained a total of four epitopes (Fig. 1A). Following three immunizations, the strongest anti-MAP-1 ELISA IgG responses were observed in mice with the C57BL/6 background (in both the HLA-A2 transgene and the wild-type genotype), while weaker responses were seen in BALB/c and CD1 mice and the vaccine was almost nonimmunogenic in A/J mice (Fig. 2A). Since the NANP repeat sequence was the only known B-cell epitope present in MAP-1, we think that the majority of anti-MAP-1 and anti-PfCSP ELISA IgG responses detected were directed against this particular epitope. Our finding that the strongest ELISA response was generated in the C57BL/6 background is in agreement with an earlier report demonstrating that the antibody response to the NANP epitope was under MHC regulation and mapped to the I-Ab gene product (17). While (NANP)n ≥2-mer has been shown to contain the B-cell epitope, (NANP)n ≥16-mer is essential for optimal T-cell activity (17). Since MAP-1 contained only four repeats of NANP, the strong anti-CSP ELISA IgG response observed in this study (Fig. 2A) could be attributed to T-cell help provided by the other immunodominant CD4+ T-cell epitopes in this vaccine. Alternatively, it is possible that fewer repeat units of NANP are needed for effective T-cell function when presented as macromolecules such as MAP. The inability of peptide S-8 to recognize anti-NANP antibodies can be explained by the fact that >40 NANP repeats are needed to serve as an effective ELISA coating antigen (10). Immunizations with the MAP-1 vaccine also induced antibodies against the native sporozoite surface antigens as measured by IIF. The highest antibody levels were observed in C57BL/6 HLA-A2 mice (mean endpoint titer, 409,600), while the lowest antibody levels were seen in A/J mice (mean endpoint titer, 100).
MAP-1 immunization-induced antibodies were also highly effective in preventing the invasion of liver cells by P. falciparum sporozoites. Again, the strongest sporozoite invasion-inhibitory effect was seen in the sera from C57BL/6 HLA-A2 mice (95.16% invasion inhibition), and in fact these antibodies were almost equivalent to anti-CSP MAb NFS1 (positive control) in their inhibitory effect. Although the generation of sporozoite invasion-inhibitory antibodies was somewhat dependent on the MHC-background, some inhibitory effect was present in sera from all of the mouse strains tested (Table 2). MAP-1 vaccine also induced effective cellular responses, as measured by the frequency of IFN-γ- and IL-4-producing splenocytes. In a recall ELISPOT assay following stimulation with peptide S-8, the highest frequencies of IFN-γ-producing spleen cells were observed in C57BL/6 HLA-A2 and wild-type C57BL/6 mice. IFN-γ-producing cells, albeit at a lower level, were also observed with the other three peptides containing T-cell epitopes. Generally lower frequencies of IL-4-producing cells were seen in all of the mouse strains when spleen cells were stimulated against the four individual peptides. IFN-γ is known to play a critical role in immunity against pre-erythrocytic-stage malaria (37), and the fact that MAP-1 immunizations induced a high frequency of IFN-γ-producing T cells raises the prospect that in clinical studies, this vaccine might induce effective responses that target liver stage parasites.
The most interesting aspect of this study was that, contrary to our expectation, the presence of HLA-A2 molecules in the C57BL/6 background facilitated the generation of higher anti-MAP-1 antibody levels (1.3-fold by ELISA and 16-fold by IIF) and slightly greater ISI activity (P > 0.027) than their wild-type C57BL/6 counterparts. Surprisingly, expression of the HLA-A2 product did not influence either overall MAP-1-specific or peptide S-10-specific cellular responses, as measured by the frequency of IFN-γ-producing spleen cells by ELISPOT assay. How the presence of the HLA-A2 product influenced the magnitude and biological activity of anti-MAP-1 antibodies in C57BL/6 mice is not clear. One possibility is that the cytokines synthesized by CD8+ T cells that were generated following the interactions between HLA-A2 and the peptide S-8 complex had influenced the quality and quantity of antibodies synthesized by primed B cells. However, additional studies are needed to address this question.
MAP-2, which included B-cell and T-cell epitopes from CSP and LSA-1 (pre-erythrocytic stage), and MSP-1 and MSP-3 (blood stage antigens) induced high ELISA IgG titers in all four strains of mice tested. Among the four epitopes that constituted MAP-2, no antibody response was detected against peptide S-11, which is composed of two B-cell epitopes from PfCSP. The first epitope (S-11a) is located at the NH2 terminus to the repeat region and constitutes a part of the RI domain. This sequence was identified as a B-cell epitope in two independent studies in Africa (9, 47) but was found to be nonresponsive in C57BL/6 mice when delivered in complete Freund's adjuvant (9). The second epitope (S-11b) was first identified based on its reactivity with an anti-CSP MAb generated in BALB/c mice (53) and recognition by sera from individuals living in Papua New Guinea and Brazil (47). Our studies suggest that while these B-cell epitopes are individually recognized by some individuals living in areas where malaria is endemic, when presented as a combination epitope in a MAP vaccine, they are not effective B-cell epitopes in mice. A moderate antibody response was detected in three out of four strains of mice against peptide S-12, a known T-cell epitope from LSA-1, suggesting the presence of a previously unidentified B-cell epitope within this sequence. In a study of children in Gabon, IFN-γ secretion in response to the LSA-J and Ls6 epitopes was associated with resistance to reinfection in mild malaria but not in severe malaria (28). The Ls6 epitope of LSA-1 has been shown to generate CTL responses in HLA-B53- and HLA-B35-positive individuals (23), and CTL responses to it are associated with protection against severe malaria in HLA-B53-restricted individuals in Gambia (22, 23). Peptide S-14 was immunogenic in all four strains of mice and induced the strongest ELISA IgG responses among the four peptides that constituted MAP-2 (Fig. 2B). Peptide S-14 is based on the well-characterized B-cell and T-cell epitopes from MSP-3. This epitope is recognized by the predominantly cytophilic IgG1 and IgG3 subclasses of antibodies in sera from adults immune to malaria (35). Antibodies that were isolated by immunopurification of immune sera against this epitope are known to kill P. falciparum parasites in vitro through an ADCI mechanism that requires the presence of monocytes (35). The most effective cellular responses measured following MAP-2 immunizations were also against peptide S-14, i.e., 436 IFN-γ-secreting SFC/106 splenocytes in C57BL/6 mice. A lower level of response against peptide S-12 (a T-cell epitope from LSA1) was detected, i.e., 77 SFC/106 splenocytes. The most effective response was against peptide S-13 in BALB/c mice, which had 205 SFC/106 splenocytes. Anti-MAP-2 antibodies had no GIA activity.
MAP-3, composed of four antigens from the asexual blood stage of P. falciparum, was highly immunogenic in all of the mouse strains tested, with the highest titers achieved in A/J and BALB/c mice. It appears that most of this response was directed against peptide S-16, a major B-cell and T-cell epitope in RAP-1 and RAP-2. Peptide S-17, a red-cell-binding domain from SERA, induced high levels of antibodies in BALB/c and A/J mice and moderate levels in C57BL/6 and CD1 mice (Fig. 2C). Anti-MAP-3 antibodies were also biologically relevant, with the strongest effect seen in the sera from C57BL/6 mice, a 54 to 73% reduction in the growth of different strains of blood stage P. falciparum parasites in erythrocyte cultures, while a lesser degree of GIA activity was demonstrated in sera from the other three strains of mice (Fig. 5).
In summary, we have designed and constructed three novel MAP vaccines and determined their immunogenicity in four strains of mice of disparate MHC types. These MAP vaccines were able to induce effective antibody and cellular responses, although immune responses against the individual peptides were generally MHC dependent. Sera from immunized C57BL/6 mice also exhibited a high level of ISI activity (anti-MAP-1) and a moderate level of GIA activity (anti-MAP-3). These studies have allowed the identification of a set of immunogenic peptides that could be incorporated in the next iterations of MAP constructs that may generate highly effective antimalarial responses in populations of genetically diverse HLA types.
Acknowledgments
We thank Davison Sangweme and Phuong Thao Pham for their help with the production of P. falciparum sporozoites. We thank Noel Gerald for critically reading the manuscript.
The opinions or assertions contained herein are our private views and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense. The research described here was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC publication, 1996 edition.
Editor: J. H. Adams
Footnotes
Published ahead of print on 7 September 2010.
REFERENCES
- 1.Audran, R., M. Cachat, F. Lurati, S. Soe, O. Leroy, G. Corradin, P. Druilhe, and F. Spertini. 2005. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect. Immun. 73:8017-8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballou, W. R., J. Rothbard, R. A. Wirtz, D. M. Gordon, J. S. Williams, R. W. Gore, I. Schneider, M. R. Hollingdale, R. L. Beaudoin, W. L. Maloy, et al. 1985. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science 228:996-999. [DOI] [PubMed] [Google Scholar]
- 3.Bejon, P., J. Lusingu, A. Olotu, A. Leach, M. Lievens, J. Vekemans, S. Mshamu, T. Lang, J. Gould, M. C. Dubois, M. A. Demoitie, J. F. Stallaert, P. Vansadia, T. Carter, P. Njuguna, K. O. Awuondo, A. Malabeja, O. Abdul, S. Gesase, N. Mturi, C. J. Drakeley, B. Savarese, T. Villafana, W. R. Ballou, J. Cohen, E. M. Riley, M. M. Lemnge, K. Marsh, and L. von Seidlein. 2008. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N. Engl. J. Med. 359:2521-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum-Tirouvanziam, U., C. Servis, A. Habluetzel, D. Valmori, Y. Men, F. Esposito, L. Del Nero, N. Holmes, N. Fasel, and G. Corradin. 1995. Localization of HLA-A2.1-restricted T cell epitopes in the circumsporozoite protein of Plasmodium falciparum. J. Immunol. 154:3922-3931. [PubMed] [Google Scholar]
- 5.Bongfen, S. E., P. M. Ntsama, S. Offner, T. Smith, I. Felger, M. Tanner, P. Alonso, I. Nebie, J. F. Romero, O. Silvie, R. Torgler, and G. Corradin. 2009. The N-terminal domain of Plasmodium falciparum circumsporozoite protein represents a target of protective immunity. Vaccine 27:328-335. [DOI] [PubMed] [Google Scholar]
- 6.Boykins, R. A., M. Joshi, C. Syin, S. Dhawan, and H. Nakhasi. 2000. Synthesis and construction of a novel multiple peptide conjugate system: strategy for a subunit vaccine design. Peptides 21:9-17. [DOI] [PubMed] [Google Scholar]
- 7.Caro-Aguilar, I., S. Lapp, J. Pohl, M. R. Galinski, and A. Moreno. 2005. Chimeric epitopes delivered by polymeric synthetic linear peptides induce protective immunity to malaria. Microbes Infect. 7:1324-1337. [DOI] [PubMed] [Google Scholar]
- 8.Corradin, G. 2007. Peptide based malaria vaccine development: personal considerations. Microbes Infect. 9:767-771. [DOI] [PubMed] [Google Scholar]
- 9.Del Giudice, G., Q. Cheng, D. Mazier, N. Berbiguier, J. A. Cooper, H. D. Engers, C. Chizzolini, A. S. Verdini, F. Bonelli, A. Pessi, et al. 1988. Immunogenicity of a non-repetitive sequence of Plasmodium falciparum circumsporozoite protein in man and mice. Immunology 63:187-191. [PMC free article] [PubMed] [Google Scholar]
- 10.Del Giudice, G., A. S. Verdini, M. Pinori, A. Pessi, J.-P. Verhave, C. Tougne, B. Ivanoff, P.-H. Lambert, and H. D. Engers. 1987. Detection of human antibodies against Plasmodium falciparum sporozoites using synthetic peptides. J. Clin. Microbiol. 25:91-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Guercio, M. F., J. Sidney, G. Hermanson, C. Perez, H. M. Grey, R. T. Kubo, and A. Sette. 1995. Binding of a peptide antigen to multiple HLA alleles allows definition of an A2-like supertype. J. Immunol. 154:685-693. [PubMed] [Google Scholar]
- 12.Doolan, D. L., S. L. Hoffman, S. Southwood, P. A. Wentworth, J. Sidney, R. W. Chesnut, E. Keogh, E. Appella, T. B. Nutman, A. A. Lal, D. M. Gordon, A. Oloo, and A. Sette. 1997. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA-B supertype alleles. Immunity 7:97-112. [DOI] [PubMed] [Google Scholar]
- 13.Doolan, D. L., S. Southwood, D. A. Freilich, J. Sidney, N. L. Graber, L. Shatney, L. Bebris, L. Florens, C. Dobano, A. A. Witney, E. Appella, S. L. Hoffman, J. R. Yates III, D. J. Carucci, and A. Sette. 2003. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc. Natl. Acad. Sci. U. S. A. 100:9952-9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan, J. E., J. L. Weber, W. R. Ballou, M. R. Hollingdale, W. R. Majarian, D. M. Gordon, W. L. Maloy, S. L. Hoffman, R. A. Wirtz, I. Schneider, et al. 1987. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science 236:453-456. [DOI] [PubMed] [Google Scholar]
- 15.Fidock, D. A., H. Gras-Masse, J. P. Lepers, K. Brahimi, L. Benmohamed, S. Mellouk, C. Guerin-Marchand, A. Londono, L. Raharimalala, J. F. Meis, et al. 1994. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J. Immunol. 153:190-204. [PubMed] [Google Scholar]
- 16.Freund, J., H. E. Sommer, and A. W. Walter. 1945. Immunization against malaria: vaccination of ducks with killed parasites incorporated with adjuvants. Science 102:200-202. [DOI] [PubMed] [Google Scholar]
- 17.Good, M. F., J. A. Berzofsky, W. L. Maloy, Y. Hayashi, N. Fujii, W. T. Hockmeyer, and L. H. Miller. 1986. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J. Exp. Med. 164:655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Good, M. F., J. A. Berzofsky, and L. H. Miller. 1988. The T cell response to the malaria circumsporozoite protein: an immunological approach to vaccine development. Annu. Rev. Immunol. 6:663-688. [DOI] [PubMed] [Google Scholar]
- 19.Good, M. F., W. L. Maloy, M. N. Lunde, H. Margalit, J. L. Cornette, G. L. Smith, B. Moss, L. H. Miller, and J. A. Berzofsky. 1987. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science 235:1059-1062. [DOI] [PubMed] [Google Scholar]
- 20.Haynes, J. D., J. K. Moch, and D. S. Smoot. 2002. Erythrocytic malaria growth or invasion inhibition assays with emphasis on suspension culture GIA. Methods Mol. Med. 72:535-554. [DOI] [PubMed] [Google Scholar]
- 21.Herrington, D. A., D. F. Clyde, G. Losonsky, M. Cortesia, J. R. Murphy, J. Davis, S. Baqar, A. M. Felix, E. P. Heimer, D. Gillessen, et al. 1987. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature 328:257-259. [DOI] [PubMed] [Google Scholar]
- 22.Hill, A. V., C. E. Allsopp, D. Kwiatkowski, N. M. Anstey, P. Twumasi, P. A. Rowe, S. Bennett, D. Brewster, A. J. McMichael, and B. M. Greenwood. 1991. Common west African HLA antigens are associated with protection from severe malaria. Nature 352:595-600. [DOI] [PubMed] [Google Scholar]
- 23.Hill, A. V., J. Elvin, A. C. Willis, M. Aidoo, C. E. Allsopp, F. M. Gotch, X. M. Gao, M. Takiguchi, B. M. Greenwood, A. R. Townsend, et al. 1992. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature 360:434-439. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman, S. L., P. F. Billingsley, E. James, A. Richman, M. Loyevsky, T. Li, S. Chakravarty, A. Gunasekera, M. Li, R. Stafford, A. Ahumada, J. E. Epstein, M. Sedegah, S. Reyes, T. L. Richie, K. E. Lyke, R. Edelman, M. Laurens, C. V. Plowe, and B. K. Sim. 2010. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum. Vaccin. 6:97-106. [DOI] [PubMed] [Google Scholar]
- 25.Howard, R. F., K. C. Jacobson, E. Rickel, and J. Thurman. 1998. Analysis of inhibitory epitopes in the Plasmodium falciparum rhoptry protein RAP-1 including identification of a second inhibitory epitope. Infect. Immun. 66:380-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jalloh, A., M. Jalloh, and H. Matsuoka. 2009. T-cell epitope polymorphisms of the Plasmodium falciparum circumsporozoite protein among field isolates from Sierra Leone: age-dependent haplotype distribution? Malar. J. 8:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi, M. B., A. A. Gam, R. A. Boykins, S. Kumar, J. Sacci, S. L. Hoffman, H. L. Nakhasi, and R. T. Kenney. 2001. Immunogenicity of well-characterized synthetic Plasmodium falciparum multiple antigen peptide conjugates. Infect. Immun. 69:4884-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luty, A. J., B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, F. Migot-Nabias, P. Deloron, R. S. Nussenzweig, and P. G. Kremsner. 1999. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J. Infect. Dis. 179:980-988. [DOI] [PubMed] [Google Scholar]
- 29.Lyke, K. E., R. B. Burges, Y. Cissoko, L. Sangare, A. Kone, M. Dao, I. Diarra, M. A. Fernandez-Vina, C. V. Plowe, O. K. Doumbo, and M. B. Sztein. 2005. HLA-A2 supertype-restricted cell-mediated immunity by peripheral blood mononuclear cells derived from Malian children with severe or uncomplicated Plasmodium falciparum malaria and healthy controls. Infect. Immun. 73:5799-5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCutchan, T. F., J. C. Kissinger, M. G. Touray, M. J. Rogers, J. Li, M. Sullivan, E. M. Braga, A. U. Krettli, and L. H. Miller. 1996. Comparison of circumsporozoite proteins from avian and mammalian malarias: biological and phylogenetic implications. Proc. Natl. Acad. Sci. U. S. A. 93:11889-11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno, C. A., R. Rodriguez, G. A. Oliveira, V. Ferreira, R. S. Nussenzweig, Z. R. Moya Castro, J. M. Calvo-Calle, and E. Nardin. 1999. Preclinical evaluation of a synthetic Plasmodium falciparum MAP malaria vaccine in Aotus monkeys and mice. Vaccine 18:89-99. [DOI] [PubMed] [Google Scholar]
- 32.Nardin, E. 2010. The past decade in malaria synthetic peptide vaccine clinical trials. Hum. Vaccin. 6:27-38. [DOI] [PubMed] [Google Scholar]
- 33.Nardin, E. H., D. A. Herrington, J. Davis, M. Levine, D. Stüber, B. Takacs, P. Caspers, P. Barr, R. Altszuler, P. Clavijo, et al. 1989. Conserved repetitive epitope recognized by CD4+ clones from a malaria-immunized volunteer. Science 246:1603-1606. [DOI] [PubMed] [Google Scholar]
- 34.Nardin, E. H., G. A. Oliveira, J. M. Calvo-Calle, Z. R. Castro, R. S. Nussenzweig, B. Schmeckpeper, B. F. Hall, C. Diggs, S. Bodison, and R. Edelman. 2000. Synthetic malaria peptide vaccine elicits high levels of antibodies in vaccinees of defined HLA genotypes. J. Infect. Dis. 182:1486-1496. [DOI] [PubMed] [Google Scholar]
- 35.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 36.Oeuvray, C., H. Bouharoun-Tayoun, H. Grass-Masse, J. P. Lepers, L. Ralamboranto, A. Tartar, and P. Druilhe. 1994. A novel merozoite surface antigen of Plasmodium falciparum (MSP-3) identified by cellular-antibody cooperative mechanism antigenicity and biological activity of antibodies. Mem. Inst. Oswaldo Cruz 89(Suppl. 2):77-80. [DOI] [PubMed] [Google Scholar]
- 37.Overstreet, M. G., I. A. Cockburn, Y. C. Chen, and F. Zavala. 2008. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol. Rev. 225:272-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozaki, L. S., R. W. Gwadz, and G. N. Godson. 1984. Simple centrifugation method for rapid separation of sporozoites from mosquitoes. J. Parasitol. 70:831-833. [PubMed] [Google Scholar]
- 39.Perlaza, B. L., J. P. Sauzet, A. T. Balde, K. Brahimi, A. Tall, G. Corradin, and P. Druilhe. 2001. Long synthetic peptides encompassing the Plasmodium falciparum LSA3 are the target of human B and T cells and are potent inducers of B helper, T helper and cytolytic T cell responses in mice. Eur. J. Immunol. 31:2200-2209. [DOI] [PubMed] [Google Scholar]
- 40.Potocnjak, P., N. Yoshida, R. S. Nussenzweig, and V. Nussenzweig. 1980. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J. Exp. Med. 151:1504-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puentes, A., J. Garcia, R. Vera, Q. R. Lopez, M. Urquiza, M. Vanegas, L. M. Salazar, and M. E. Patarroyo. 2000. Serine repeat antigen peptides which bind specifically to red blood cells. Parasitol. Int. 49:105-117. [DOI] [PubMed] [Google Scholar]
- 42.Quang, N. D., P. T. Hoa, M. S. Tuan, N. X. Viet, A. Jalloh, and H. Matsuoka. 2009. Polymorphism at the apical membrane antigen 1 gene (AMA1) of the malaria parasite Plasmodium falciparum in a Vietnamese population. Biochem. Genet. 47:370-383. [DOI] [PubMed] [Google Scholar]
- 43.Riley, E. M., O. Olerup, S. Bennett, P. Rowe, S. J. Allen, M. J. Blackman, M. Troye-Blomberg, A. A. Holder, and B. M. Greenwood. 1992. MHC and malaria: the relationship between HLA class II alleles and immune responses to Plasmodium falciparum. Int. Immunol. 4:1055-1063. [DOI] [PubMed] [Google Scholar]
- 44.Sattabongkot, J., N. Yimamnuaychoke, S. Leelaudomlipi, M. Rasameesoraj, R. Jenwithisuk, R. E. Coleman, R. Udomsangpetch, L. Cui, and T. G. Brewer. 2006. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am. J. Trop. Med. Hyg. 74:708-715. [PubMed] [Google Scholar]
- 45.Shi, Y. P., P. Das, B. Holloway, V. Udhayakumar, J. E. Tongren, F. Candal, S. Biswas, R. Ahmad, S. E. Hasnain, and A. A. Lal. 2000. Development, expression, and murine testing of a multistage Plasmodium falciparum malaria vaccine candidate. Vaccine 18:2902-2914. [DOI] [PubMed] [Google Scholar]
- 46.Shi, Y. P., S. E. Hasnain, J. B. Sacci, B. P. Holloway, H. Fujioka, N. Kumar, R. Wohlhueter, S. L. Hoffman, W. E. Collins, and A. A. Lal. 1999. Immunogenicity and in vitro protective efficacy of a recombinant multistage Plasmodium falciparum candidate vaccine. Proc. Natl. Acad. Sci. U. S. A. 96:1615-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi, Y. P., V. Udhayakumar, M. P. Alpers, M. M. Povoa, A. J. Oloo, T. K. Ruebush II, and A. A. Lal. 1993. Natural antibody responses against the non-repeat-sequence-based B-cell epitopes of the Plasmodium falciparum circumsporozoite protein. Infect. Immun. 61:2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silva-Flannery, L. M., M. Cabrera-Mora, M. Dickherber, and A. Moreno. 2009. Polymeric linear peptide chimeric vaccine-induced antimalaria immunity is associated with enhanced in vitro antigen loading. Infect. Immun. 77:1798-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva-Flannery, L. M., M. Cabrera-Mora, J. Jiang, and A. Moreno. 2009. Recombinant peptide replicates immunogenicity of synthetic linear peptide chimera for use as pre-erythrocytic stage malaria vaccine. Microbes Infect. 11:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh, S., S. Soe, J. P. Mejia, C. Roussilhon, M. Theisen, G. Corradin, and P. Druilhe. 2004. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 190:1010-1018. [DOI] [PubMed] [Google Scholar]
- 51.Stephens, H. A., A. E. Brown, D. Chandanayingyong, H. K. Webster, M. Sirikong, P. Longta, R. Vangseratthana, D. M. Gordon, S. Lekmak, and E. Rungruang. 1995. The presence of the HLA class II allele DPB1*0501 in ethnic Thais correlates with an enhanced vaccine-induced antibody response to a malaria sporozoite antigen. Eur. J. Immunol. 25:3142-3147. [DOI] [PubMed] [Google Scholar]
- 52.Stowers, A. W., J. A. Cooper, T. Ehrhardt, and A. Saul. 1996. A peptide derived from a B cell epitope of Plasmodium falciparum rhoptry associated protein 2 specifically raises antibodies to rhoptry associated protein 1. Mol. Biochem. Parasitol. 82:167-180. [DOI] [PubMed] [Google Scholar]
- 53.Stüber, D., W. Bannwarth, J. R. Pink, R. H. Meloen, and H. Matile. 1990. New B cell epitopes in the Plasmodium falciparum malaria circumsporozoite protein. Eur. J. Immunol. 20:819-824. [DOI] [PubMed] [Google Scholar]
- 54.Takala, S. L., D. Coulibaly, M. A. Thera, A. H. Batchelor, M. P. Cummings, A. A. Escalante, A. Ouattara, K. Traore, A. Niangaly, A. A. Djimde, O. K. Doumbo, and C. V. Plowe. 2009. Extreme polymorphism in a vaccine antigen and risk of clinical malaria: implications for vaccine development. Sci. Transl. Med. 1:2ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takala, S. L., and C. V. Plowe. 2009. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria’. Parasite Immunol. 31:560-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tam, J. P., P. Clavijo, Y. A. Lu, V. Nussenzweig, R. Nussenzweig, and F. Zavala. 1990. Incorporation of T and B epitopes of the circumsporozoite protein in a chemically defined synthetic vaccine against malaria. J. Exp. Med. 171:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Udhayakumar, V., D. Anyona, S. Kariuki, Y. P. Shi, P. B. Bloland, O. H. Branch, W. Weiss, B. L. Nahlen, D. C. Kaslow, and A. A. Lal. 1995. Identification of T and B cell epitopes recognized by humans in the C-terminal 42-kDa domain of the Plasmodium falciparum merozoite surface protein (MSP)-1. J. Immunol. 154:6022-6030. [PubMed] [Google Scholar]
- 58.Urquiza, M., L. E. Rodriguez, J. E. Suarez, F. Guzman, M. Ocampo, H. Curtidor, C. Segura, E. Trujillo, and M. E. Patarroyo. 1996. Identification of Plasmodium falciparum MSP-1 peptides able to bind to human red blood cells. Parasite Immunol. 18:515-526. [DOI] [PubMed] [Google Scholar]
- 59.Vaughan, A. M., R. Wang, and S. H. Kappe. 2010. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum. Vaccin. 6:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaughan, K., M. Blythe, J. Greenbaum, Q. Zhang, B. Peters, D. L. Doolan, and A. Sette. 2009. Meta-analysis of immune epitope data for all plasmodia: overview and applications for malarial immunobiology and vaccine-related issues. Parasite Immunol. 31:78-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, R., Y. Charoenvit, G. Corradin, R. Porrozzi, R. L. Hunter, G. Glenn, C. R. Alving, P. Church, and S. L. Hoffman. 1995. Induction of protective polyclonal antibodies by immunization with a Plasmodium yoelii circumsporozoite protein multiple antigen peptide vaccine. J. Immunol. 154:2784-2793. [PubMed] [Google Scholar]
- 62.Young, J. F., W. T. Hockmeyer, M. Gross, W. R. Ballou, R. A. Wirtz, J. H. Trosper, R. L. Beaudoin, M. R. Hollingdale, L. H. Miller, C. L. Diggs, et al. 1985. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science 228:958-962. [DOI] [PubMed] [Google Scholar]
- 63.Zavala, F., J. P. Tam, M. R. Hollingdale, A. H. Cochrane, I. Quakyi, R. S. Nussenzweig, and V. Nussenzweig. 1985. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 228:1436-1440. [DOI] [PubMed] [Google Scholar]